How useful are biomarkers for the diagnosis of Alzheimer’s disease and especially for its therapy?
Marta Valenza, Caterina Scuderi
Alzheimer’s disease key facts: Alzheimer’s disease (AD) is a slowly progressive neurodegenerative disease with no available effective treatment. It is possible to distinguish an early-onset AD that affects a limited number of subjects of young age, and a sporadic or late-onset form of the disease that affects the vast majority of subjects who are diagnosed with AD. As life expectancy has increased considerably over the past century,the number of people diagnosed with AD has grown exponentially. So, AD and AD-related pathologies represent a huge social and economic burden. The number of individuals waiting for effective disease-modifying therapy is impressive. It is estimated that 50 million people worldwide live with dementia,the majority of these cases are caused by AD (World Health Organization, 2021). In the US about 6 million individuals are living with AD, and more than 9 million people are in EU member states (OECD and European Union,2020). The costs of health care and longterm care are substantial. Given this massive societal impact, enormous efforts have been made to understand the pathogenetic mechanisms of the disease with the hope of identifying new targets and, therefore,developing effective drugs. However, despite huge preclinical and clinical scientific efforts,therapeutic advances are truly modest, and the clinical practice is still anchored to the use of drugs modulating the cholinergic and glutamatergic systems.
AD is a progressive disease in which symptoms worsen over time as neurological degeneration advances. Several risk factors have been identified such as genetics, family history, age, lifestyle, and environment. AD is characterized by peculiar histopathological modifications that occur well in advance of the clinical symptoms. It is well established that AD spans decades; thus,several clinical stages have been classified,each characterized by specific molecular alterations that change as the disease progresses. These include the deposition of amyloid-beta (Aβ) peptides in senile plaques,the formation of neurofibrillary tangles (NFTs)caused by the aberrant phosphorylation of the cytoskeletal neuronal tau protein, and neuronal loss, especially affecting cholinergic neurons. Senile plaques typically tend to accumulate extensively throughout the entire cortex, with the occipital and temporal lobes being the most affected. Clinical investigations suggest that Aβ accumulation is the earliest event that is followed by synaptic dysfunction and increased tau phosphorylation. The latter places neurons at increased risk of degeneration due to the formation of intraneuronal NFTs, leading to neurodegeneration and the onset of cognitive symptoms (Jack and Holtzman,2013). Since NFTs appearance in the brain seems to follow a pattern, Braak and Braak proposed to classify AD in six stages based on neuropathological findings (Braak and Braak,1991). The first area affected by NFTs is the entorhinal cortex (stages I and II, no cognitive symptoms). Then, NFTs seem to affect the hippocampus, firstly in the CA1 region, and limbic regions (stage III, first AD symptoms).Once the amygdala and the thalamus are filled up with NFTs (stage IV), these tangles spread also into neocortical association areas (mainly layers III and V) (stages V and VI, full-blown and advanced AD). The early Aβ deposit and the subsequent NFTs formation with neuronal degeneration constitute the so-called amyloid cascade hypothesis (Hardy and Higgins, 1992), which has been largely confirmed in familial forms of the disease that are caused by mutations in genes encoding for Aβ turnover-related proteins. The most common late-onset AD form appears to be characterized by a more intricate interplay between genetic susceptibility, Aβ aggregation, activation of both microglia and astrocytes, vascular changes, and other factors that impact brain health, such as hypertension, dyslipidemia and obesity, diabetes mellitus, smoking,physical inactivity, depression, and low levels of education (Kivipelto et al., 2018; Valenza et al., 2021). All these contribute to the complexity of the disease.
Aβ represents one of the preferred targets for the development of diseasemodifying drug candidates against AD(Loera-Valencia et al., 2019). After waiting almost twenty years, the last Food and Drug Administration-approved drug for AD, aducanumab, is indeed a monoclonal antibody directed against Aβ oligomers and fibrils. Aducanumab approval gives hope to the many patients waiting for a cure, but it must be emphasized that aducanumab approval has been accompanied by numerous controversies mostly related to the sampling of subjects enrolled in clinical trials and, in particular, to the drug ability to slow quite modestly the cognitive decline in symptomatic AD patients.
