Research Progress on the Function of Melatonin and Its Receptor in Animal Reproduction
Tian LIN Hefeng ZHANG Xiaoliang PEI Huina BO
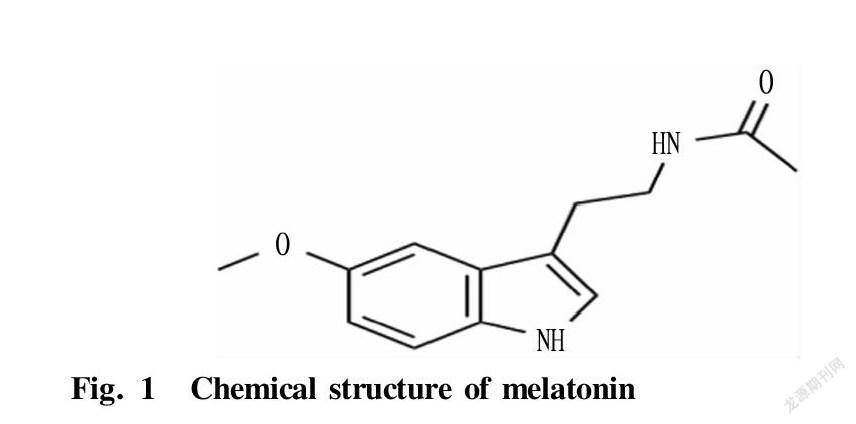
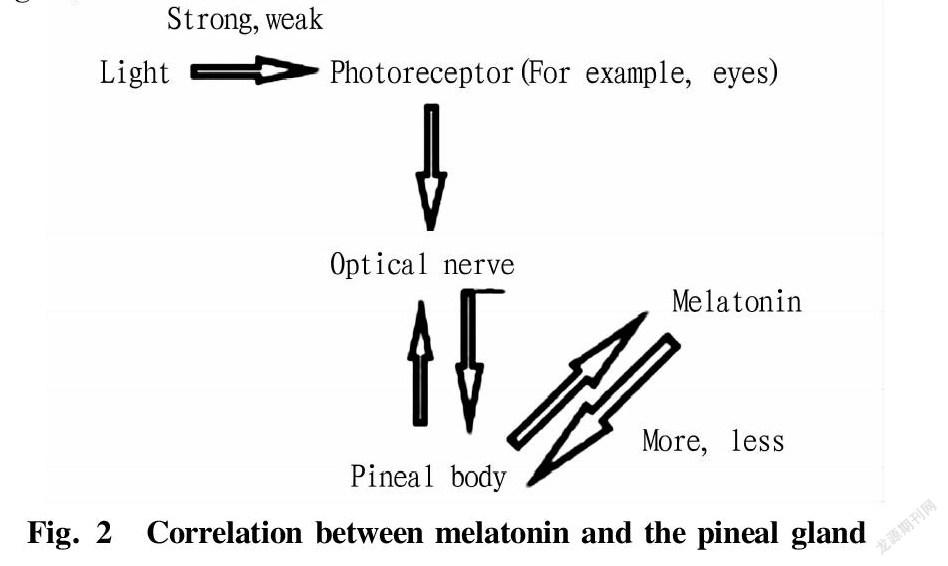
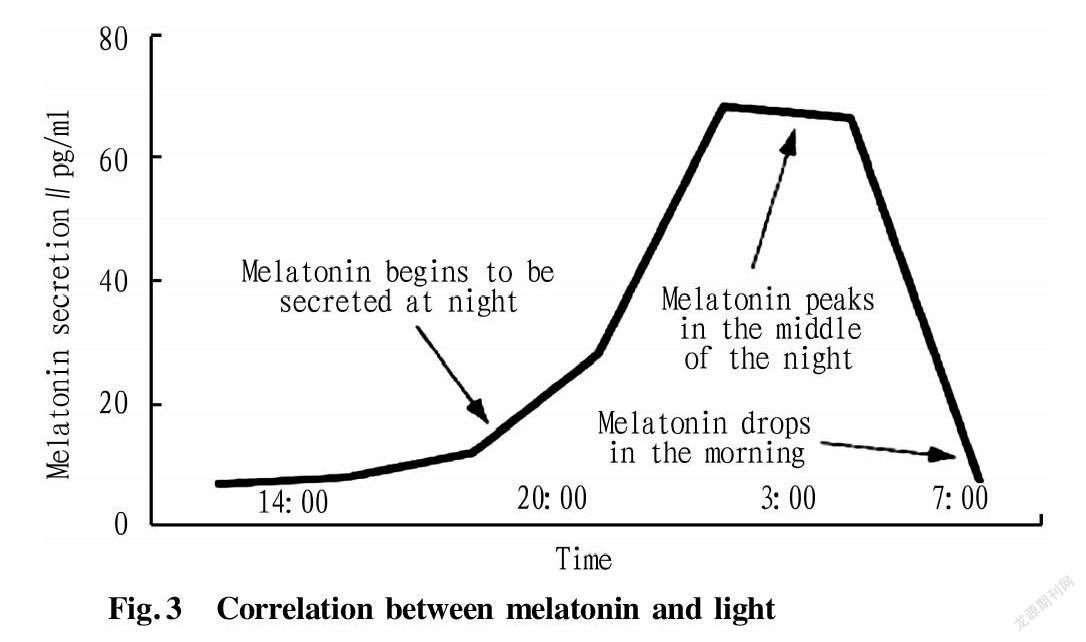
Abstract Melatonin has been a research hotspot in the field of medicine and life sciences for more than 60 years since it was discovered. In order to accelerate the increase of economic animal productivity, a large number of studies also have focused on the regulation of melatonin and its receptors during animal reproduction in recent years. In this paper, the relevant characteristics of melatonin and the latest research progress in animal reproduction were discussed, and the biological functions of melatonin were reviewed from the aspects of germ cells, reproductive endocrine and embryo development, laying a theoretical foundation for further exploring the regulation mechanism of melatonin on reproductive process.
Key words Melatonin; Germ cells; Hormones; Embryo development
Received: October 9, 2021 Accepted: December 7, 2021
Tian LIN (1982-), female, P. R. China, veterinarian, devoted to research about livestock inspection and testing.
*Corresponding author.
Melatonin is mainly an indoleamine hormone produced by the hypothalamic pineal gland of mammals. Its chemical component is N-acetyl-5-methoxytryptamine. In 1957, the dermatologists at Yale University separated and detected its molecular structure for the first time. Since then, melatonin has become a research hotspot in the fields of medicine and life sciences. Melatonin is an important regulator that mediates the effects of light on animal reproduction, and its synthesis and secretion show a rhythmic change of high during the day and low at night. For seasonal breeding animals, the duration of sunshine determines the activity of melatonin to inhibit or promote reproduction. In high latitudes, the photoperiod is the main environmental factor that determines the start and duration of the breeding season. The pineal gland controls reproductive activities by changing the duration of nocturnal secretion of melatonin[1]. In recent years, with the deepening of exploration, it has been discovered that melatonin plays an important role in animal sexual maturation, hormone secretion, gamete formation, early embryonic development, pregnancy and childbirth[2-3]. In modern animal husbandry, techniques such as cryopreservation of sperm, in-vitro maturation of oocytes and in-vitro development of embryos are the basis of artificial breeding. Improving the vitality of sperm after freezing and improving the maturation of oocytes in vivo and in vitro and the quality of embryonic development are of great significance to animal production. This paper mainly summarized related characteristics of melatonin and its latest research progress in animal reproduction, laying a theoretical foundation for the in-depth exploration of melatonin’s regulatory function on mammalian reproduction, the optimization of in vitro embryo production system, and the improvement of the economic benefits of animal husbandry production, and also providing a theoretical reference for human reproductive health.
