Polyphenols as potential enhancers of stem cell therapy against neurodegeneration
Diana Rodríguez-Vera, Antonio Abad-García, Nancy Vargas-Mendoza,Rodolfo Pinto-Almazán, Eunice D. Farfán-García, José A. Morales-González,*,Marvin A. Soriano-Ursúa,*
Abstract The potential of polyphenols for treating chronic-degenerative diseases (particularly neurodegenerative diseases) is attractive. However, the selection of the best polyphenol for each treatment, the mechanisms by which they act, and their efficacy are frequently discussed. In this review, the basics and the advances in the field, as well as suggestions for using natural and synthetic polyphenols alone or in a combinatorial strategy with stem cell assays, are compiled and discussed.Thus, stem cells exhibit several responses when polyphenols are added to their environment, which could provide us with knowledge for advancing the elucidation of the origin of neurodegeneration.But also, polyphenols are being included in the innovative strategies of novel therapies for treating neurodegenerative diseases as well as metabolic diseases related to neurodegeneration. In this regard, flavonoid compounds are suggested as the best natural polyphenols due to their several mechanisms for acting in ameliorative effects; but increasing reports are involving other polyphenols.Even if some facts limiting bioactivity prevent them from conventional use, some natural polyphenols and derivatives hold the promise for being improved compounds, judged by their induced effects. The current results suggest polyphenols as enhancers of stem cell therapy against the targeted diseases.
Key Words: Alzheimer’s disease; chronic degenerative diseases; combinatorial therapy; metabolism;neurodegenerative diseases; neuronal damage; neuroprotection; Parkinson’s disease; polyphenols;stem cell assays
Introduction
Polyphenols comprise an enormous family of phytocompounds that are found in the plant kingdom; many of these are reported to be biologically active.Polyphenols are abundantly found in fruits, vegetables, nuts, cocoa, wine, tea,and coffee; thus, the ingestion of the food sources of polyphenols has been widely recommended to prevent cardiovascular diseases (CVD) (Crowe et al.,2011).
Polyphenols have been extensively studied as a possible natural option in the therapy of a wide range chronic diseases such as diabetes, but also for cardiovascular and neurodegenerative disorders and cancer. However, prior to reviewing such therapeutic effects, it is necessary to determine whether consumption from dietary sources has an association in the origin of the previously mentioned diseases. In this respect, the Mediterranean diet, which is rich in fruits, vegetables, polyunsaturated fats, and low-saturated fat, is considered as a protective diet related to a low risk of cardiovascular and cerebrovascular events, diabetes, neurodegenerative diseases and cancer(Bonaccio et al., 2017; Guasch-Ferré et al., 2017).
Polyphenols has been explored as potential drugs from ancient times, albeit they have not been defined and classified to date (Figure 1). Many natural compounds have been testedin vitro; in that they possess multiple beneficial effects. However, in fact, during only the last 40 years, natural polyphenols have been administered to humans to measure biological effects, and many of these are added to food as supplemental protective agents. Only in the last 15 years, some synthetic polyphenols have been approved for human diseases (Hu et al., 2017).
On the other hand, stem cells were described in 1961 and the advance in their applications are expanding (Pierce and Verney, 1961). The modulation of cellular development and differentiation (including the reversion of differentiated cells into pluripotent cells) through the addition of chemical compounds has opened new perspectives in cellular intervention. Some polyphenols are found among compounds exerting effects on stem cells;notwithstanding this, their mechanisms of action are unclear to date.
Regarding chronic degenerative diseases, protection, damage-limiting,and repairing are cornerstone processes; all of which are approached to understand their etiology or progress, but also to identify potential targets and generate new therapeutic strategies. Thus, the history of both polyphenols and stem cells has converged to understand and treat these disrupted processes (Figure 1), and the numbers of articles and projects considering them as a combination are increasing (Figure 2). Polyphenols have mainly demonstrated acting as preventive and protective agents, while therapy with SC to repair damage is increasing. Thus, unsurprisingly, their combinatorial use is studied for application in some diseases entailing a high global burden, such as obesity, diabetes mellitus, Alzheimer’s disease (AD),and Parkinson’s disease (PD).
Search Strategy
In this manuscript, we review the effects of polyphenols on stem cell systems linked to the study of metabolic disorders related to neurodegeneration or neurodegenerative diseases (Information was collected and revised from National Center of Biotechnology Information, PubMed, Global health,Embase, Web of Science, Google scholar and clinical trials databases) but additionally, the potential for them to act as a potential combinatorial therapy on these diseases.
Polyphenols in Metabolic Disorders Related to Neurodegeneration
The reported effects of polyphenols are clearly linked to their potential for regulating metabolic disorders, and these disorders to neurodegenerative diseases (Del Bo et al., 2019). In a systematic review regarding polyphenol intake, it was found that Japan appears to be the major polyphenol consumer(1500 mg/d), followed by North America (900 mg/d). It was found that the greatest polyphenol consumption (1170 mg/d) was associated with less risk of CVD and mortality, and higher polyphenol intake (2632 mg/d) increased protection against metabolic disorders. Nevertheless, some studies referred the mentioned effect at doses of 1200 and above mg/d. Among polyphenols analyzed in diet, lowest risk for cardiovascular events were observed when flavonoids were consumed in a range of 115-944 mg/d. It is noteworthy that the high variability among the results is probably the result of intrinsic factors such as population characteristics, different habits among the countries,markers/endpoint measures for diabetes and CVD, and food sources.
