Respiratory plasticity following spinal cord injury:perspectives from mouse to man
Katherine C. Locke, Margo L. Randelman, Daniel J. Hoh,Lyandysha V. Zholudeva, Michael A. Lane,*
Abstract The study of respiratory plasticity in animal models spans decades. At the bench, researchers use an array of techniques aimed at harnessing the power of plasticity within the central nervous system to restore respiration following spinal cord injury. This field of research is highly clinically relevant.People living with cervical spinal cord injury at or above the level of the phrenic motoneuron pool at spinal levels C3-C5 typically have significant impairments in breathing which may require assisted ventilation. Those who are ventilator dependent are at an increased risk of ventilator-associated comorbidities and have a drastically reduced life expectancy. Pre-clinical research examining respiratory plasticity in animal models has laid the groundwork for clinical trials. Despite how widely researched this injury is in animal models, relatively few treatments have broken through the preclinical barrier.The three goals of this present review are to define plasticity as it pertains to respiratory function post-spinal cord injury, discuss plasticity models of spinal cord injury used in research, and explore the shift from preclinical to clinical research. By investigating current targets of respiratory plasticity research, we hope to illuminate preclinical work that can influence future clinical investigations and the advancement of treatments for spinal cord injury.
Key Words: breathing; phrenic; plasticity; rehabilitation; respiration; spinal cord injury; translation
Introduction
Each year there are more than 17,000 new spinal cord injury (SCI) cases in the United States and an estimated 294,000 people living with some level of SCI today (National Spinal Cord Injury Statistical Center, 2020). The most common location of traumatic injury to the spinal cord is the cervical region (National Spinal Cord Injury Statistical Center, 2020). Injury to the spinal cord disrupts ascending and descending neural pathways and can affect motor and sensory function. Injuries occurring in people are complicated and heterogeneous,varying substantially in neuropathology. People living with cervical SCI at or above the level of the phrenic motoneuron (PhMN) pool (cervical levels C3-C5) typically have significant impairments in breathing which may require assisted ventilation. Respiratory input from the brainstem innervates the spinal phrenic network controlling the diaphragm (the primary muscle of inspiration) and more caudal motor networks that control intercostal and abdominal muscles (Figure 1). Direct damage to the phrenic network, loss of supraspinal drive to this network, and denervation to more caudal circuits(intercostal and abdominal) result in associated muscle paresis or paralysis and subsequent muscle atrophy. Such injuries not only usually necessitate assisted ventilation, but these individuals have a higher risk of secondary complications including pneumonia, and may suffer from additional deficits including impaired cough reflex, impaired mucociliary clearance, and sleepdisordered breathing (Baydur and Sassoon, 2010; Chiodo et al., 2016; Sankari et al., 2019). The National Spinal Cord Injury Statistical Center reports that the life expectancy of a 20-year-old, ventilator-dependent patient falls from 59.4 years with no injury to just 10 years post-injury (National Spinal Cord Injury Statistical Center, 2020). With this in mind, research centered on the restoration of independent breathing and improvement in quality of life has become a nexus of both clinical and biochemical research.
The very earliest clinical case reports from ancient Egypt recognized the brainstem as an essential component of breathing (Imhotep, 2650BC). In the mid 1800s, Flourens (1858) also suggested that the “noeud vital” (vital node)for respiration was located between the V of the grey matter in the medulla(obex) and the pyramidal decussation. It is now known that respiratory nuclei are distributed throughout the pons and medulla, with those comprising the ventral respiratory column (VRC) being responsible for the generation and maintenance of ongoing rhythmic respiratory drive, and input to respiratory networks in the spinal cord (Hilaire and Monteau, 1976, 1997; Feldman et al., 2003; Alheid et al., 2004; Feldman and Del Negro, 2006; Alheid and McCrimmon, 2008; Feldman, 2011). Continued advances in our understanding of the respiratory system and its underlying neural networks have made it one of the more frequently used systems to study spinal cord injury,plasticity, and repair, while also being highly clinically relevant. The central pattern generator for breathing and the PhMNs primarily receive impulses from the VRC in the medulla. These impulses mostly descend ipsilaterally to synapse on their PhMN targets which then send a motor impulse through the phrenic nerve to the diaphragm. Notably, pre-clinical studies in rodents(Goshgarian et al., 1991; Vinit and Kastner, 2009; Darlot et al., 2012), cats(Cohen, 1973; Janczewski and Karczewski, 1990), and rabbits (Janczewski and Karczewski, 1990) have revealed that some of these descending VRC projections cross (decussate) at the brainstem and spinal cord levels to innervate the PhMN pool on the other side (contralateral) of the spinal cord,and a small subset even re-cross the spinal midline again (Boulenguez et al.,2007; Lane et al., 2009). Supraspinal projections from either the ipsilateral or contralateral VRC that descend the contralateral spinal cord and cross to the ipsilateral spinal cord below the level of injury are considered the anatomical basis for the spontaneous crossed phrenic phenomenon (CPP). Mounting preclinical evidence is also exploring the contribution of spinal interneurons(Darlot et al., 2012; Buttry and Goshgarian, 2014; Zholudeva et al., 2017;Satkunendrarajah et al., 2018; Jensen et al., 2019).This network and plasticity via the CPP help to explain spontaneous contraction of a previously paralyzed hemidiaphragm following SCI seen both clinically and in research. In 1868, Brown-Sequard noted that a hemi-lesion between C1-C4 did not always affect diaphragmatic contraction (Brown-Sequard, 1868).Porter eventually confirmed the findings and systematically documented the CPP in a pre-clinical model of complete C2 Hemisection (C2Hx) (Porter, 1895).It is important to note that the cases Brown-Sequard reported on were likely neurologically complete injuries, but also anatomically incomplete hemilesions (although often misleadingly represented as anatomically complete).For 56 years the CPP went relatively unexplored until 1951 when Lewis and Brookhart published work concluding that the extent of functional recovery attributable to the CPP varies in proportion to the discharge from supraspinal respiratory centers (Lewis and Brookhart, 1951; Goshgarian, 2009). Since then,this system has evolved to include inducible CPP by methods such as hypoxia,hypercapnia, and asphyxia (Lane et al., 2008a, 2009; Goshgarian, 2009).