Melatonin and Its Receptors
Melatonin (MEL) is an indoleamine hormone synthesis of which is regulated by light and which has obvious circadian rhythm. After the pineal gland cells take up tryptophan from the blood, they produce serotonin under the action of hydroxylase and decarboxylase. Serotonin produces N-acetylserotonin under the action of N-acetyltransferase (NAT)(Fig.1). Then, melatonin is produced under the action of methylase, and this process requires the participation of hydroxyindole-O-methyltransferase (HIOMT). HIOMT regulates the periodic secretion of melatonin, and the synthesis of melatonin in the pineal gland increases significantly after increasing the activity of HIOMT[4](Fig.2). The molecular weight of melatonin is 232.27. It has the characteristics of high lipophilicity, and being soluble in organic solvents and easily damaged by heat. Melatonin and its receptors are distributed in the retina, gastrointestinal tract, ovary and testis of male and female animals[5], but melatonin is only periodically secreted in the pineal gland and retina. Studies have found that there are two types of receptors for melatonin. Membrane receptors include MT1, MT2 and possibly MT3, and another type of nuclear receptor, RZR/ROR, belongs to the orphan receptor subfamily. MT3 is mainly found in non-mammalian bodies, and mammals are only found in the liver and kidney of hamsters. Melatonin acts by binding to G-coupled protein receptors MT1, MT2 and nuclear receptor RZR/ROR in vivo[6]. Among them, MT1 and MT2 receptors can inhibit the activity of adenylate cyclase (cAMP). The difference is that MT1 receptor can activate protein kinase C-β, while MT2 receptor can inhibit the activity of guanylate cyclase and stimulate protein kinase C[7]. MT1 receptor is a high-affinity receptor that can regulate ion flow and ion channels. MT2 receptor is a low-affinity receptor, which can regulate intracellular Ca2+ and inhibit the release of neurotransmitters[8]. Studies have shown that melatonin has a highly effective antioxidant effect and exerts a wide range of physiological functions in the body. Because of its hydrophilicity and lipophilicity, melatonin can easily pass through all physiological barriers, protect DNA, fatty acids and proteins in the nucleus and mitochondria from free radical damage, and reduce cell oxidative damage[9]. A large number of studies have confirmed that melatonin is involved in a variety of physiological processes, such as animal sleep, neuroendocrine, animal immune regulation[10], species evolution[11], anti-cancer[12], anti-aging, skin color formation, cell growth cycle regulation, energy metabolism, angiogenesis[13], neuroprotection and depression treatment[14], and goat cashmere-producing performance[15-17]. Melatonin plays an active role in a variety of animal disease models and clinical research(Fig.3).
Biological Function and Research Status of Melatonin in the Regulation of Animal Reproduction
Melatonin and its receptors with male animal germ cells
Melatonin and its receptors MT1 and MT2 are expressed in ram sperm[18], indicating that melatonin may have a direct effect on sperm cells. Studies have shown that melatonin can significantly reduce the rate of sperm deformity and degree of lipid peroxidation and improve sperm stability, and can also improve the stability of mouse sperm DNA in the case of continuous or intermittent hypoxia in mouse sperm[19]. The antioxidant properties of melatonin can prevent the quality of epididymal sperm in the rat testicular ischemia-reperfusion model[20]. In the process of transplanting spermatogonial stem cells into the testis of azoospermic mice, melatonin can effectively reduce ROS and lipid peroxides, thereby increasing the efficiency of transplantation and improving the structural characteristics of testicular tissue[21]. For boar sperm for in-vitro fertilization, melatonin can affect its vitality and the stability of nucleoprotein independently of its antioxidant properties[22]. In addition, in the study of human sperm, it was found that melatonin was significantly related to sperm motility, and compared with the fertile group, the level of melatonin in the seminal plasma of the sterile group was significantly reduced[23]. Adding melatonin to diluted semen can increase the percentage of motile sperm and fast motile sperm[24].
In the process of sperm freezing, melatonin can protect sperm from damage. The study by Ashrafi et al. proved that melatonin could be added to a bovine sperm freezing liquid to offset the adverse effects on bovine sperm during the freezing and thawing process, and the total antioxidant capacity and antioxidant enzyme activity were improved after the addition of melatonin, resulting in a protection effect on frozen sperm. Melatonin can stimulate the activity of SOD, GPx and CAT and other enzymes involved in the elimination of ROS metabolism, thereby maintaining the fluidity of cell membranes[25]. In the process of dyeing, sorting and freezing, melatonin can protect buffalo sperm from the damage of active oxygen, and improve the quality of sperm after the process of freezing and thawing. During the cryopreservation of human sperm, melatonin can also effectively protect sperm from freezing damage through the PI3K/AKT signaling pathway[26].