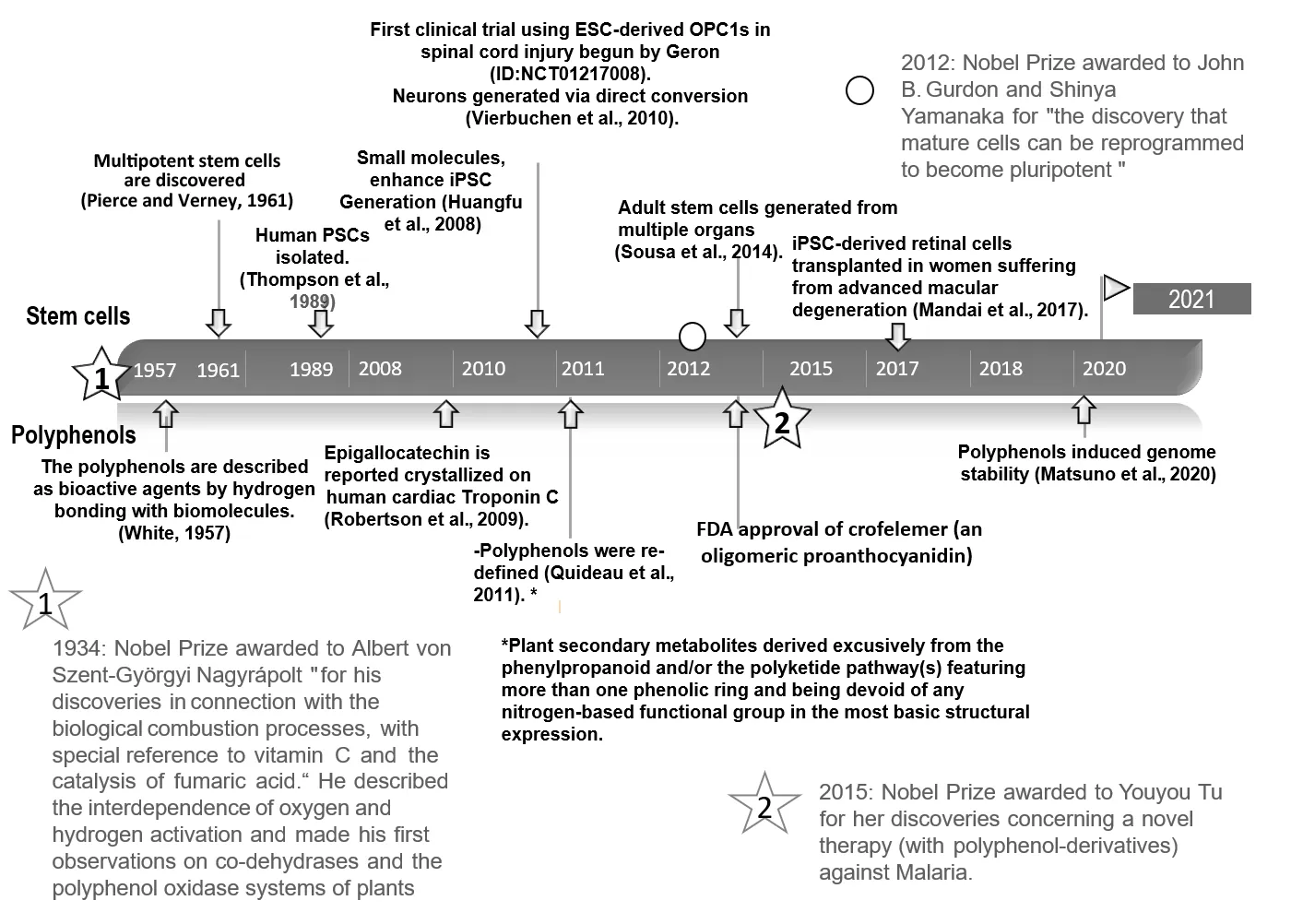
Figure 1 |Study timeline regarding polyphenols and stem cells.Some facts about the emergence of chronologically organized polyphenols and stem cells (White, 1957; Pierce and Verney, 1961; Thompson et al.,1989; Huangfu et al., 2008; Robertson et al., 2009; Vierbuchen et al., 2010;Quideau et al., 2011; Sousa et al., 2014;Mandai et al., 2017; Matsuno et al.,2020). ESC: Embryonic stem cells; FDA:Food and Drug Administration; iPSC:induced pluripotent stem cells; OPC:oligodencyte precusor cell.
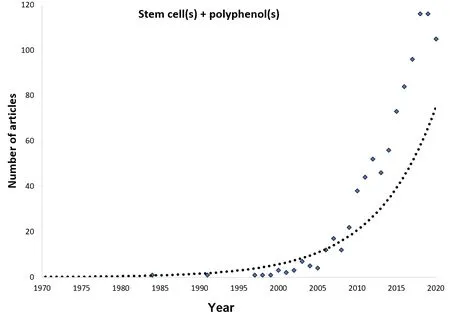
Figure 2 |Increased interest in stem cells and polyphenols.This plot presents the number of articles by year for which the terms “Stem cells” and“polyphenols” are found together in the PubMed database.
Another meta-analysis reported that 100 mg/d of flavonoids leads to a linear decrease of all-cause and CVD mortality (6% and 4%, respectively),highlighting the intake of different forms of flavonoids: flavonols, flavones,flavanones, anthocyanidins, and proanthocyanidins (Grosso et al., 2017).The ingestion of a variety of flavonoids (anthocyanidins, proanthocyanidins,flavonols, flavones, flavanones, and flavan-3-ols) of between 139 and 604 mg/d attenuates the risk of CVD (Wang et al., 2014). The mechanisms linked to the previously mentioned effects are mostly that polyphenols may exert antioxidant, anti-inflammatory, and vasodilatory activities (Figure 3). In this manner, a relationship has been found between a low grade of inflammation based on the use of plasmatic (C-reactive protein) and cellular (leukocyte and platelet counts and the granulocyte:lymphocyte ratio) biomarkers and the high intake of polyphenols deriving from the Mediterranean diet (Bonaccio et al., 2017).
Assuredly, based on the importance of dietary polyphenols in disease prevention, they are being studied individually to elucidate their specific functions and the mechanisms of action by which they exert their activity.Numerous investigations, includingin vitroandin vivoresearches and human trials, have been conducted to evaluate polyphenols from different sources,for example, some catechins from green tea, such as (-)-epigallocatechin-3-gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate, and(-)-epicatechin have been recognized as offering multiple health effects mainly, due to the high antioxidant activity attributed to them. Different studies reported the potential in cancer therapy exerted by catechins (Khan and Mukhtar, 2018). EGCG is described as the major compound (50-70%)of catechins in green tea; in non-small cell lung cancer, EGCG from a pretreatment with a green-tea extract inhibited epidermal growth factor receptor/Akt signaling, reducing the expression of epidermal growth factorinduced programmed cell death ligand 1 (PD-L1) expression. Also, EGCG affected Janus kinase/signal transducers and activators of transcription signaling. This was possible due to the decline of mRNA and protein levels of Interferon γ (IFN-γ)-induced PD-L1 (Rawangkan et al, 2018). PD-L1 and programmed cell death 1 (PD-1) are immune checkpoints that are expressed in tumor and immune cells, playing a crucial role in tumor progression. Thus,the blockade of PD-L1/PD-1 has succeeded as immunotherapy in the early stages of several types of cancer (Baumeister et al., 2016). Pre-treatment with EGCG inhibited PD-L1/PD-1 signaling, enhancing T-cell activity, and resulting in the inhibition of the growth of lung cancer (Rawangkan et al., 2018).In models of metabolism disorders, EGCG treatment appears to enhance hepatic glucose homeostasis and inhibit gluconeogenesis by blocking PEPCK and G-6-Pase and lipogenesis through SREBP-1C, FAS, and ACC1 in the liver.Additionally, molecular simulations revealed that EGCG is capable of bonding to the active side of α-glucosidase and α-amylase (Li et al., 2018). EGCG also improves insulin resistance by promoting glucose uptake and the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase, attenuating oxidative stress and the inflammatory markers malondialdehyde, tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and nuclear factor kappa B (NF-κB), in addition to the increase of the glucose transporter 2 protein and its downstream proteins peroxisome proliferator-activated receptor and coactivator PGC-1β in human hepatoma HepG2 cells (Zhang et al., 2018).EGCG also modulates macrophage accumulation, inflammation, and the Notch signaling pathway (Huang et al., 2019). Numerous studiesin vitroandin vivowere carried out, showing the positive effects of polyphenols from green tea in cancer prevention/treatment involving changes in metabolic rates. The modulation of some of these proteins (particularly enzymes and cytokines) have been linked to neurodegenerative diseases. Unfortunately,little evidence from human studies is available, thus rendering it necessary to run perform investigations to establish safety limits for doses employed within in a clinical context, particularly for modulating those cell metabolic disorders linked to neurodegeneration (Zhang et al., 2014).