Figure 1 |Respiratory muscles and their innervation.Three-dimensional rendering of the muscles contributing to breathing separated by their relative depth (superficial layers, left and deep layers, right), with the spinal distribution of motoneurons that innervate them. Primary respiratory networks are highlighted in blue font, while secondary networks (e.g., accessory muscles) that can contribute to breathing are shown in green font.
In addition to these examples of phrenic plasticity, similar spontaneous improvements have been seen in other respiratory networks such as the intercostals, following cervical or thoracic injury. Pre-clinical studies revealed spontaneous improvement in intercostal function weeks after a high cervical SCI (Dougherty et al., 2012). This functional plasticity was attributed to a“crossed-intercostal” pathway (Dougherty et al., 2012), which may consist of direct input from brainstem respiratory centers, or polysynaptic pathways with spinal interneurons that are known to exist within these spinal networks(Lane et al., 2008b). Progressive recovery of cough has also been seen following thoracic SCI in the cat model (Jefferson et al., 2010). Compared to the evidence for plasticity within the phrenic network, less is known about the intercostal and abdominal respiratory pathways, which networks contribute to plasticity, and how it translates to changes in ventilation or recovery from impaired expiratory functions (e.g., cough). What is unfortunately clear is that spontaneous plasticity is limited and deficits after SCI persist in both preclinical models (Kowalski et al., 2007) and the human population (Brown et al., 2006; Baydur and Sassoon, 2010).
While injury at cervical, thoracic and lumbar spinal levels can compromise respiratory networks, the most devastating consequence arises following cervical injury, which is the focus for the remainder of this review. Cervical SCI can lead to respiratory failure and several secondary complications making them targets of therapy vast. The three goals of this review are to define plasticity as it pertains to respiratory function post-SCI, discuss plasticity models of SCI used in research, and explore the shift from preclinical to clinical research. By investigating current targets of respiratory plasticity research, we hope to illuminate preclinical work that can influence future clinical investigations and the advancement of treatments for SCI.were searched based on known content or using search terms relevant to the statements being made. No exclusion criteria were used in searches.
Choosing a Pre-Clinical Model of Spinal Cord Injury
Search Strategy and Selection Criteria
PubMed database was used to search for all references cited in this manuscript, and citations were added using Endnote software. References
Besides considering the spinal level of injury (e.g., cervicalvs. thoracic),the type of injury used to model SCI can also have important differences in neuropathological and functional outcomes (Table 1). The most frequently used model of respiratory dysfunction and plasticity after SCI is the lateral C2Hx (Goshgarian, 2003; Vinit and Kastner, 2009; Hoh et al., 2013; Warren et al., 2014; Figure 2). Given the early studies by Porter in adult canines, the C2Hx model has essentially been used for almost 130 years (Porter, 1895).This incomplete injury compromises direct (monosynaptic) projections from the VRC in the medulla to ipsilateral PhMNs, resulting in ipsilateral hemidiaphragm paralysis (Ellenberger and Feldman, 1988; Ellenberger et al.,1990; Lane et al., 2008a, 2009; Vinit and Kastner, 2009; Figure 3). This injury model has since been used to study respiration and neuroplastic potential(see below) post-SCI in a number of species, most commonly rats (Golder et al., 2001; Fuller et al., 2008, 2009; Lane et al., 2008b; Lee et al., 2013)and mouse (Minor et al., 2006; Seeds et al., 2009; Zholudeva et al., 2017;Satkunendrarajah et al., 2018; Michel-Flutot et al., 2021a).
In more recent years, a growing number of studies have used a clinically comparable contusion injury model (Kwon et al., 2002; Scheff et al., 2003),instead of the C2Hx. This injury model damages both spinal respiratory circuitry (moto- and interneurons) and descending respiratory axons from the VRC. El-Bohy et al. (1998) were the first to show that lateral or midline cervical contusion resulted in reduced phrenic motor output and attenuated phrenic motor response to the respiratory challenge. For example, a lateral contusion injury between cervical levels 3 and 4 (C3/4) disrupts the descending respiratory bulbospinal axons and damages both grey and white matter within the cervical spinal cord. The resulting neuropathology and asymmetric lesion are more comparable to injuries usually occurring in people (Sassoon and Baydur, 2003; DiMarco, 2009; Austin et al., 2013),and may even be more comparable to the injuries originally described by Brown-Sequard (Figure 2). Contusive damage typically leads to cystic cavity formation in most mammalian species including humans (Backe et al., 1991;Kwon et al., 2002; Scheff et al., 2003; Lane et al., 2008a, 2012; Talekar et al., 2016; Burks et al., 2019), which at mid-cervical levels results in a loss of phrenic moto- and interneurons at the lesion epicenter and denervation of phrenic network caudal to injury (Figure 3). This cervical contusion model used by us (Lane et al., 2012; Spruance et al., 2018; Zholudeva et al., 2018b)and others (Baussart et al., 2006; Golder et al., 2011; Nicaise et al., 2012a,b, 2013; Awad et al., 2013; Wen and Lee, 2018; Wen et al., 2019; Wu et al.,2020a) has revealed that contusion injury results in reproducible attenuation of diaphragm function both during breathing of room air (eupneic) and respiratory challenge (hypoxia and hypercapnia).Given that contusive injuries span longer rostro-caudal differences, the extent of disruption and neuronal loss, and the contribution of spared pathways to plasticity may vary between injury models. Using a dual contusion and hemisection injury, Alilain and colleagues (2011) demonstrated that crossed phrenic pathways appear to at least partially contribute to the motor function after injury (Awad et al., 2013). Retrograde, transneuronal tracing of the phrenic motor network also reveals an increase in the number of labeled phrenic spinal interneurons (SpINs) connected with the motor network on the side of a contusive injury, highlighting a role for SpINs in plasticity post-injury(Lane et al., 2012).