The effect of melatonin on sperm is closely related to the added concentration of its external source. Martín-Hidalgo et al.[27] observed that although 1 μM melatonin could increase the proportion of live cells with intact acrosomes, it could not improve the function of pig semen stored at 17 ℃ for 7 d. Li et al.[28] found that 10-4 M melatonin could significantly improve the sperm quality of Holstein cattle. Micromolar concentrations of melatonin can regulate the capacitation of ram sperm induced by cAMP elevating agents by reducing the levels of ROS and cAMP, while at lower concentrations, melatonin changes the subpopulation of motile sperm. These findings provide a reference for further study of melatonin in controlling sperm capacitation in artificial insemination systems[29]. For human semen, after adding 2 mM melatonin, it was found that the proportions of motile sperm, forward motile sperm and fast motile sperm increased, the number of dead sperm decreased, and endogenous NO significantly decreased, but the ROS level did not change significantly. It shows that 2 mM melatonin can directly or indirectly remove excess NO, thereby protecting sperm. Thus, it can be inferred that different species have different sensitivity to melatonin, leading to differences in the concentration of melatonin that plays a beneficial role.
Melatonin with its receptors and oocytes
Melatonin has a positive effect on mammalian germ cells. The presence of melatonin membrane receptors and nuclear receptors was detected on mature oocytes, corpus luteum granulosa cells and membrane cells, suggesting that melatonin may directly affect the biological functions of the ovary. Studies have found that melatonin can be synthesized by the ovary and released into the follicular fluid, and accumulates concentration as the follicle develops, and the concentration of melatonin in large follicles is higher than that in small follicles[30]. It is currently hypothesized that serotonin synthesized in granulosa cells is used to synthesize melatonin, and melatonin acts as a calmodulin inhibitor and free radical scavenger in oocytes to participate in the growth and maturation of oocytes[31-32]. Melatonin can not only remove excessive free radicals produced by oocytes during ovulation and improve the nucleus and cytoplasm of oocytes[33], but also can promote granular cell mitosis and follicular development by increasing the secretion of insulin-like growth factor IGF-1[34]. Many research reports have shown that high concentrations of melatonin in follicular fluid can inhibit follicular atresia and protect oocytes from free radical damage during ovulation, which is very important for the full development of oocytes[35]. Melatonin can significantly improve the nuclear maturation of oocytes in polyovarian syndrome. After melatonin treatment, the division rate of oocytes was significantly higher than that of the control group without melatonin, indicating that melatonin has the effect of inducing oocyte nuclear maturation and can ensure its fertilization ability[36].
Mammalian oocytes have a complex subcellular structure. When the temperature and osmotic pressure change slightly, oocytes will change. In the process of cryopreservation of oocytes, with the change of the external environment, the internal environment of cells will change significantly, and ROS will be generated at the same time, which will affect the balance between the redox reaction and the antioxidant system in cells, and the imbalance of the system will cause the occurrence of oxidation reactions, which will significantly reduce the viability of cells[37]. The addition of melatonin to the oocyte maturation media of porcine, bovine and sheep species can reduce the production of ROS, indicating that melatonin plays an important role in promoting oocyte maturation in vitro[38]. Supplementing a certain concentration of melatonin to the oocyte maturation fluids can promote the nuclear development from MI to MII stage, and increase the maturation rate of nucleus and the uniform distribution of cytoplasmic mitochondria[39]. In the in-vitro maturation systems of oocytes, in addition to being affected by the concentration, whether the added melatonin can play a beneficial role also depends on the oxygen concentration in the culture conditions. Under too high or too low oxygen conditions, the addition of melatonin may reduce blastocyst rate[40]. Therefore, to ensure that the melatonin added in vitro plays a positive and beneficial role, it is necessary to further explore the appropriate concentration of melatonin added to different species and suitable in-vitro culture conditions.