Grape and wine polyphenols comprise another vast group that has been studied in depth; available data support the benefits on CVD by regulating metabolism in cells, decreasing inflammation, promoting optimal blood lipids, and inhibiting platelet aggregation, oxidative stress and low density lipoprotein oxidation (Bohn et al., 2015). In fact, resveratrol is considered one of the most outstanding polyphenols, and the core of the recommended moderate consumption of wine is found in the so-called “French Paradox”(Pastor et al., 2019). This compound possesses many properties, including the ability to reduce oxidative stress and inflammation, prevent glycation, and diabetes, neurodegeneration, and to interfere in the progress of several cellcycle events (Galiniak et al., 2017). In H9c2 cardiac cells treated with high glucose and palmitate as a model of diabetic cardiomyopathy, resveratrol proved to restore autophagy, suppressing mTOR and its downstream effectors p70S6K1 and 4EBP1 through the activation of AMPK and JNK1.A disruption was observed of the Beclin1-Bcl-2 complex, attenuating apoptosis in resveratrol- treated cells. The authors suggest that resveratrol could protect in diabetic cardiomyopathy (Xu et al., 2018). It is supposed that its main mechanisms of action include the prevention of apoptosis by downregulation of the expression of the anti- or pro-apoptotic proteins involved in cytoprotective antioxidant systems, hampering ROS production and enhancing mitochondrial function (Xu et al., 2018).

Figure 3 |Summary of effects from polyphenols supporting medical hypotheses for chronic degenerative diseases.In the center are common sources of polyphenols from diet. Actions of polyphenols in specific tissues or organs is depicted as their role for specific pathologies has been reported. LDL:Low-density lipoprotein; NF-κB: nuclear factor kappa B.
On this basis, it could not be assured that the absence of the intake of dietary polyphenols can be the cause of metabolic diseases linked to neurodegenerative processes; however, it is known that they play an important role in the prevention or treatment of metabolic diseases, since the metabolism is clearly affected by polyphenol administration, as tested in several stem cells. Therefore, several studies link the effects of polyphenol administration to cancer SC and dental stem cells; the main effects lie in the mitochondrial processes, and carbohydrate metabolism, particularly the glycolysis pathways (Menéndez and Joven, 2014; Bonuccelli et al., 2018;Liu et al., 2019), but also, the lipid metabolism (Cheng et al., 2020) and the antioxidant effects regulating the cell cycle have been reported as changing after polyphenol administration in stem cells (Bao et al., 2013). Thus,among dietary molecules, polyphenols are prominent due to their multiple reported biological activities and modes of action, as well as a great deal of epidemiological data showing the association of polyphenols with lower rates of chronic diseases and mortality (Silva and Pogacnik, 2020).
Even though polyphenols seem not essential for humans, they exert a positive impact on nutrition and a more profound understanding of their effects and of their absorption, distribution, metabolism, and excretion in humans has been reported by the scientific community in recent years (Fox et al., 2010). The way in which polyphenols play a key role in the occurrence and progression of non-transmissible diseases is often linked to the maintenance of homeostasis at the cellular level (Teixeira et al., 2019). Evidence also reveals that dietary polyphenols can prevent or delay disease progression through actions on cell organelles, as well as improve the functionality of hypothalamic signals and of several hormones implicated in the dysregulation of the energy balance present in metabolic diseases (Panickar, 2013; Guash-Ferré et al.,2017). As well, they are useful in maintaining health through mitigating the inflammation and cellular damage induced by the production of reactive oxygen species (ROS), commonly observed with increased levels in metabolic disorders (Queen and Tollefsbol, 2010). Thus, recent findings suggest that polyphenols exert a pleiotropic effect (Figure 4) (Sears, 2017). Moreover,Sears (2017) suggest the mechanisms by which polyphenol therapeutic applications limit inflammation and oxidative stress; polyphenols can act in a non-specific fashion as a free radical scavenger, but their effects may lie in three forceful facts: their effect on gene expression in human cells; their influence on the gut microbiota, and their ability to maintain mitochondrial processes.
Besides, it is well known that the replacement of beta cells with some derived stem cells, is a widely studied strategy to find potential therapy for diabetes mellitus; while those differentiated to produce catecholamines have been proposed to neurodegenerative disorders (lacking endogenous catecholamine-producer cells with a disordered metabolism). Nonetheless,some facts have been limiting to date, as the adult cells are heterogenous(Angelova et al., 2018). Intercellular communication and intracellular pathways are a challenge in which full cell maturity can be regulated,combination with polyphenols could regulate to achieve effective therapy (see later).
For ending this section, it should be considered that the role of polyphenols in the gut, and probably key for regulating the absorption of nutrients and substances regulating metabolism, is due to their unique regulation of bacterial strains in the gut microbiota, in part through action on the barrier integrity involving cell-regulation effects, and for the prevention of the entry of bacterial fragments such as lipopolysaccharide into the blood (Cardona et al., 2013; Roopchand et al., 2015; Schneeberger et al., 2015; Zhu et al., 2015;Anhe et al., 2016). It should be emphasized that, regardless of the polyphenol type, adequate intake levels depend on the desired gene-to-modulate (Sears and Ricordi 2012; Sears, 2017).
Polyphenols Regulating Processes Linked to the Origin of Neurodegeneration
Neurodegenerative disorders are diseases of the nervous system (brain,spinal cord, and peripheral nerves) whose origin can derive form metabolic,infectious, toxic, or even hereditary processes (Hou et al., 2019). In recent decades, these pathologies represent a significant public health, social, and financial problem, due to the increase in life expectancy, increasing their prevalence, morbidity, and mortality (Hou et al., 2019; Hung et al., 2019).Some factors such as environmental and genetic factors play a key role for suffering from and for the progression of neurodegenerative diseases (Migliore and Coppede, 2009). In developed countries, pathologies such as Alzheimer disease, cerebrovascular accidents, and Parkinson disease comprise a great clinical problem (Migliore and Coppede, 2009; Hung et al., 2019). Patients with neurodegenerative diseases generally experience a gradual loss of memory and reasoning capability, mood changes, and personality alterations(Losada-Barreiro and Bravo-Díaz, 2017).
Although most neurodegenerative diseases have different pathological characteristics and their origins are not totally clear, most of them entertain important points of convergence. Modifications in neuronal electrical activity(Duffy et al., 1984; Surmeier and Schumacker, 2013; Kumar et al., 2017),mitochondrial dysfunction (Wu et al., 2019), inflammation (Swardfager et al.,2010; Green et al., 2019), protein-handling abnormalities (Guerra-Araiza et al., 2013; Song and Zou, 2015; Hetz and Saxena, 2017), and oxidative stress(Song and Zou, 2015) are some of the changes observed in neurodegenerative diseases.
Dysfunctions in voltage-dependent ion channels are common in neurological pathologies (Duffy et al., 1984; Kumar et al., 2017). The primary function of voltage-gated ion channels is the generation and propagation of action potential in neurons. Reports indicate that changes in voltage-gated potassium (Kv) channels are important components in the physiological changes in AD (Surmeier and Schumacker, 2013). Also, electrical impairment in K-selective voltage-gated channels and voltage-gated sodium channels are critical in multiple sclerosis (Duffy et al., 1984). In PD, electric disturbances are reported in L-type calcium channels (Surmeier and Schumacker, 2013).Likewise, K-selective voltage-gated channels and voltage-gated sodium channels have been proposed as key elements in the pathogenesis of (Duffy et al., 1984). In this sense, quercetin, catechin and resveratrol blocked sodium current; and quercetin also has effect on calcium channels; suggesting a keyrole as modulator in neurodegeneration through action on ion channels(Wallace et al., 2006).