Table 1 |Mouse to man: considerations for translation
Spinal Interneurons and Respiratory Plasticity after Spinal Cord Injury
Recent studies have shown that pre-motor SpINs, that innervate the phrenic and other respiratory networks, are found throughout the neural axis,and receive input from neurons in the VRC and other respiratory-related supraspinal nuclei (e.g., serotonergic axons from the raphe, several reticular nuclei including the gigantocellular nucleus) (Zholudeva et al., 2018a). While the contribution of these SpINs to breathing remains elusive, several studies have now shown that they modulate respiratory output, integrate the phrenic networks on each side of the spinal cord, and integrate phrenic with other respiratory networks (Lane et al., 2008a, 2009; Lane, 2011; Darlot et al.,2012; Buttry and Goshgarian, 2015; Zholudeva et al., 2018a; Jensen et al.,2019). They do so under normal, eupneic breathing, and can change their contribution to output with changing conditions (e.g., hypoxia) (Lane et al.,2009; Sandhu et al., 2009; Streeter et al., 2017). Even more pertinent to the present discussion, these cells have been shown to contribute to both spontaneous and therapeutically driven plasticity post-SCI, and SpINs are being increasingly considered to be key therapeutic targets for promoting functional recovery (Harkema, 2008; Zholudeva et al., 2018a, 2021; Zavvarian et al., 2020).
Enhancing Respiratory Neuroplasticity after Spinal Cord Injury
Neuroplasticity is the ability of the central nervous system to change either anatomically or functionally (or both) resulting in persistent alterations in function. This ability is an integral part of normal physiology and, following injury, can be either adaptive or maladaptive (Table 2). The CPP, as described above, and the plasticity that has been reported following contusive injury,have provided extensive preclinical data on adaptive plasticity within the phrenic motor network. Moreover, clinical reports of progressive respiratory improvement following traumatic SCI are beginning to highlight similar examples of plasticity among the human population. Several strategies including neural interfacing, physical stimuli, and growth-promoting agents(pharmacological or genetic) have been identified as promising ways of enhancing neural plasticity and are subject of ongoing research. We will now discuss the targets of SCI research as they apply to spontaneously occurring and therapeutically driven neuroplasticity.

Table 2 |Types of functional plasticity
Neural interfaces
Stimulation of neuronal networks via neural interfaces (e.g., electrical stimulation) activates spared networks and contributes to modest anatomical and functional plasticity. These stimulation strategies include epidural,intraspinal, functional electrical stimulation, and transcranial magnetic stimulation (Edgerton and Harkema, 2011; Young, 2015; Hormigo et al., 2017;Courtine and Sofroniew, 2019; Zavvarian et al., 2020; Figure 4A). The last decade has seen rapid advances in the engineering of hardware for interfacing with the injured spinal cord (Courtine and Sofroniew, 2019). With the development of more biologically compatible materials, invasive implantation is becoming more translationally viable. The resolution at which we can stimulate components of the injured respiratory networks is significantly improving. Simultaneously, preclinical research has seen a rapid advancement in the development of less invasive hardware, including optrodes for epidural stimulation of neural tissues (Mondello et al., 2018). Coming years may also see the preclinical development of neural interfacing strategies with other optogenetic, chemogenetic, and ultrasonic capabilities for activating injured networks. Within the respiratory networks, a host of neural interfacing strategies have been explored, including diaphragm stimulation (DiMarco et al., 2005b; Onders, 2012; DiMarco, 2018), intercostal muscle stimulation(DiMarco et al., 2005a; DiMarco and Kowalski, 2010, 2015), epidural stimulation (DiMarco and Kowalski, 2009; Kowalski and DiMarco, 2011;Kowalski et al., 2013, 2016; Gonzalez-Rothi et al., 2017; Bezdudnaya et al.,2018, 2020), and to a lesser extent intraspinal stimulation (Mercier et al.,2017). In an effort to develop and characterize less invasive, yet translationally relevant means of stimulation, Vinit and colleagues are exploring the use of transmagnetic stimulation as well (Vinit et al., 2014; Lee et al., 2021; Michel-Flutot et al., 2021b). For a more detailed discussion on neural interfacing for respiratory networks, please see (Onders, 2012; DiMarco and Kowalski, 2013;Hormigo et al., 2017).
Activity-based therapies
Respiratory training encompasses rehabilitative, resistive, and activity-based training methods to strengthen the neuromuscular respiratory circuitry.These include respiratory resistive devices, exposure to gas (e.g., hypoxia or hypercapnia training) as well as non-respiratory strategies such as locomotor training (Randelman et al., 2021; Figure 4B). Resistance-based training strategies have been shown to significantly improve respiratory function in people with a wide range of underlying impairments (Sapienza and Wheeler,2006), and can assist in weaning from assisted ventilation. As ongoing clinical studies explore how and when muscle strength training can be used, it may become an important component of conventional therapies for people living with SCI. Locomotor training for people with SCI has been shown for decades to improve motor outcomes (Behrman and Harkema, 2000; Harkema, 2001;Barbeau et al., 2006; Edgerton et al., 2006, 2008; Harkema et al., 2012;Behrman et al., 2017) and in more recent years these benefits have been seen across a wider range of functions in both the clinical population (Harkema et al., 2008; Terson de Paleville et al., 2013) and in pre-clinical studies (Ward et al., 2014, 2016; Hubscher et al., 2016; Harman et al., 2021).