Biomarkers for Alzheimer’s disease: AD begins many years before the clinical onset of the disease. The results of both preclinical and human studies converge towards this point. This evidence prompted researchers to identify and standardize biomarkers useful for diagnosing the disease as early as possible, because it is now likewise clear that the treatment must be started very early, during the asymptomatic stages of the disease. Any treatment started when AD is already fully overt may just slightly slow the course of the disease. As AD pathogenesis is complex, there is an urgent need for further research to understand all the important mechanisms underlying it, and at what stage they occur and become measurable.
Although AD is typically defined by specific protein accumulation, it shares with other age-related neurodegenerative diseases many fundamental processes associated with progressive neuronal dysfunction and death (e.g., neuroinflammation, oxidative stress, proteotoxic stress, programmed cell death) that can be present before the onset of clinical features. As discussed below, other diseases may culminate into AD in some cases and patients with positive AD biomarkers do not necessarily develop AD. All these aspects should be taken into account as they complicate the identification and interpretation of biomarkers. Hence,specific non-invasive biomarkers to diagnose neurodegenerative diseases and to monitor their progression in clinical trials are a major research priority. Moreover, biomarkers for early diagnosis could help in preventing or limiting disease progression through preventive or early treatment.
Currently, the diagnostic gold standard for AD is a neuropathological evaluation at autopsy or the measurement of cerebrospinal fluid (CSF) proteins which requires invasive procedures. Blood proteins determination, genetics diagnostics markers,and neuroimaging techniques have also demonstrated possible utility. Recently,several circulating small non-coding RNAs(sncRNAs) have been considered as potential biomarkers for neurodegenerative disorders(Watson et al., 2019). Indeed, sncRNAs exert diverse roles and participate in gene regulation. So, their expression changes during disease progression. MicroRNAs(miRNAs) are the most studied sncRNAs with regulatory functions. They are differentially regulated in the brain and blood of patients suffering from diverse neurodegenerative disorders, including AD. The involvement of miRNA in disease development represents a promise for future studies as these sncRNAs have shown their capability of discriminating between disease subtypes and stages(Watson et al., 2019).
The A/T/N score system: In 2016, an unbiased descriptive classification scheme for AD biomarkers has been elaborated. This scheme called the A/T/N system divides the major AD biomarkers into three categories based on the nature of the pathophysiology that each measure, namely biomarkers of Aβ plaques labeled “A”, biomarkers of fibrillar tau labeled “T”, and biomarkers of neurodegeneration or neuronal injury labeled “N” (Jack et al., 2016). This system is flexible in that new biomarkers can be added to the three existing groups, and new biomarker groups beyond A/T/N could be added when available. Starting from this scheme, the 2018 National Institute on Aging-Alzheimer’s Association research group develops a research framework that defines and stages AD across its entire spectrum at a biological level, by neuropathologic changes or biomarkers, and considers cognitive impairment as a symptom of the disease rather than the definition of the disease (Jack et al., 2018). In other words, this framework defines AD by biomarkers indicative of neuropathologic changes independent from clinical symptoms. It is now well established that the diagnostic criteria (e.g., Mini-Mental State Examination and others) used by physicians to define probable AD do not necessarily correspond to AD pathologic changes. Indeed, up to 30% of patients diagnosed with AD dementia do not reveal AD hallmarks at autopsy (Nelson et al.,2011). In this context, the use of the A/T/N score system may be advantageous to make more accurate diagnoses.
The use of biomarkers in the context of Alzheimer’s disease continuum:Several CSF and plasma biomarkers that,to different extents, measure different pathogenic mechanisms are available.Biomarker abnormalities for AD-related pathophysiological processes, such as biomarkers for Aβ and tau pathology,synaptic dysfunction, neuroinflammation,and neurodegeneration have been identified(Zetterberg and Bendlin, 2021). Interestingly,scientists suggested a model of the temporal staging of AD-related biomarker modifications along with the phases of the AD continuum(Palmqvist et al., 2019) (Figure 1).