Tian LIN et al. Research Progress on the Function of Melatonin and Its Receptor in Animal Reproduction
Melatonin and its receptors with reproductive endocrine
Melatonin affects cell proliferation and energy metabolism, so melatonin is an important player in the regulation of reproductive hormone synthesis. The effects of melatonin on reproductive hormones depend on a variety of factors, including physiological conditions and animal species. For long-day seasonally breeding animals such as rodents, female rats experience anestrus and inhibited reproductive system under short-day cycles[41]. After melatonin injection, ovarian weight is reduced, and sexual maturation is delayed[42]. Melatonin reduces the expression of androgen receptor and testicular androgen-binding protein in male rats[43]. Injecting melatonin into the testes of male rats in the reproduction period results in reduced testicular volume and significantly lower testosterone levels[44]. Thus, melatonin has inhibitory effects on reproduction in long-day mammals. For short-day animals, implantation of melatonin in non-estrus doe can increase the content of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in vivo[45]. Melatonin is positively correlated with androgen levels in animals with short-day periods, and testosterone concentrations were twice as high in sheep treated with melatonin as in controls[46]. Sustained melatonin supplementation in short-day-reared animals can promote gonadal function[47-48], long-term melatonin treatment can induce early testicular development in sika deer[49], and subcutaneous melatonin injection increases testosterone concentrations in male goats[50]. The above results indicate that melatonin is an active factor in the reproduction of short-day animals.
Melatonin is the key to synergizing photoperiod with reproductive activity. Melatonin regulates the hypothalamic-pituitary-gonadal axis by activating the gonadotrophin releasing hormone (GnRH) receptors on hypothalamic neurons to regulate the secretion of FSH and LH, thereby affecting the secretion of gonadal reproductive hormones[51]. FSH and LH play an important role in animal reproduction. For female animals, FSH, LH and their receptors can regulate the level of cAMP in granulosa cells and theca cells, and cAMP can activate steroid synthase, thereby regulating the synthesis of progesterone, estradiol and so on. The LH peak before ovulation induces a high level of MT1 expression in granulosa cells, and at the same time, the expression level of the melatonin synthase SNAT gene in cumulus granulosa cells also increases significantly, resulting in an increase in the level of melatonin in the follicular fluid[52], which thereby regulates the luteinization of granulosa cells. Melatonin treatment can significantly increase the expression level of LH receptor in human granulosa cells, which can increase the likelihood of follicle ovulation[53]. In male animals, the long-term effects of exogenous melatonin in sheep can lead to the release of GnRH and LH, thereby activating reproductive activity[54]. There is evidence that melatonin has an inhibitory effect on reproduction in long-day animals, possibly through the inhibition of GnRH[55]. However, the exact mechanism by which melatonin regulates GnRH remains unclear. Kisspeptins (Kp) are upstream factors that promote GnRH secretion and are the key to transmitting melatonin messages[56]. It was found that photoperiod can modulate kiss1 signaling through melatonin to drive the reproductive axis[57]. After inducing zebrafish with melatonin, the Kiss1, kiss2 and GnRH3 genes in the brain and the expression of LHβ in the pituitary are enhanced, thereby promoting gonadal maturation and significantly improving the reproductive capacity of zebrafish[58]. Therefore, melatonin can cause changes in Kp expression, thereby affecting the reproductive system, but the relationship between melatonin and Kp still needs to be further explored.