One example of a non-invasive respiratory activity-based therapy uses decreased oxygen levels (hypoxia). This form of respiratory training - known as intermittent hypoxia (IH) - consists of brief intermittent exposures to hypoxia with alternating levels of normoxia. These IH protocols can vary by the severity of hypoxia (e.g., percent of O2), duration of hypoxic episodes,number of hypoxia/normoxia cycles per day, longevity of treatment, and/or start time of treatment following injury (Dale-Nagle et al., 2010; Dale et al.,2014; Gonzalez-Rothi et al., 2015, 2021). One of the hallmarks of respiratory plasticity with IH training is the ability to elicit persistent (hours) increase in phrenic nerve activity called phrenic long-term facilitation (Fuller et al.,2000; Mitchell et al., 2001; Devinney et al., 2013). Many of the IH-facilitated respiratory plasticity pathways are serotonin (Ling et al., 2001; Baker-Herman and Mitchell, 2002; Golder and Mitchell, 2005) and BDNF dependent (Baker-Herman et al., 2004). Several lines of evidence have shown that daily acute intermittent training with hypoxia can enhance respiratory motor output and tidal volume (Lovett-Barr et al., 2012) and restore breathing capacity after preclinical SCI (Vinit et al., 2009; Navarrete-Opazo et al., 2015; Dougherty et al., 2018).
An alternative form of gas training to hypoxia is to increase levels of carbon dioxide (i.e., intermittent hypercapnia) while maintaining normoxia(Randelman et al., 2021). While early studies using chronic hypercapnia revealed little efficacy (Bach and Mitchell, 1996, 1998; Baker et al., 2001),intermittent hypercapnia appears to promote some adaptive respiratory plasticity (Baker and Mitchell, 2000; Baker et al., 2001). Combining hypercapnia with hypoxia has also been shown to enhance respiratory function, tidal volume, and frequency after SCI (Lee et al., 2017; Wen et al.,2019; Wu et al., 2020b; Lin et al., 2021). Therefore, understanding the proper dose (level of gas) and duration (exposure time) of different types of gas as a“chemical” stimulant is crucial for effectively modulating respiratory plasticity.As preclinical studies continue to refine these therapeutic approaches there will be a strong potential for rapid translation into the clinic.
Pro-regenerative treatments
The goal of pro-regenerative treatments is typically to enhance axon outgrowth for the repair of neural networks capable of restoring function.The barriers to growth can be broadly divided into intrinsic and extrinsic barriers. Various neuronal intrinsic mechanisms contribute to structural plasticity including gene transcription, neuronal chromatin, and cytoskeletal architecture, intrinsic neuronal molecular brakes, and mitochondrial energy deficits. Extrinsic barriers to axonal growth such as cellular damage and death,upregulated matrix molecules (e.g., perineuronal net), myelin and protein debris, and fibroastrocytic scarring also contribute to attenuated neuronal growth. Both intrinsic and extrinsic mechanisms controlling structural plasticity and axon growth have been targeted with pharmacological and genetic strategies (e.g., viral vectors) and are described in detail elsewhere(Tedeschi and Bradke, 2017), and have been applied as anatomical and functional repair strategies for respiratory networks (Nantwi and Goshgarian,1998; Alilain et al., 2011; Gransee et al., 2013; Warren et al., 2018; Charsar et al., 2019; Urban et al., 2019).