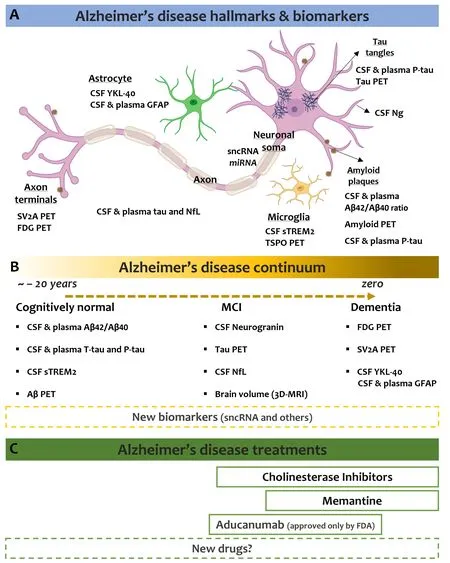
Figure 1 |Key Alzheimer’s disease facts.Cellular elements involved in AD pathology and biomarkers identified so far related to modifications of the activity of these cells (A) that correlate with AD progression (B). The figure also reports the treatments for AD (C). Data reported in this figure apply only to individuals in the AD continuum. 3D-MRI: Volumetric magnetic resonance imaging; AD:Alzheimer’s disease; Aβ: amyloid beta; CSF: cerebrospinal fluid; FDG: fluorodeoxyglucose; GFAP: glial fibrillary acidic protein; MCI: mild cognitive impairment; miRNA: microRNA; NfL: neurofilament light; Ng: neurogranin; PET: positron emission tomography; P-Tau: phosphorylated tau; sncRNA: small non-coding RNA; sTREM2: soluble triggering receptor expressed on myeloid cells 2; SV2A: synaptic vesicle glycoprotein 2A; TSPO: translocator protein; T-Tau: total tau; YKL-40: chitinase-3-like protein 1. Part of the figure was created using Biorender.com that granted permission to publish it.
The first changes regard alterations in both CSF and plasma of the Aβ42/Aβ40ratio, closely followed by soluble tau modifications.Indeed, in response to Aβ pathology,neurons increase both phosphorylation and secretion of tau, resulting in increased total tau and phosphor ylated tau concentrations in CSF and plasma. CSF or plasma amyloid abnormalities precede change in amyloid positron emission tomography (PET). Moreover, only after amyloid PET turns positive, the biomarkers for neuroinflammation, synaptic dysfunction,and neurodegeneration result altered.Indeed, it is believed that microglia react and secrete the soluble triggering receptor expressed on myeloid cells 2, which reaches its maximum in the mild cognitive impairment stage and then decreases as the disease progresses to the dementia stage. Another best-established biomarker for microglia is the translocator protein PET.Markers for synaptic dysfunction are believed to be CSF neurogranin (which increases in close association with amyloid PET positivity)and SV2A and fluorodeoxyglucose PET, while neuroaxonal degeneration is detected by measurement of neurofilament light into the CSF and blood. The volumetric magnetic resonance imaging of the brain is thought to be the par excellence imaging biomarker to detect neurodegeneration. Lastly, astrocyte activation/degeneration is studied through the biomarker YKL-40 in the CSF, whereas studies looking at dosing the glial fibrillary acidic protein levels in both CSF and plasma are in progress.
The availability of AD biomarkers has certainly improved the diagnostic tools that clinicians can utilize. However, their use in routine clinical practice is scant, mainly because they are based on imaging or CSF investigations, both invasive and expensive.Another issue to be addressed regards the real predictivity of these biomarkers.Indeed, comorbidities frequently associated with AD make the clinical picture intricate.For instance, a patient diagnosed with frontotemporal dementia syndrome with positive AD biomarkers is likely to have a silent comorbid AD that does not contribute to the clinical syndrome at all. Moreover,a depressed patient with some cognitive problems may display symptoms due to depression or have preclinical AD. The role of comorbid AD in other neurological disorders represents a big challenge for physicians.
Another factor to be strongly considered is age, in particular with the oldest old. Indeed,the relationship between AD pathological brain lesions and clinical status wanes with old age. Lifetime risks of AD vary considerably by age and most persons with preclinical AD will never develop AD dementia during their lifetimes (Brookmeyer and Abdalla, 2018).Postmortem studies have well documented that Aβ deposition and tau pathology could be seen in almost all aged brains, from both cognitively unimpaired and impaired subjects (Elobeid et al., 2016). This should be considered when developing diagnostic biomarkers, particularly for identifying the early stages of the disease.