Figure 4 |Polyphenols exerting potential therapeutic effects on metabolic and neurodegenerative diseases.Polyphenols have the ability to activate genes such as Nrf2, which generates and increases antioxidative enzymes such as glutathione peroxidase (GPX), superoxide dismutase (SOD),and catalase (CAT). As anti-inflammatory actions,polyphenols have shown their effects in binding nuclear factor kappa B (NF-κB) and also, these actions are associated with the stimulation of peroxisome proliferator activated receptor gamma (PPAR-γ). CAT: Catalase; ROS: reactive oxygen species.
On the other hand, mitochondrial dysfunction and inflammation are constant in neurodegenerative diseases. In neurodegenerative diseases, a reduction in mitochondrial function may be produced by the following: 1) maintenance of the loss of the chemical and/or electrical transmembrane potential of the inner membrane; 2) dysfunction of the electron transport chain, or 3)decrease in the carriage of vital metabolites into mitochondria (Kumar et al.,2017; Wu et al., 2019). Regarding inflammation, reports indicate an increase of IL-1, TNF-α, and transforming growth factor beta in AD, PD, multiple sclerosis, ictus, and Huntington’s disease (Swarfager et al., 2010; Chen et al., 2018). In addition, dysregulation in the concentrations of IL-6 has been associated with neurodegenerative diseases (Green et al., 2019).
Protein aggregation and misfolding are common in neurodegenerative diseases. Misfolded proteins are generally inactive; however, the accumulation of these proteins can cause stress in cells and organelles such as the endoplasmic reticulum (Hetz and Saxena, 2017). Other important changes that might affect protein aggregation are their posttranslational modifications.These posttranslational modifications are both reversible and irreversible post-translational chemical changes that occur in the later stages of protein biosynthesis, which have been associated in many neurodegenerative diseases (Hetz and Saxena, 2017).
Even though the etiology of neurodegenerative diseases is not fully elucidated, oxidative stress is one of the most important mechanisms that has been associated with neurodegenerative diseases. Reports indicate that tissues possess distinct oxygen requirements that vary in their metabolic demands. The central nervous system is composed of a large number of fatty acids and with low levels of antioxidant defenses (Guerra-Araiza et al., 2013).The brain requires more than 20% of the total oxygen consumption, in that the neurons and astrocytes are mostly responsible for the oxygen and glucose consumed. Thus, an increase in the production of free radicals or ROS may easily change the redox homeostasis (Gandhi and Abramov, 2012; Song and Zou, 2015). The oxidative stress is originated by the loss of balance in the cell redox homeostasis due to two causes 1) the overproduction of ROS or free radicals, and 2) by antioxidant-system dysfunction (Song and Zou, 2015).
Neurodegenerative diseases consistently involve free-radical reactions are initiated by redox, thermal, or photochemical processes, increasing the oxidative stress (Guerra-Araiza et al., 2013). The accumulation of oxidative stress can produce cellular impairment and may affect the DNA repair system,accelerating aging and neurodegeneration (Gandhi and Abramov, 2012; Song and Zou, 2015). Therefore, oxidative stress can damage lipids, proteins, DNA,and other biomolecules, producing neural injury and death (Losada-Barreiro and Bravo-Díaz, 2017).
Depending in the oxidation of the biomolecules mentioned previously, several products can be observed. Malondialdehyde and 4-hydroxynonenal are the most important subproducts of the oxidation of polyunsaturated fatty acids.Nitrotyrosine is the product of protein oxidation through the action of reactive nitrogen oxide species (Filgueiras et al., 2020). With respect to DNA, oxidation can produce protein-DNA crosslinks, mutations, or the breaking of strands.The most common oxidative product of DNA is 8-oxo-2′-deoxyguanosine.8-Oxo-2′-deoxyguanosine results because guanine has a lower one-electron reduction potential than the other nucleosides in DNA (Wang et al., 2020b).At present, no cure exists for neurodegenerative diseases, and treatments only offer symptomatic relief. Thus, further understanding of the pathophysiology of these diseases is essential for controlling the menace caused by these. Polyphenols have been suggested as effective agents limiting some neurological processes linked to the origin of the neurodegenerative diseases. Thus, EGCG is attractive by the properties mentioned above. But also, in terms of antioxidant effects as a key potential mechanism of action,polyphenols can activate genes in stem cells, among these Nrf2, as well as modulate its activity (Schottker er al., 2015; Kahroba et al., 2020; Qader et al., 2020). When activated, it generates and increases antioxidative enzymes as glutathione peroxidase, SOD and catalase (CAT). In fact, curcumin and quercetin can increase the activity of antioxidant enzymes such as glutathione peroxidase, SOD, CAT, or glutathione reductasein vitroandin vivoin cells linked to metabolic processes (i.e., hepatocytes); and they can even activate endogenous defense systems as well. Then, the reduction of free radicals also limits cell damage, which is linked to a decreasing morbidity and mortality(Schottker et al., 2015; Sears, 2017). As anti-inflammatory actions, some polyphenols have exhibited their effects, binding the inflammatory gene transcription factor identified as NF-κB to its binding sites directly in the nucleus (Sears, 2017; Ye et al., 2020). In addition, these actions are associated with a stimulation of peroxisome proliferator activated receptor gamma(Serra et al., 2016; Wang et al., 2018). Thus, it is clear a multi-mechanisms potential role of polyphenols to modulate metabolic disorders linked to cell dysfunction, including those occurring in neurodegenerative diseases.
Effects of Polyphenols on Neurons and Stem Cells Suggesting Their Potential Against Neurodegeneration
At the beginning of this century, Choi et al. (2001) reported that epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. During the last 15 years, stem cells have been converted into neural or glial stem cells, and these have been investigated as models to study neural disorders (Joannides et al., 2007; Murry et al.,2008; Prajumwongs et al., 2016). In such systems, the roles of polyphenols as antioxidant, potential anti-inflammatory agents, and in limiting proteins unfolding, signaling, and accumulation have been demonstrated (Ray et al., 2018; Sarubbo et al., 2018; Henriquez et al., 2020; García-Aguilar et al.,2021). Related to that, and as is above mentioned, some conditions have been identified as risk factors for neurodegenerative diseases. Thus, aging is linked to a progressive loss of neurons in the central nervous system and peripheral nervous system, also, genetic and environmental factors are included as key factors. In fact, aging is linked to cognitive dysfunction with compromised neural plasticity, decreased neurogenesis and increased neuronal death. There is a limited regenerative capacity of the mature human brain to assume the functions of damaged brain tissue. In this sense, just a few data are known regarding mammalian-stock stem cells; they are found in the rostral subventricular zone of the lateral ventricles and in the subgranular zone of the dentate gyrus (DG) in the hippocampus (Elmann et al., 2018). The migration and repopulation of neural stem cells (NSC) are regulated by both intrinsic and extrinsic factors; these include learning, physical exercise, stress seizures, physical activity, neurotransmitters, glucocorticoids, sex hormones,growth factors, neurotrophins, antidepressants, opioids, and compounds from the diet (including polyphenols) (Peiris-Pages et al., 2016).
The motivation to study risk and modulatory factors is due to the limited results with available therapies for disrupted neurogenesis in patients with neurological diseases. Moreover, aberrant neurogenesis is shared hallmark for the majority of neurodegenerative diseases, if well distinct proteins and different neuronal nuclei are linked to different diseases (Bonaccio et al.,2017).