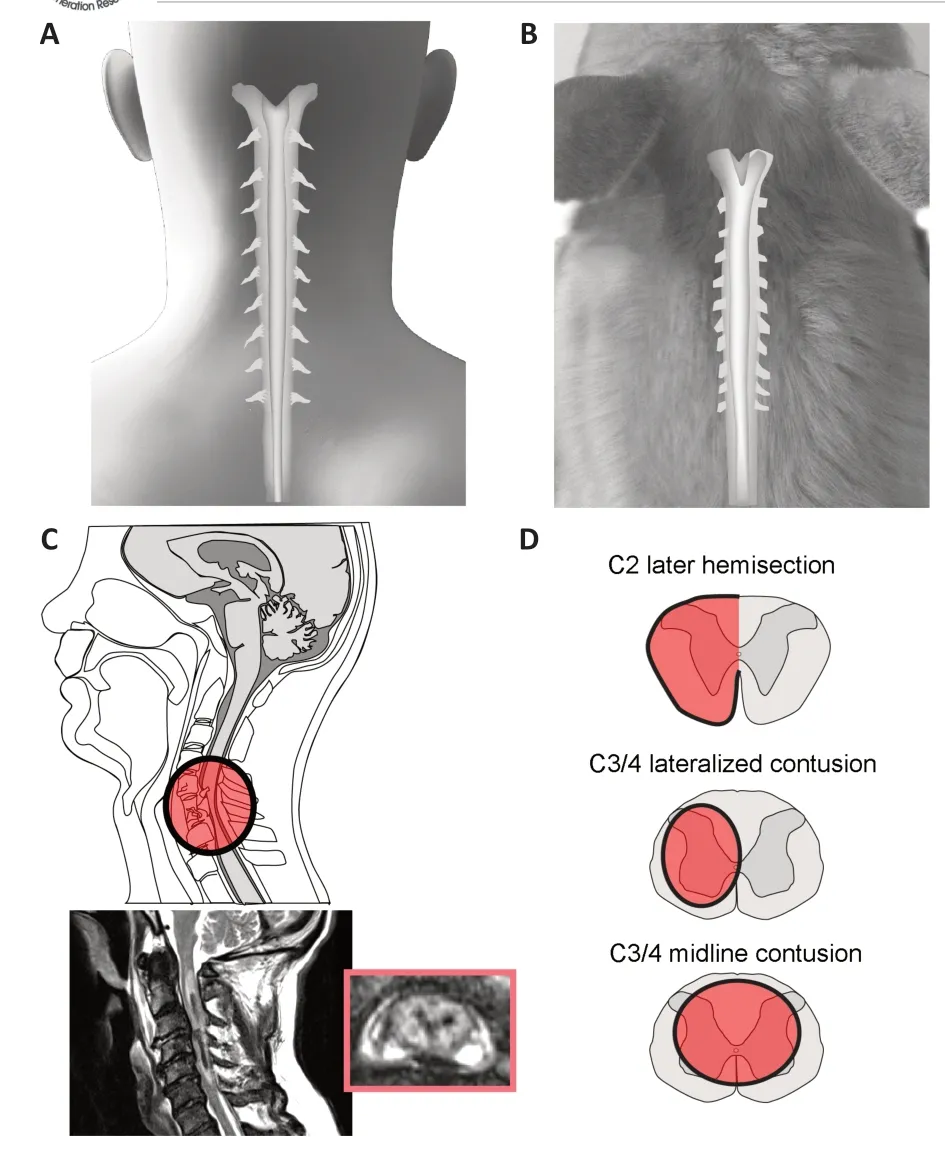
Figure 2 |Cervical spinal cord injury in “mouse” and “man”.Comparison of the cervical spinal cord in man (A, C) and rodent (B, D). These representations highlight gross morphology of the cervical spinal cord in each species (A,B) and show examples of clinically occurring traumatic spinal cord injury (C), and common pre-clinical models of cervical spinal cord injury used to study respiratory dysfunction (D).MRI of the human spinal cord (inset, C) from patient seen at Shands Hospital, University of Florida, USA. Unpublished data.

Figure 3 |Models of cervical spinal cord injury and resulting respiratory deficits.Schematic diagram of the cervical spinal cord highlighting the phrenic network,comprising phrenic motoneurons (green), pre-motor spinal interneurons (purple), and descending bulbospinal input (grey, VRC). These images compare two spinal cord injury(SCI) models that are commonly used to examine respiratory function and plasticity. The C2 hemisection (A) is the most frequently used model of SCI for examining respiratory plasticity after SCI. This model completely disrupts all descending pathways from the ventral respiratory group (VRC) in the medulla, to the phrenic motoneurons (green)on the same side (ipsilateral). Electrophysiological recording from this denervated network shows diaphragm paralysis, but sustained activity on the contralateral side(likely undergoing compensation). In contrast, contusive models of SCI (B) more closely resemble the neuropathological deficits associated with human SCI. Most human injuries also occur at mid-cervical levels denervating some phrenic motoneurons and resulting in loss of others. Recording diaphragm activity in this pre-clinical model reveals deficits ipsilateral to injury. Ongoing research is studying the neuroplastic potential of this injured anatomical substrate and progressive functional changes. dEMG: Diaphragm electromyography; SCI: spinal cord injury; VRC: ventral respiratory column.
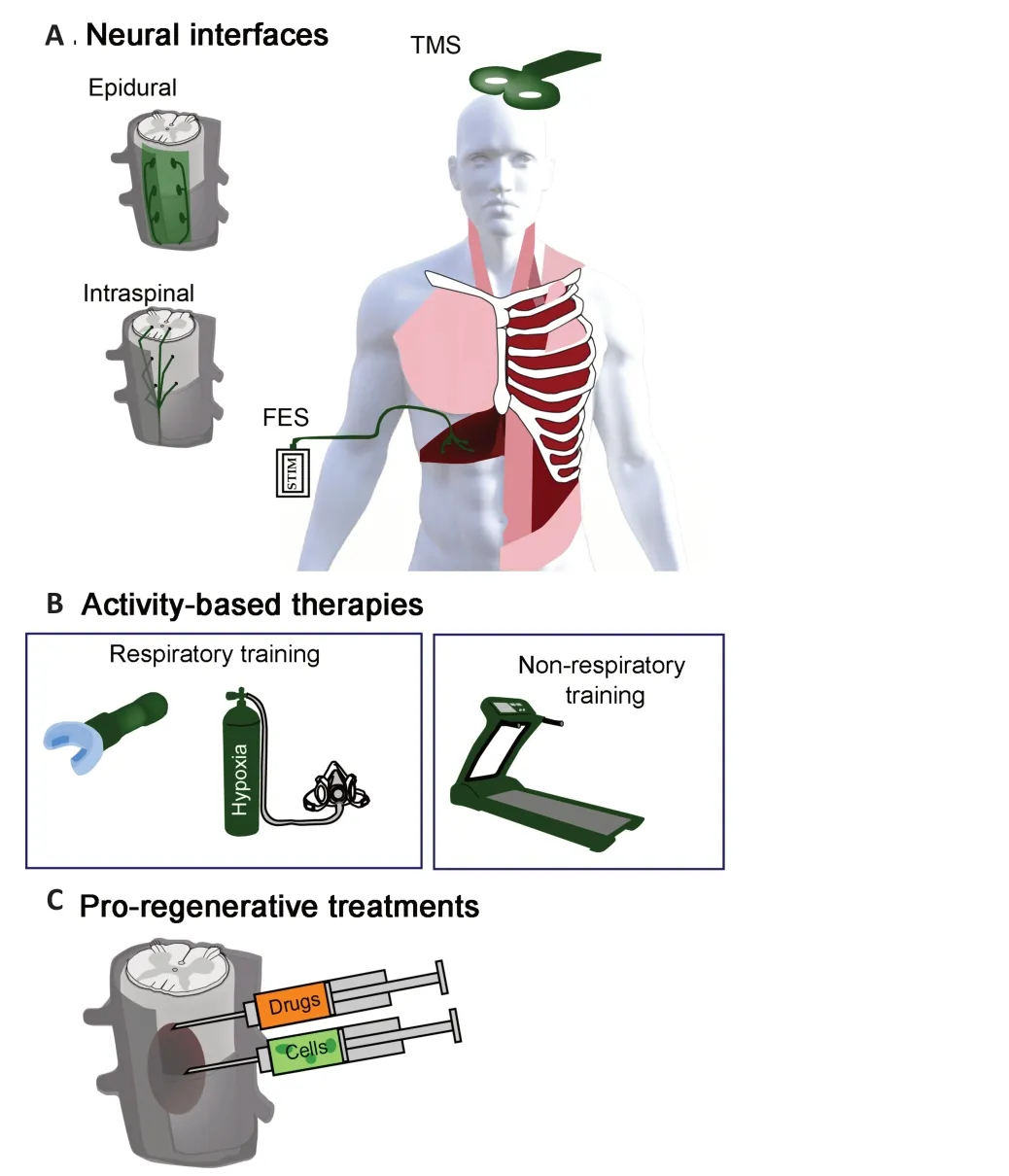
Figure 4 |Therapeutic strategies to enhance neuroplasticity.Neural interfaces (A), activity-based therapies (B), and pre-regenerative treatments (C)are subject of ongoing pre-clinical and clinical investigations for enhancing neuroplasticity after spinal cord injury. Recent years have seen an increased translation of neural interfaces for people with spinal cord injury and diaphragm pacing (FES) represents one of the greatest advances in ventilating people with impaired breathing (A). A growing number of activity-based therapies are being developed to enhance plasticity and are often used to strengthen respiratory muscles and wean people form assisted ventilation(B). While there is a vast array of pro-regenerative treatments under pre-clinical development, there is growing motivation to explore translating some of these to people with spinal cord injury (C). FES: Functional electrical stimulation; TMS: transcranial magnetic stimulation.