Melatonin with embryonic development
Melatonin has antioxidant properties that decompose various free radicals and plays a key role in embryo transfer and embryonic development. High concentrations of maternal melatonin after fertilization can regulate the expression of antioxidant genes and antioxidant enzymes[59]. In addition to directly scavenging free radicals, it can also promote the secretion of progesterone by granulosa cells of the corpus luteum[60], which is very important for the healthy development of embryos. During maternal pregnancy, melatonin can down-regulate the expression of the pro-apoptotic genes Bax and Caspase-3 and up-regulate the expression of the anti-apoptotic gene Bcl-2, increase the level of intracellular glutathione and reduce the level of ROS[61-62], and in turn reduce the apoptosis rates of blastocysts and improve embryo quality and subsequent embryo implantation rate[63]. In order to meet the energy metabolism required for fertilization and early embryo development, there are a large number of lipid droplets in livestock embryos, especially in the process of in vitro preservation and maturation of gametes and embryos that are popular in modern farming. In this biochemical process, oxidative stress that damages embryos is unavoidable[64]. When gametes and embryos are exposed to relatively high oxidative stress conditions, intense ROS react with lipid droplets to damage critical cellular structures, disrupt cell membrane integrity, and alter the structure and function of nucleic acids and proteins[65]. Protein peroxidation leads to changes in protein structure and function, nucleic acid damage, and impacts on gene expression and genetic stability[66]. The free radical damage of the plasma membrane will affect the balance between the intracellular redox reaction and the intracellular antioxidant system, resulting in increased sensitivity to lipids. The external environment has undergone tremendous changes, which directly affect the function and viability of gametes and embryos[67]. Decreased antioxidant activity and elevated ROS in oocytes are the main causes of embryonic developmental defects, apoptosis, and embryonic developmental arrest in vitro[68], of which apoptosis is considered to be one of the main factors for embryo loss and reduced viability of thawed embryos during in-vitro culture[69]. The addition of exogenous melatonin can improve the in-vitro oocyte maturation rate, blastocyst rate, embryo development and embryo survival rate of pigs, cattle, sheep and other livestock[70-72], and significantly improve embryo development quality in in-vitro culture systems. In in-vitro culture system of mouse embryos, the addition of 10-6 melatonin can increase the fertilization rate, improve early blastocyst development, significantly improve the development of mouse pronuclear embryos and blastocyst hatching rate, and increase the implantation rate of embryos and pregnancy rate after transplantation. In pigs, melatonin at a concentration of 10-9 in vitro can significantly promote embryonic cleavage and blastocyst rates, but cells are damaged above or below this concentration[73]. In a 20% hyperoxia environment, adding appropriate melatonin to culture media can significantly reduce the effects of oxidative stress, thereby improving the blastocyst development rate and quality of bovine embryos in vitro. The addition of exogenous melatonin can improve the survival rate of goat embryos after thawing and the hatching rate of blastocysts after 24 h of culture, and reduce the rate of developmentally arrested embryos[74]. For embryos of different species, the concentration of melatonin added and the in-vitro culture conditions are different. Therefore, the establishment of an efficient and stable in-vitro culture system for different livestock still needs to be solved urgently.
Conclusions and Prospects
Based on previous research reports, a certain dose of melatonin can ensure normal body metabolism in mammals and improve reproductive performance. Scientists have been working on the direct regulation of melatonin in male sperm quality, oocyte maturation and embryonic development. In the body, as an antioxidant, melatonin reduces oxidative stress caused by reactive oxygen species generated during ovulation, reduces oxidative damage in follicles, and improves fertilization rate. In in-vitro sperm preservation and oocyte culture systems, adding a certain concentration of exogenous melatonin can reduce the oxidative stress caused by environmental changes, balance the relationship between the redox reaction and the antioxidant system in the system, and reduce damage to nucleic acid and protein structure and function from environmental shocks. As an antioxidant and endocrine hormone, the biological functions of melatonin in mammalian reproductive systems are obvious, and further studies on melatonin in regulating steroid hormone synthesis and exploring the mechanism of small molecule melatonin receptors may also contribute to the treatment of reproductive disorders and diseases caused by steroid hormone disorders. However, the effect of melatonin on sperm function, oocyte maturation and embryo development and its mechanism of action still need to be further understood, and how culture conditions and exogenous melatonin concentrations specifically affect the developmental capacity of oocytes and embryos also requires more research and exploration.
References
[1] CHAI K, LIU X, ZHANG Y, et al. Day-night and reproductive cycle profiles of melatonin receptor, kiss, and gnrh expression in orange-spotted grouper (Epinephelus coioides)[J]. Molecular Reproduction & Development, 2013, 80(7): 535-548.
[2] VANDEVOORT CA, MTANGO NR, MIDIC U, et al. Disruptions in follicle cell functions in the ovaries of rhesus monkeys during summer[J]. Physiological Genomics, 2015, 47(4): 102-112.
[3] BASINI G, BUSSOLATI S, CICCIMARRA R, et al. Melatonin potentially acts directly on swine ovary by modulating granulosa cell function and angiogenesis[J]. Reproduction Fertility & Development, 2017, 29(12): 2305-2312.