Results are promising for therapy development, as an example regeneration from endogenous NSC in the brain of AD patients and AD transgenic mice is reduced, and the genetic modification of NSC expressing the Aβ-degrading enzyme neprilysin in transgenic murine models (as 3xTg-AD and Thy1-APP)reduces Aβ pathology and increases synaptic density (Msuya and Mndolwa,2005). However, the use of stem cells to ameliorate the lack of adult mammalian neurogenesis is restricted, since it has described with effects on cognitive function, particularly with the intervention of hippocampal formation and on nuclei linked to memory storage and processing (affected in the majority of neuropathologies). Main mistakes for transplanted NSC are related to inability to differentiate into specific types of neurons, malignant transformation, and immune rejection (Jugran et al., 2021).
Thus, dietary phytochemicals exerting a neuromodulatory effect and neurogenic properties are attractive for exploration as promised therapy.Some compounds, such as curcumin, resveratrol, blueberry polyphenols,sulforaphanes, salvionic acids, and some polyunsaturated fatty acids have shown to induce neurogenesis in the adult brain (Renaud and Martinoli,2019). The latter have demonstrated to reduce oxidative stress and neuroinflammation, hence cell signaling, the activation of autophagy, and the affectation of grown factors (Ebrahimi and Schluesener, 2012). Dietary polyphenols promote cell repair and survival, enhancing the ability of the brain to resist severe stress; those induced effects are suggested by means of conducting and activating trophic factors, antioxidant and DNA-repair enzymes, and proteins involved in mitochondrial biogenesis (Bhular and Rupasinghe, 2013).
While the mechanisms of action of polyphenols are unclearly revealed,certain clues have been discovered in the understanding of neurogenesis hallmarks. Thus, a proinflammatory state (the activation of microglia and the release of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6) induced in mice by the injection of lipopolysaccharide in the central nervous system induced decreased hippocampal neurogenesis with marked inhibition of NSC differentiation (Rawangkan et al., 2018). The inclusion of polyphenols in dietary habits appears to increase neurogenesis, limiting neuroinflammation and extending the survival of newly generated cells in the DG; this translates into hippocampal neurogenesis, and this effect may be mediated by some neurotransmitter and hormone receptors (Ross et al., 2018).
The effects of specific polyphenols on neurogenesis have been recently revised (Khan and Mukhtar, 2018). Some of the examples involving evaluations of stem cell system (including regions in stem cells that are in adult brain of mammals) are found later. Blueberry and strawberry polyphenols have been shown to increase neurogenesis, and recently, the grape-seed extract has demonstrated to improve adult neurogenesis in the DG (Kahroba et al.,2020). Curcumin could promote brain health by increasing or stabilizing adult neurogenesis, that could be via the activation of the Akt/GSK-3beta signaling pathway and increased the expression of brain-derived neurotrophic factor(BDNF). In rats, curcumin proved to improve perform on several memory tasks after administration for 12 weeks (Bhullar and Rupasinghe, 2013); while resveratrol, a stilbene, acts as a sirtuin-activator and to induce neurogenesis(Teixeira et al., 2020).
Polyphenols in vegetable oils exert action on the oleic acid-producing enzyme,stearoyl-CoA desaturase, which rescued NSC proliferation impairments in the AD brain (Georges et al., 2019). Epigenetic factors also play a crucial role in regulating stem-cell proliferation and differentiation. Some studies suggest that NSC can be stimulated for proliferation and neural differentiation for the mitigation of cognitive dysfunctions. The use of cocoa polyphenols effects in AD has exhibited protective effects in cell viability and cell morphology against injury induced by amyloid-beta accumulation and regulating the BDNF/TrkB/ERK5 pathway. Also, olive oil polyphenols increased the levels of BDNF, but additionally increased nerve growth factor levels, linked to the proliferation and migration of endogenous stem cells in mice (Kahroba et al., 2020). Green tea EGCG inhibited sevoflurane-induced neurodegeneration, while improved memory related to the activation of CREB/BDNF/TrkB-PI3K/Akt signaling.In an AD mouse model, resveratrol significantly increased estradiol and neprilysin levels, which resulted in decreased estradiol and neprilysin levels and in Aβ deposition, and a reversal in the decline of memory due to the high levels of neurophysins found. There are reports on the oral administration of green tea in neuroblastoma- induced mouse models. Isohamnetin, a flavonol isolated from the leaves ofGingko bilobaL., could potentiate nerve growth factor-induced neurite outgrowth and could increase the expression of neurofilaments in PC12 cells (Georges et al., 2019). Quercetin promotes neurite growth by significantly increasing the expression of cellular cyclic adenosine monophosphate (cAMP) and GAP-43 in N1E-115 neuroblastoma cells (Elmann et al., 2018).
Rosmarinic acid reduced amyloid β aggregation by increased monoamine secretion, in turn disrupting AD development in a murine model. This also could have implications in PD, in that theaflavin, a black-tea polyphenol,protects nigral monoaminergic neurons against MPTP-induced parkinsonism(Shin and Chung, 2012). Otherwise, there has been considerable interest in the neuroprotective effects of polyphenols, especially in their impact on cognitive deficits by activating the phosphorylation of protein kinase C and by stimulating AMP kinase activity in Neuro2a cells and in primary neurons with resveratrol. Curcumin can also disrupt plaques and restore distorted neurites in Alzheimer mouse models. Both of the, latter are considered therapeutic agents for altering brain-aging processes and neuroprotective agents (Renaud and Martinoli, 2019).
On the other hand, the best performance of molecules for the prevention or treatment of neurodegenerative diseases is a hot topic due to the weak advance known at present in terms of the improvement of the quality of life in patients.
Chemicals and small molecules that target signaling pathways related to the cell’s cycle, fate, state, and function can be evaluated for cell reprogramming.
This could imply a strategy to revert the evolution of neurons affected in neurodegenerative diseases, or at least for them to be a source of neural cells with the potential to play the key role of cells involved in disease manifestation. In this regard, Hou et al. (2013) reported the reprogramming of mouse cells into induced pluripotent stem cells (iPS) by means of a cocktail with seven small molecules (VPA, CHIR99021, E616452, Tranylcypromine,Forskolin, 3-deazaneplanocin A, and TTNPB). In addition, Zhao et al. (2015)promoted an improved method by adding four small molecules (AM580,EPZ004777, SGC0946, and 5-aza-2-deoxycitidine). Other combinations of drugs have been reported in such cells (Inoue and Yamanaka, 2011; Biswas and Jiang, 2016; Farkhondeh et al., 2019). The resulting cell systems could be used to cell replacement, but they can also be employed as neuron-like systems to evaluate responses to polyphenol administration. In the best of our knowledge this has been poorly explored; but the effects of polyphenols during the transforming process, and on the transformed cells seem attractive as some of the used compounds have several rings and hydroxyl groups (like polyphenols).
Structure-(Protective) Activity Relationship of Polyphenols
The group of polyphenols represent a very complex group of phytochemical compounds (Figure 5).