The biggest challenge for scientists remains AD therapy. Any progress in the diagnostic field must necessarily be accompanied by advancements in pharmacology,otherwise, there is a risk of increasing unmet clinical needs. Despite many years of intense research, no AD disease-modifying treatments are available. Current therapy still relies on cholinesterase inhibitors(donepezil, rivastigmine, and galantamine),and the N-methyl-D-aspartate antagonist memantine, or a manufactured combination of memantine and donepezil (Forloni, 2020;National Institute on Aging, 2021). To those aducanumab has recently been added, but only in the US. We are still waiting to know what the European Medicines Agency will decide. The currently used drugs just slightly improve behavioral symptoms and mental skills and poorly slow down the course of the disease. All these drugs are prescribed to people who receive a diagnosis of mild cognitive impairment or AD. However, their effects are reversible and lessen over time,because of the progression of the disease. As mentioned before, AD begins decades before clinical onset and its therapy must be started very early to be effective, precisely during the asymptomatic stage. In agreement, the National Institute on Aging (2021) prompts scientists to study the earliest stage of dementia, named prodromal, a stage in which the cellular and molecular alterations leading to the disease have already been triggered, but the affected person is still asymptomatic. This poses two issues, the first, how to be sure that that person will actually develop the AD over time, and the second one, what to administer. Even if AD will be accurately diagnosed at the prodromal stage, there is no therapeutics strongly supported by the scientific evidence to be administered.
In addition, people with prodromal AD who do not experience any symptoms may refuse invasive therapies or drugs that have serious side effects or disabling daily life. The safety and tolerability of these drugs must be considered with great attention. If new effective drugs should ever be identified and approved for prodromal AD, they would be used for decades and, therefore, patient compliance becomes a crucial aspect.
Concluding remarks: AD biomarkers should be examined largely within the same individuals, and preferably in longitudinal cohort studies. Additional investigations are much needed to help identify the very early signs of AD, better understand the course of the disease, and, most importantly,clarify which biomarkers reliably predict AD development and which may be linked either to other conditions or to normal brain aging.Despite the scientific efforts, conflicting data still exist and highlight the need to explore new classes of biomarkers, in particularly for the very early stages of AD. The current answer to the question embedded in the title is that none of the biomarkers is as sensitive as a direct examination of tissue at autopsy. However, they are much useful for observational and interventional research,and we hope soon for clinical practice.
Finally, in order not to run the risk of increasing the unmet clinical needs, it will be necessary to perform further pharmacological investigations aimed at developing new disease-modifying drugs to be used already in cognitively normal subjects and, therefore, endowed with safety as well as tolerability.
Marta Valenza, Caterina Scuderi*Department of Physiology and Pharmacology “V.Erspamer”, SAPIENZA University of Rome, Rome,Italy
*Correspondence to: Caterina Scuderi, PhD,caterina.scuderi@uniroma1.it.https://orcid.org/0000-0002-7314-1539(Caterina Scuderi)
Date of submission: July 19, 2021
Date of decision: September 23, 2021
Date of acceptance: October 27, 2021
Date of web publication: February 28, 2022
https://doi.org/10.4103/1673-5374.335791
How to cite this article:Valenza M, Scuderi C(2022) How useful are biomarkers for the diagnosis of Alzheimer’s disease and especially for its therapy?Neural Regen Res 17(10):2205-2207.
Availability of data and materials:All datagenerated or analyzed during this study are included in this published article and itssupplementary information files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Nitin K Saksena,Neurodegenerative Disease Section, Iggy Get Out,Australia; Lotfi Hacein-Bey, Sutter Medical Group,USA; Paulina Carriba, Cardiff University, UK.
Additional file:Open peer review reports 1, 2.
- 中国神经再生研究(英文版)的其它文章
- Functional in vivo assessment of stem cell-secreted prooligodendroglial factors
- iGluR expression in the hippocampal formation, entorhinal cortex,and superior temporal gyrus in Alzheimer’s disease
- Exploiting Caenorhabditis elegans to discover human gut microbiotamediated intervention strategies in protein conformational diseases
- N-methyl-D-aspartate receptor functions altered by neuronal PTP1B activation in Alzheimer’s disease and schizophrenia models
- Aminopeptidase A and dipeptidyl peptidase 4: a pathogenic duo in Alzheimer’s disease?
- Ubiquitin homeostasis disruption,a common cause of proteostasis collapse in amyotrophic lateral sclerosis?