[4] CEINOS RM, CHANSARD M, REVEL F, et al. Analysis of adrenergic regulation of melatonin synthesis in Siberian hamster pineal emphasizes the role of HIOMT[J]. Neurosignals, 2004, 13(6): 308-317.
[5] REITER RJ, TAN DX, ROSALES-CORRAL S, et al. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives[J]. Mini Rev Med Chem, 2013, 13(3): 373-384.
[6] REITER RJ, TAN DX, MANCHESTER LC, et al. Medical implications of melatonin: receptor-mediated and receptor-independent actions[J]. Adv Med Sci, 2007, 52(6): 11-28.
[7] DUBOCOVICH ML, MARKOWSKA M. Functional MT 1 and MT 2 melatonin receptors in mammals[J]. Endocrine, 2005, 27(2): 101-110.
[8] DUBOCOVICH ML, DELAGRANGE P, KRAUSE DN, et al. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors[J]. Pharmacological Reviews, 2010, 62(3): 343-380.
[9] REITER RJ, ROSALES-CORRAL SA, MANCHESTER LC, et al. Peripheral reproductive organ health and melatonin: ready for prime time[J]. International Journal of Molecular Sciences, 2013, 14(4): 7231-7272.
[10] FAREZ M, MASCANFRONI I, M NDEZ-HUERGO S, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses[J]. Cell, 2015, 162(6): 1338-1352.
[11] TOSCHES MA, BUCHER D, VOPALENSKY P, et al. Melatonin signaling controls circadian swimming behavior in marine zooplankton[J]. Cell, 2014, 159(1): 46-57.
[12] BRITTNEY J, NIHAL A. Melatonin in cancer management: progress and promise[J]. Cancer Research, 2006, 66(20): 9789-9793.
[13] LERNER AB. Hormones and skin color[J]. Scientific American, 1961, 205(1): 98.
[14] BARCHAS JD, LERNER AB. Localization of melatonin in the nervous system[J]. Journal of Neurochemistry, 2010, 11(6): 489-491.
[15] YUE CW, ZHANG W, KONG XH, et al. Effect of melatonin on cashmere performance in inner mongolia white cashmere goats[J]. Chinese Journal of Animal Science, 2007, 43(7): 32-34.
[16] PARADIES G, PETROSILLO G, PARADIES V, et al. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease[J]. Journal of Pineal Research, 2010, 48(4): 297-310.
[17] DUAN C, XU J, SUN C, et al. Effects of melatonin implantation on cashmere yield, fibre characteristics, duration of cashmere growth as well as growth and reproductive performance of Inner Mongolian cashmere goats[J]. Animal Producition Science, 2015, 6(22): 1-6.
[18] CASAO A, GALLEGO M, ABECIA JA, et al. Identification and immunolocalisation of melatonin MT(1) and MT(2) receptors in Rasa Aragonesa ram spermatozoa[J]. Reproduction Fertility & Development, 2012, 24(7): 953-61.
[19] REITER RJ. Melatonin: Lowering the high price of free radicals[J]. News Physiol Sci, 2000, 15(5): 246-250.
[20] KURCER Z,HEKIMOGLU A, ARAL F, et al. Effect of melatonin on epididymal sperm quality after testicular ischemia/reperfusion in rats[J].Fertility & Sterility,2010, 93(5): 1545-1549.
[21] MOHAMMADGHASEMI F, JAHROMI SK. Melatonin ameliorates testicular damages induced by nicotine in mice[J]. Iranian Journal of Basic Medical Sciences, 2018, 21(6): 639-644.
[22] MARTINA R, RAFAEL B, ANNA P, et al. Melatonin affects the motility and adhesiveness of in vitro capacitated boar spermatozoa via a mechanism that does not depend on intracellular ROS levels[J]. Andrology, 2018, 6(5): 720-736.
[23] AWAD H, HALAWA F, MOSTAFA T, et al. Melatonin hormone profile in infertile males[J]. International Journal of Andrology, 2010, 29(3): 409-413.
[24] DU PLESSIS SS, HAGENAAR K, LAMPIAO F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS[J]. Andrologia, 2010, 42(2): 112-116.
[25] ASHRAFI I, KOHRAM H, ARDABILI FF. Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa[J] Animal Reproduction Science,2013, 139(1-4): 25-30.