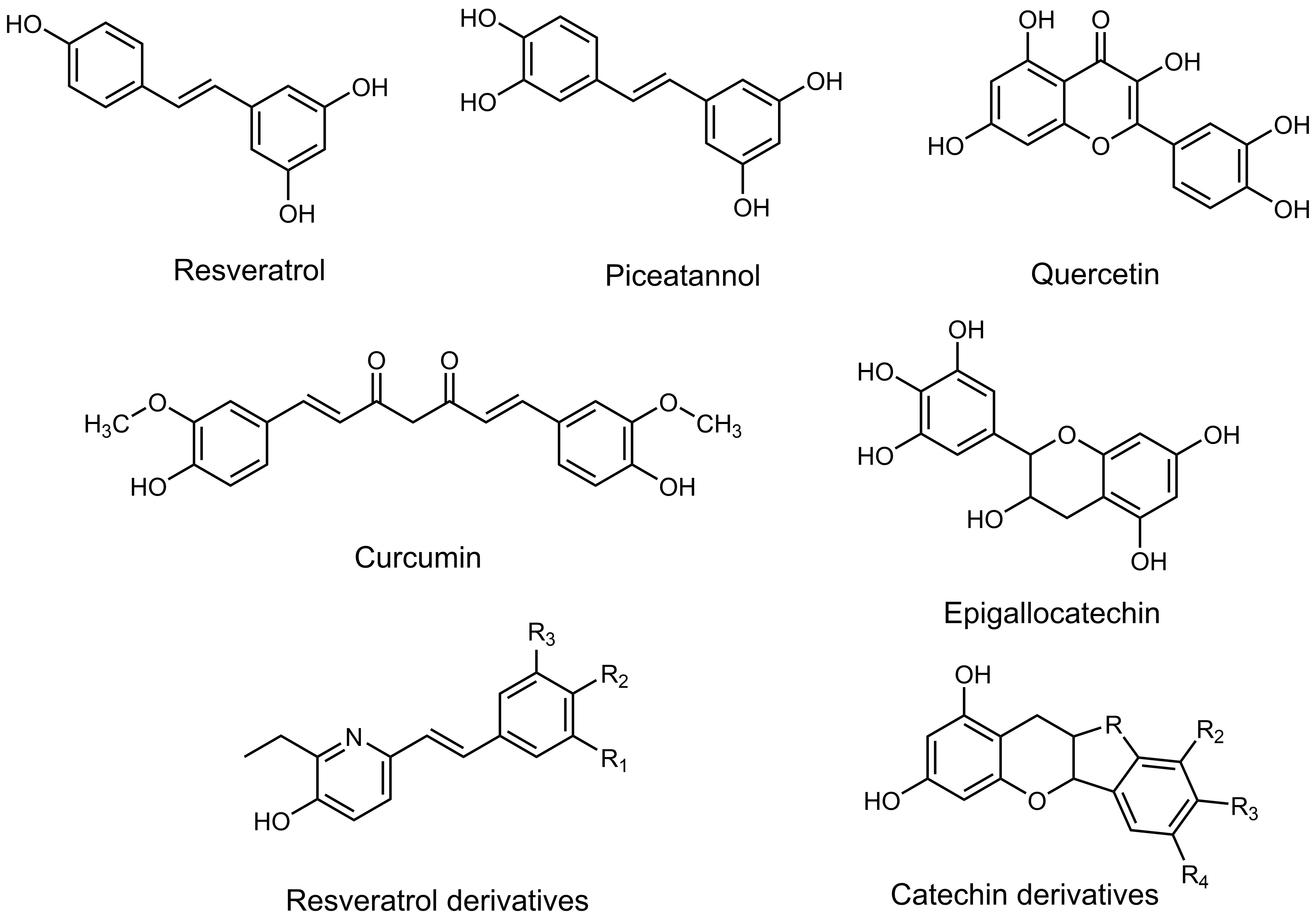
Figure 5 |Natural and synthetic polyphenols: structure-efficacy to treat chronicdegenerative diseases.
They are also nutrients known to be powerful activators of the human genes involved in the synthesis of antioxidant enzymes, in the modulation of anti-inflammatory pathways, and in the activation of anti-aging genes, as well as critical factors for maintaining the efficacy of some organelles (González-Sarrías et al., 2020). Their first two actions, as an antioxidant and as an anti-inflammatory, are noteworthy, due to neurodegenerative diseases such as PD,AD, amyotrophic lateral sclerosis, and multiple sclerosis; in these diseases the concentrations of free radicals are cumulative. These compounds can act in a non-specific manner as free radical scavengers for human health (Maraldi et al., 2014). Additionally, some specific mechanisms have been described to a subgroup of polyphenols; some of them are commented below.
The anabolism of polyphenols is complex in both plants and animals. Their classification is dictated by the number of rings (C6) that they contain, as well as the structural elements binding these rings to each another. Essential classes include phenolic acids (C6-C3 and C6-C1), flavonoids (C6-C3-C6),stilbenes (C6-C2-C6), and lignans (C6-C3-C3-C6). They are the basis of this complex group and deriving from these there can be another modifications around their structure, which include hydroxylations, O-glycosylations andcistransisomerization. This yields another at least > 8000 estimated compounds(Gorzynik-Debicka et al., 2018). These molecules have at least one galloyl or catechol group (with hydroxyl groups in the ortho position), which confer on them the ability to react with ROS and metals, mainly by chelation processes(Ebrahimi and Schluesener, 2012). In addition, initially, antioxidant properties were linked to the pharmacological activities of polyphenols. Their chemical structures confer them several activities as metal-chelators, antioxidants,protein complexes-destabilizing, estrogen-like, enzyme-binding, which let us to infer their usefulness in some neurodegenerative diseases treatments(González-Sarrías et al., 2020). Unsurprisingly, additional ways for there to be action in biological systems have been reported.
Thus, although structure-activity has been poorly explored among all polyphenols, there are some data for specific compounds. Among recently supported mechanisms, flavolignans have been shown to inhibit the binding of ligands to the inflammatory complex system; on yielding a result, the reduction of the transcription of NF-κB and the activation of antioxidant genes such asNrf2(Vargas-Mendoza et al., 2020). These actions are associated with the activation of the peroxisome p[roliferator-activated receptor gamma and,with a dietary intake of polyphenols, this attenuates oxidative stress, since these polyphenols generate an increased expression of antioxidative enzymes such as glutathione peroxidase, SOD, and CAT, an enzyme complex that is very effective in removing excessive free radicals and in reducing the risk for certain metabolic and neurodegenerative diseases (Bhullar and Rupasinghe,2013).
Likewise, 7,8-dihydroxyflavones have been reported as modulators in PC12 neuronal-like cells, with a direct agonistic effect on Trk receptors, the main receptors of neurotrophic factors including nerve growth factor and BDNF.However, in the same evaluation, several other polyphenolic compounds activated the extracellular signal-regulated kinase and phosphoInositide 3-kinase/Akt pathways (Moosavi et al., 2016).
It has been demonstrated that, in neural stem cells and in the studies of mouse models, polyphenols deriving from grapes have improved synaptic transmission through the cAMP response element-binding protein. In transgenic models of AD, these polyphenols have improved in reducing the oligomerization of Aβ peptides and have contributed to reduction in cognitive impairments. The main component of these grape-seed extracts has been denominated 3′-O-methyl-epicatechin-5-O-β-glucuronide; this component has shown improvement in reducing the abnormal folding of tau proteins(Elmann et al., 2018). Resveratrol, inhibits β42 fibril formation and protects from Aβ neurotoxicity by inhibiting inducible nitric oxide synthase inhibition,the activation of AMPK-dependent pathways, and direct action on sonic hedgehog signaling for modulating cell differentiation (Konyalioglu et al.,2013; Cheng et al., 2015; Chiang et al., 2018). Probably, Piceatannol (with only one hydroxyl group different from resveratrol) induces greater effects in these processes that are linked to metabolic and neurodegenerative disorders(Arai et al., 2016; Kershaw and Kim, 2017).