One strategy that has been explored to enhance the repair of respiratory pathways is pharmacologically targeting phosphatase and tensin homolog(PTEN), which is a negative regulator of mammalian target of rapamycin(Park et al., 2008, 2010). Inhibition of PTEN with PTEN antagonist peptide has been shown to promote robust growth of damaged axons in both chronic(Du et al., 2015) and acute (Urban et al., 2019) SCI and has even been shown to significantly restore respiration following diaphragm hemiparesis.Though promising in the treatment of respiratory dysfunction, bringing PTEN antagonist peptide from preclinical models to clinical trial would be a dangerous endeavor as PTEN inhibition is directly linked to the development of some cancers (Milella et al., 2015). Therefore, longitudinal studies evaluating residual effects of PTEN antagonist peptides are necessary prior to application in humans. Alternatively, there may be other means of targeting such pathways. There is mounting evidence to suggest that activity-based strategies may be a viable non-pharmacological alternative to targeting the mechanisms of enhanced axonal growth (Liu et al., 2012; Gutierrez et al.,2013).
Another strategy that can target both intrinsic and extrinsic mechanisms of repair is cell or tissue transplantation. Within the respiratory networks, preclinical studies have explored the reparative potential of olfactory ensheathing glia (Polentes et al., 2004; Stamegna et al., 2011; Stamegna et al., 2018), fetal spinal cord tissue (White et al., 2010; Lee et al., 2014; Spruance et al., 2018),and their more-refined counterpart neural progenitor cells (Zholudeva et al., 2018b; Goulão et al., 2019), and peripheral nerve bridges (Gauthier and Rasminsky, 1988; Lammari-Barreault et al., 1991; Gauthier and Lammari-Barreault, 1992; Decherchi and Gauthier, 1996; Decherchi et al., 1996, 1997;Gauthier et al., 1996, 2002; Decherchi and Gauthier, 2002; Alilain et al., 2011).While diverse in their mechanism of action (and elusive for some), each of these approaches has demonstrated repair within phrenic motor networks and improvements in respiratory function. Although untested in the clinical arena for respiratory functions, ongoing pre-clinical studies continue to refine and optimize transplantation strategies and improve their translational potential (Charsar et al., 2017; Lane et al., 2017; Zholudeva and Lane, 2019;Fischer et al., 2020). Despite what is quickly becoming a remarkable potential,translating cell therapies are not without difficulties (discussed in Fischer et al., 2020). A major hurdle faced in translating cellular therapies to the clinical population has been producing large enough numbers of purified human cells in a good manufacturing practice facility, that are identical to those that were shown to have pre-clinical efficacy. Maintaining the survival of donor cells (when needed for the proposed therapeutic effects) is another difficulty that is not easily overcome without significant immunosuppression, and it remains unclear as to how long that is required. Ongoing pre-clinical research is broadening its focus to start exploring some of these hurdles in the hope of paving a smoother translational path.