On the other hand, quercetin, a naturally occurring flavonoid, is known as a potent protector against metabolic and neural disorders by means of its critical role in attenuating oxidative stress, inflammation, and metabolic dysregulation (Yang et al., 2017). Some flavonoids share structural features with cAMP or nucleoside triphosphates, providing them ability to activate or inhibit some enzymes, in which they can act with modulatory effects.As examples are the effects of resveratrol on cAMP phosphodiesterase, of theaflavins on ATP synthase and the respiratory chain, and of curcumin on glyoxalase 1 (Msuya and Mndolwa, 2005). With respect to the dose for the previously mentioned effects, some reports on stem cells and other cell systems also suggest that high circulating concentrations of polyphenols are not required to exert these effects. Their interaction with diverse enzymatic targets, in very small doses of polyphenols, may benefit from the different pathways that ensue in cells (Del Río et al., 2013).
Albeit the structure-relationship regarding kinetics has been less explored, it is known that polyphenol pharmacokinetics (particularly biotransformation and binding to plasma proteins and lipids) modulate bioefficacy (Jannin et al., 2004; Delmas et al., 2011). Differences in polyphenol uptake and bioavailability are in dependence of the route of administration and the analytical methods for measurement (Abd El-Mohsen et al., 2006). In addition, it has been proposed that polyphenols are retained at the cell membrane by albumin and lipoprotein receptors, offering a carrier-mediated mechanism to reach the intracellular space, in addition to passive diffusion(Lançon et al., 2004). Moreover, polyphenols modify the pharmacokinetics of co-administered drugs; thus, this mechanism could be involved in the synergistic pharma-effects proposed for developing multi-drug therapies;including those in which different polyphenols or polyphenols with drugs and hormones are administered in combination to treat a specific malady(Yang et al., 2014). Consequently, it is predicted that some physiological and pathological conditions impact polyphenol bioavailability and bifunctionality(Ulrich-Merzenich et al., 2010; Elmann et al., 2018). Recent works describe promising natural and synthetic polyphenol structures by designing that takes into consideration both pharmacodynamic and pharmacokinetic properties(Martelli and Giacomini, 2018; Silva et al., 2019; Li et al., 2019; Farfán-García et al., 2020; Mizuno et al., 2020; Balaha et al., 2021; Gansukh et al., 2021).
Combination of Polyphenols and Stem Cells As an Innovative Therapy
As is above sentenced, more than 8000 polyphenols have been identified in fruits, vegetables, teas, chocolates, herbs, and spices as free monomers or oligomers (Pandey and Rizvi, 2009), and their use is proposed in isolated or polyphenol combinations for degenerative disorders (Conte et al.,2003). Among their multiple modes of action, their antioxidant properties are extensively described; these effects take place through several mechanisms, such as the hypoxia inducible factor 1 alpha pathway. The decrease of oxidative stress/NS and many other mechanisms mentioned in this manuscript appears to play a key role in the pathophysiology of many diseases (Ebrahimi and Schluesener et al., 2012), and it is clear that the increased consumption of polyphenols in diet is associated with decreases in metabolic disorders related to neurodegeneration (Renaud and Martinoli,2019), neurodegenerative and psychiatric disorders (Gansukh et al., 2021).
In addition to their attractive results, it has been noted that polyphenols must be safe, well-tolerated, and must possess biostability and effectiveness in humans in order to propose novel therapies with polyphenols at the core. Some studies have found that they are safe and tolerable in the short,medium, and long term of use. It has even been proposed that adverse effects are not common, but the reported cases do include minimal gastrointestinal problems and, more rarely, cephalea, dizziness, and rashes (Galati and O’Brien, 2004; Koga et al., 2011). In contrast, their low bioavailability and modest effects in clinical assays, as well as the difficulty to evaluate the protection conferred by natural molecules without effects from diet source,detracts from the appeal of polyphenols for pharmaceutical use. Therefore,several strategies have been employed by pharmaceutical scientists and drug developers to solve these issues (Renaud and Martinoli, 2019). Here it has come to light that new medical-engineering progress in vehicle formulation has advanced to the extent that it allows polyphenols to be contained in lipid nanocapsules, nanoparticles, exosomes, nanocomposites, emulsified formations, or in gel form, and also rectal suppositories. The aim of this was an effort to achieve efficient systemic distribution, procure bone marrow for immunomodulatory effects, and possess controlled-release implant strategies(Augustin et al., 2013; Frozza et al., 2013; Neves et al., 2013; Souto et al.,2013).
Another controversial point comprises the balance between the focus on improving bioavailability and strategies to identify the appropriate doseresponse of polyphenols under certain clinical conditions. It is suggested that higher doses are potentially harmful, in that they could be stress factors,toxins precursors, or that they may induce an unbalanced defense response or an extended life for cells favoring neoplasia (DiMeo et al., 2013).
Due to these, other complementary strategies for the administration of polyphenols have been studied. It is in this respect that treatment with stem cells has been proposed and explored. Stem cells are undifferentiated and unspecialized cells with a plastic potential to become diverse body cells.In this regard, human stem cells entertain multilineage differentiation and proliferation potential that have been studied for cell therapy. Some authors also, have studied and demonstrated specific systems as key to enhance the ability of cells to act as a therapy. One example of this is that with a decrease of Nox4 activity in stem cells, where polyphenol action can positively affect and even help to reach a declination of nuclear ROS production and DNA damage (Renaud and Martinoli, 2019). This fact states that nuclear Nox4 regulation exerts an important pathophysiologic effect on stem proliferation through the modulation of nuclear signaling and DNA damage (Sears et al.,2017). In relation to the amazing potential of stem cells, their capacity for self-renewal and their ability in terms of differentiation have contributed to consideration of their use in clinical applications such as cell-based therapies,drug discovery, and tissue engineering. Because the objective of stem cellsbased therapies is to treat, repair, and replace tissues in disease or in organs,with novel strategies that are healthy and functional. Therefore, polyphenol in combination as a therapy can be an option (Eckel et al., 2005).
Meanwhile, for neurodegenerative diseases linked to an extended state of metabolic disorders and chronic inflammation, stem cell replacement therapies have emerged as promising methods for their cure by means of replacing damaged cells, tissues, or organs (Beshlawy et al., 2019). Since certain other tissue/organ transplantations entail many complications,such as those related to immunosuppression, surgical complications, organ unavailability, prolonged recovery, and high surgical costs (Quaglia et al.,2008; Houben et al., 2015; Bobbio et al., 2019), cell replacement (from stem cell therapy) appears to be effective solely with the appropriate number of cells and validated and standardized protocols that take into account the efficacy of the enzyme, receptor, neurotransmitter, or hormone replacement after manipulating the cellsin vitro, which is in an early stage of development,considering that many of these assays have not been fully investigated in the clinic.