From Mouse to Man: Plasticity in Clinical Research
The medical management of SCI is constantly evolving. In recent years,clinicians have begun bringing the discoveries of benchwork scientists into clinical research and practice. Particular emphasis has been placed on using external triggers to stimulate plasticity including exercise, virtual reality exposure, and hypoxia/hypercapnia training for the ease of application and relatively minimal invasive nature of the therapy.
IH and hypercapnia have shown promising results in inducing spinal plasticity post-SCI. In 2021, Christiansen et al. found that paired corticospinal motor neuronal stimulation-induced plasticity could be potentiated by acute (short exposures) IH. In this study, sixteen people with cervical SCI were treated with either paired corticospinal motor neuronal stimulation-acute IH or paired corticospinal motor neuronal stimulation-sham-acute IH, and then measured motor evoked potentials as an amplifier of plasticity. This study builds upon a strong foundation of research supporting IH as a method of inducing plasticity in humans (Mateika et al., 2015; Mateika and Komnenov, 2017) and improving respiratory function (Tester et al., 2014; Sutor et al., 2021). While IH has been more readily translated clinically, hypercapnia training (intermittent or sustained exposures) has also been demonstrated to evoke respiratory plasticity and ventilatory long term facilitation with and without exposure to hypoxia (Harris et al., 2006; Griffin et al., 2012; Sankari et al., 2015; Bascom et al., 2016; Vermeulen et al., 2020; Welch, 2021). With ongoing clinical use of hypercapnic treatments, and mounting evidence of intermittent hypercapnia demonstrating therapeutic efficacy in pre-clinical models of SCI, coming years may see this as a newly translated treatment of individuals living with injury.Beyond activity-based or training strategies, pharmacological interventions have also been explored as means for improving respiratory function after SCI. Theophylline is a phosphodiesterase inhibiting drug commonly used in therapy for chronic obstructive pulmonary disease and asthma (Jilani et al.,2021). It is theorized that theophylline improves respiratory muscle strength by enhancing descending bulbospinal pathways and/or increasing the inotropy of the skeletal muscle in the diaphragm, intercostal, and transversus abdominis muscles (Jilani et al., 2021). Theophylline has previously been shown in animal models to restore phrenic nerve activity by inhibiting the adenosine A1 and A2-receptors (Nantwi and Goshgarian, 1998, 2002) and activating quiescent tracts in the contralateral spinal cord via the inducible CPP previously discussed (Moreno et al., 1992). Despite evidence of recovery in animal models, clinical trials have failed to find a significant improvement in pulmonary function following treatment protocols of oral theophylline(Moxham et al., 1985; Tzelepis et al., 2006) though there is some evidence to suggest that higher success rates of ventilator weaning in high cervical SCI patients can be attributed to theophylline use (Zakrasek et al., 2017).
Another therapeutic strategy that has seen rapid pre-clinical advances and translation to the clinical arena is the use of the vast array of neural interfacing strategies. The primary therapeutic goal of neural stimulation post-SCI is to restore sufficient activity to denervate spinal motor networks and activate otherwise silenced/paralyzed circuitry and muscles. When applied under the right conditions, the beneficial effects can also persist after treatment termination (Dobkin, 2003; DiMarco, 2005; Courtine et al., 2009;Onders, 2012; Mondello et al., 2014; Posluszny et al., 2014). There are many ways to stimulate the spinal cord, including functional electrical stimulation,intraspinal, epidural, or transcutaneous stimulation, or trans magnetic stimulation (DiMarco, 2005; Martin et al., 2012; Onders, 2012; Tator et al.,2012; Hormigo et al., 2017) (outlined in Figure 4A). Prior studies demonstrate that electrical stimulation can facilitate plasticity in respiratory (Hormigo et al.,2017) and non-respiratory networks (Taccola et al., 2018; Jack et al., 2020).
Within respiratory circuitry, functional electrical stimulation of respiratory muscles, particularly the diaphragm (diaphragm pacing, DP) has been successfully translated and employed for more than 30 years and can facilitate ventilator weaning and respiratory recovery (Onders, 2012;Posluszny et al., 2014). After cervical SCI diaphragm paralysis/paresis often leads to muscle atrophy within hours, especially when the injured individual is on mechanical ventilation (Powers et al., 2009; Gill et al., 2014; Smuder et al.,2016). However, DP stimulates regular muscle contractions and accordingly attenuates disuse atrophy (Dalal and DiMarco, 2014; Lu et al., 2016; Zambon et al., 2016). Stimulation of these paralyzed or impaired muscles uses the same pattern and magnitude of normal respiratory functional activity (Moe and Post, 1962). The beneficial effects of DP have even been reported to persist after the treatment’s termination (Posluszny et al., 2014). However,muscle stimulation still requires the sparing of sufficient spinal (lower)motoneurons to achieve muscle contraction, so not all patients with cervical level SCI are eligible. While preclinical assessment and development of functional electrical stimulation-based strategies continue, clinical functional electrical stimulation therapy, including DP, is proving beneficial in eligible patients (DiMarco, 2005, 2018; Onders et al., 2007; Kowalski et al., 2013;Posluszny et al., 2014). A 2018 study by Onders et al. collected data from 92 people with traumatic SCI and subsequent respiratory failure, treated with four-electrode intramuscular DP, and found complete respiratory recovery in 5 participants, with 2 able to have their electrodes removed (Onders et al., 2018). They concluded that early implantation of DP leads to favorable outcomes and improves the quality of life of people with respiratory dysfunction following traumatic SCI. The complete recovery of respiration with the use of DP is evidence of plasticity within the respiratory circuit and further propels clinical SCI research forward.
Locomotor training has also been shown to improve a range of nonlocomotor functions, including a respiratory function for individuals with chronic cervical and thoracic injuries (Terson de Paleville et al., 2013). This improvement in respiratory function is believed to be from increased heart rate and minute ventilation (i.e., increase cardiopulmonary activity) during treadmill training (Terson de Paleville et al., 2013). However, the extent of respiratory improvement may also be “dose-dependent”. Terson de Paleville saw improvements in respiratory function for subjects who received 60 minutes of stepping on a treadmill, five days a week for an average of 12 weeks (Terson de Paleville et al., 2013). In contrast, individuals who received passive robot-assisted stepping did not improve cardiopulmonary function(Jack et al., 2011). One limitation in respiratory recovery might be achieving a sufficient increase in limb afferent stimulation to encourage locomotorrespiratory coupling post-SCI (Sherman et al., 2009). This hypothesis is supported by hindlimb stimulation (a passive event) producing respiratory rhythm entrainment (Iscoe and Polosa, 1976; Morin and Viala, 2002; Potts et al., 2005) and increased phrenic motor output (Persegol et al., 1993). While there are several possible mechanisms by which locomotor training may promote improvements in breathing, better understanding what these are and how to best harness the therapeutic potential of locomotor training will require more pre-clinical and clinical investigation.
A wide range of treatments have been used in pre-clinical studies to improve respiratory function after traumatic SCI, with some limited effort to translate these to the clinical arena, but attaining the greatest degree of improvement will likely require a combination of these therapeutic approaches. Each targets unique aspects of neural repair and/or the neuroplastic potential of compromised respiratory networks. As these strategies are refined for the optimal therapeutic outcomes, future work can start to consider how they can be combined for synergistic effects.
Closing Remarks
Respiratory plasticity following cervical SCI is a devastating and widely researched trauma. Yet despite how widely researched this injury is in animal models, relatively few treatments have broken through the preclinical barrier (Table 1). This could be partial because of a lack of funding or too few incentives for academic, clinical, and industry professionals alike; or the immense costs of translation relative to the number of people that can be treated (investors make less than they would in other areas of biomedical research). This has not been helped by the failure of some treatments with pre-clinical efficacy to effectively translate to the clinic. Recent years have seen greater effort to replicate promising pre-clinical results before moving toward translation, and pre-clinical investigators are required by agencies that fund them to strive for great rigor. While there is a broad range of hurdles that must be overcome to see a pre-clinically tested treatment translated through to conventional therapy for people living with injury, there is also growing momentum in the field of SCI research to push forward with translation (Morse et al., 2021). Maintaining this momentum will require effective communication among researchers, clinicians, and those living with SCI. Though SCI may initially seem like an insurmountable scientific challenge,the studies presently discussed tell a different tale. Promising animal studies have highlighted the neuroplastic potential of respiratory networks and how they can be effectively therapeutically targeted. Clinicians have been able to use these findings to inform clinical research and intervention to the benefit of their patients, e.g., advances in diaphragm pacing (Onders et al.,2014, 2018), and respiratory training (Christiansen et al., 2021). Not only is it important for benchwork to inform clinical research, but investigators must also use clinical work to inform their research (reverse translation, a.k.a.,bedside-to-benchtop). The interactions between academic, clinical, and industry professionals, and injured individuals, must become more closely intertwined in order to propel SCI research forward and positively impact the lives of those living with SCI. The translation spectrum (Figure 5) involves a wide range of scientists and engineers in both academic and industry environments, and clinical professionals, that need to interact in a timely and effective manner with each other, and with those people living with SCI, to best pave the translational path.
Author contributions:KCL, LVZ and MAL wrote the manuscript and completed extensive literature searches and reviews. Additional input on developing treatments for respiratory dysfunction was provided by MLR, and clinical insight and perspective was provided by DJH. The figures and tables were made by LVZ and MAL. All authors contributed to the editorial process and approved the final version of the manuscript.
Conflicts of interest:There are no conflicts of interest.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Steven A. Crone, Cincinnati Children’s Hospital Medical Center, USA.
Additional file:Open peer review report 1.

Figure 5 |Translational spectrum.This relatively simple schematic diagram summarizes the quite complex translational path or “spectrum” for research in spinal cord injury. Each stage highlights the possible roles for scientists, engineers, and clinical professionals. Those living with spinal cord injury are represented at the center of this spiral, as they should influence the work being done at all stages of translation. A gradient has been used to indicate the flow between those individuals and groups associated with translation, avoiding direct arrows from one to the next, as the path is typical, not linear, and can move between these groups and in both directions. Ultimately, in addition to translating treatments to the population being treated clinically, the outcomes from ongoing work should be fed back into the pre-clinical and clinical research being done. Hurdles exist throughout the translational path and can vary in how significantly they disrupt movement from one stage to the next; but there is mounting enthusiasm for improving translation, and greater efforts are being made to better understand (i) how the translational spectrum does/should exist,(ii) the roles of those involved and how they are/should be involved, and (iii) what steps these people in the field can take to unify towards optimizing translation and providing treatment to those people living with spinal cord injury.
- 中国神经再生研究(英文版)的其它文章
- Functional in vivo assessment of stem cell-secreted prooligodendroglial factors
- iGluR expression in the hippocampal formation, entorhinal cortex,and superior temporal gyrus in Alzheimer’s disease
- Exploiting Caenorhabditis elegans to discover human gut microbiotamediated intervention strategies in protein conformational diseases
- N-methyl-D-aspartate receptor functions altered by neuronal PTP1B activation in Alzheimer’s disease and schizophrenia models
- Aminopeptidase A and dipeptidyl peptidase 4: a pathogenic duo in Alzheimer’s disease?
- Ubiquitin homeostasis disruption,a common cause of proteostasis collapse in amyotrophic lateral sclerosis?