Based on that adult cells are differentiated and play a very specific role that consists of unique biological properties such as differentiation and indefinite self-renewal, they can give rise to a functionally mature progeny and help to maintain tissue homeostasis (Peiris-Pages et al., 2016). NSC, brain cells that become neurons, astrocytes, and oligodendrocytes by a process known as neurogenesis, are particularly attractive for cell replacement therapy in metabolic and NG diseases (Bond et al., 2015). As mentioned previously,a combination of some polyphenols and stem cells could have additional benefits (Figure 6).
In that polyphenols regulate some signal pathways, such as resveratrol and baicalein, have been reported to modulate Akt/P13K/mTOR and can inhibit neural inflammation via the downregulation of the NF-κB signaling pathway(Xue et al., 2010; Capiralla et al., 2012; Simao et al., 2012). Additionally,several flavonoids can inhibit Aβ1-40and Aβ1-42, play an immunomodulatory role, and can reduce memory impairment through the downregulation of NF-κB (Paris et al., 2011; Ashafaq et al., 2012; Gelderblom et al., 2012), and could modulate differentiation in cells. This is supported in some studies on cancer stem cells and dietary polyphenols, suggesting the effect of quercetin. The latter has been elucidated, due to that it diminished ALdeHyde deHydrogenase 1 activity and reverted apoptosis resistance as detected by substrate assay. Moreover, quercetin in combination with sulforaphane, an isocyanate found to be enriched in broccoli, possesses potential synergetic effects (Dontu et al., 2003). Although it enhanced the binding of NF-κB,quercetin can also inhibit the growth of cancer stem cell-enriched xenografts associated with reduced proliferation, angiogenesis, and cancer stem-cell marker expression and the induction of apoptosis, Also, polyphenols have been reported to possess potent antioxidant activity through endogenous and exogenous mechanisms (Dontu et al., 2003).
Thus, due to several pitfalls in the polyphenols or in stem cell therapies alone,during the last few years, the evaluation of a combinatorial treatment for the treatment of metabolic and neurodegenerative diseases has been conducted.In this regard, Ge et al. (2017) reviewed the actions of iPS and polyphenols from spices. The authors suggested that iPS and spices could potentially serve as a combinatorial therapy for diabetes mellitus, because stable polyphenol compounds in spices could enhance insulin secretion, confer strong resistance on β-cell destruction, and modulate the immune response.
Supporting this additive effect of polyphenols, Drapeau et al. (2019) reported that human consumption of a proanthocyanidin-rich extract resulted in a selective mobilization of stem cell types involved in regenerative and reparative functions, with applications for preventive health, regenerative health, and postponing the aging process. The pathways for effects on stem cells appear to lie beyond the described antioxidant effect (Wang et al.,2020a), Matsuno et al. (2020) suggested that polyphenols contribute to the stability of the genome.
Regarding applications in neurodegenerative diseases, Itoh et al. (2012)described that epigallocatechin induced an increase in the number of neural stem cells in brain regions damaged by traumatic events. In this line, Wang et al. (2020a) recently described the protective effects of polyphenols, including their maintaining of the number of cells in the hippocampus, a region with preserved neural stem cells. Tandon et al. (2018) compiled and presented detailed advances (involving all of the previously mentioned pathways) with respect to the studies of polyphenols in neural, embryonic, and mesenchymal stem cells, as well as in iPSC, with potential applications for treating AD and multiple sclerosis; these authors suggest (based on more than 100 scientific works reported from 2006 to 2018), that polyphenols complement stem cell therapy and the reduction of neurodegeneration in AD and multiple sclerosis.In that AD and PD share neurodegeneration pathways, it is expected that this combinatorial therapy could be useful in applications in other neuron diseases(Moradi et al., 2021). In agreement with this, Zhuang et al. (2020) found that polyphenols alleviated the neuroinflammation induced by 6-hydroxydopamine through the suppression of the p38 MAPK signaling pathway in PC12 cells and in a murine model of PD. Yammine et al. (2020) found that, in N2a cells,polyphenols induced a mode of cell death by oxiapoptophagy, associated with mitochondrial and peroxisomal dysfunction, thus supporting the potential additive effect of combined elements and in an age-related disease therapy.That is, polyphenols could aid in opportune differentiation, extending the life and function of the stem cells (by modulating oxidative, cell-cycle and inflammatory processes), while stem cells could represent a stable and effective source of the hormones, neurotransmitters, and receptors lacking in some disorders. Emerging evidence of this combination opens the opportunity to explore and support the suggested additive action of this therapy against metabolic and neuronal diseases. The increasing number of experiments and reports combining polyphenols and stem cells has been observed during the last few years, and this tendency is expected to be maintained in the near future, as pioneering advances yield attractive results.
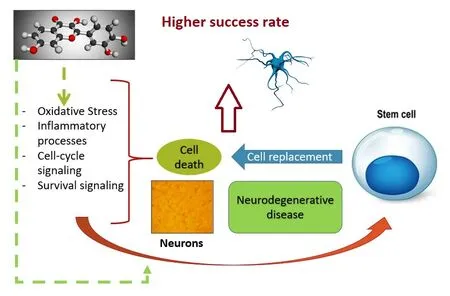
Figure 7 |The putative combinatorial strategy: polyphenols and stem cell therapy.The properties of polyphenols can enhance stem cell therapy in that they modulate mechanisms related to the origin of the disease, but are also involved in the replacement efficacy of stem cells.
Conclusions and Perspectives
Both polyphenols and stem cell therapies have exhibited improvement inin vitroandin vivomodels of neurodegenerative diseases or those metabolic disorders linked to them. Moreover, in some cases, these innovative therapies explored in more than half of century ago have been suggested as better than those used conventionally (drugs with a high incidence of adverse effects or transplantation with a high rate of short-term problems). However, there are several limitations involved in these therapies; among these, for polyphenol administration, there are limitations in instability, bioavailability, and there are toxic effects at high doses, while for stem cells, there is a dependence of the environment for establishment and survival. The multiple suggested mechanisms for polyphenols remain unclear: the most supported action is through the limitation of cell damage by oxidative stress, while only some evidence deals with direct interaction on human proteins (as is notable by the limited number of polyphenol molecules crystallized on human proteins)(Berman et al., 2000).
Interestingly, the noted weakness of single (polyphenol or stem cells) therapy can be limited by the combinatorial approach (Figure 7).
Acknowledgments:The authors are grateful to Consejo Nacional de Ciencia y Tecnología (CONACyT-México) for scholarships and grants for graduatestudents. All authors thank to Maggie Brunner for revising the use of English language in this manuscript.
Author contributions:RVD, SUMA, and MGJA conceived and drafted themanuscript. All authors provided intellectual contributions as well, in that they collected, analyzed, and discussed data, and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal, andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Functional in vivo assessment of stem cell-secreted prooligodendroglial factors
- iGluR expression in the hippocampal formation, entorhinal cortex,and superior temporal gyrus in Alzheimer’s disease
- Exploiting Caenorhabditis elegans to discover human gut microbiotamediated intervention strategies in protein conformational diseases
- N-methyl-D-aspartate receptor functions altered by neuronal PTP1B activation in Alzheimer’s disease and schizophrenia models
- Aminopeptidase A and dipeptidyl peptidase 4: a pathogenic duo in Alzheimer’s disease?
- Ubiquitin homeostasis disruption,a common cause of proteostasis collapse in amyotrophic lateral sclerosis?