[26] NAJAFI A, ADUTWUM E, YARI A, et al. Melatonin affects membrane integrity, intracellular reactive oxygen species, caspase3 activity and AKT phosphorylation in frozen thawed human sperm[J].Cell & Tissue Research, 2018, 372(1): 149-159.
[27] MARTIN HD, BARON F. The effect of melatonin on the quality of extended boar semen after long-term storage at 17 ℃[J].Theriogenology, 2011, 75(8): 1550-1560.
[28] LI XX, LIU Y, CAO PH, et al. Antioxidative effect of melatonin on IVF of bovine oocyte[J]. Acta Ecologae Animalis Domastici, 2015, 36(7): 57-62.
[29] GIMENO-MARTOS S, CASAO A, YESTE M, et al. Melatonin reduces cAMP-stimulated capacitation of ram spermatozoa[J]. Reproduction Fertility and Development, 2019, 31(2): 420-431.
[30] WANG SJ, LIU WJ, WANG LK, et al. Research progress on the regulatory effect of melatonin on the reproductive system of female animals[J]. Jiangsu Agricultural Sciences, 2016, 44(6): 15-20.
[31] RIO BD, PEDRERO JMG, MARTINEZ-CAMPA C, et al. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin[J]. Journal of Biological Chemistry, 2004, 279(37): 38294.
[32] SAKAGUCHI K, ITOH MT, TAKAHASHI N, et al. The rat oocyte synthesises melatonin[J]. Reprod Fertil Dev, 2013, 25(4): 674-682.
[33] CASAO A, ABECIA JA, PéREZ JAC, et al. The effects of melatonin on in vitro oocyte competence and embryo development in sheep[J].Spanish Journal of Agricultural Research,2010, 8(1): 35.
[34] PICINATO MC, HIRATA AE, CIPOLLA-NETO J, et al. Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets[J]. Journal of Pineal Research, 2010, 44(1): 88-94.
[35] TAMURA H, NAKAMURA Y, KORKMAZ A, et al. Melatonin and the ovary: physiological and pathophysiological implications[J]. Fertility & Sterility, 2009, 92(1): 328-343.
[36] NIKMARD F, HOSSEINI E, BAKHTIYARI M, et al. Effects of melatonin on oocyte maturation in PCOS mouse model[J]. Animal science journal = Nihon chikusan Gakkaiho, 2016, 88(4): 586-592.
[37] SOMFAI T, OZAWA M, NOGUCHI J, et al. Developmental competence of in vitro-fertilized porcine oocytes after in vitro maturation and solid surface vitrification: Effect of cryopreservation on oocyte antioxidative system and cell cycle stage[J]. Cryobiology, 2007, 55(2): 115-126.
[38] NAKANO M, KATO Y, TSUNODA Y,et al. Effect of melatonin treatment on the developmental potential of parthenogenetic and somatic cell nuclear-transferred porcine oocytes in vitro[J]. Zygote, 2012, 20(2): 199-207.
[39] ELRAEY M, GESHI M, SOMFAI T, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle[J]. Molecular Reproduction & Development, 2011, 78(4): 250-262.
[40] PAPIS K, POLESZCZUK O, WENTA-MUCHALSKA E, et al. Melatonin effect on bovine embryo development in vitro in relation to oxygen concentration[J]. Journal of Pineal Research, 2007, 43(4): 321-326.
[41] YANG Y, CHEN L, LU LZ. Review of regulation on the pineal gland of animal seasonal reproduction[J]. Journal of Agricultural Biotechnology, 2017, 25(7): 1086-1101.
[42] MEI H, LI C. Research progress of the effect of melatonin in animal[J]. Feed research, 2017, 38(1): 62-64.
[43] AHMAD R, HALDAR C. Effect of intra-testicular melatonin injection on testicular functions, local and general immunity of a tropical rodent Funambulus pennanti[J]. Endocrine, 2010, 37(3): 479-488.
[44] QIN FJ, ZHANG J, ZAN LS, et al. Inhibitory effect of melatonin on testosterone synthesis is mediated via GATA-4/SF-1 transcription factors[J]. Reproductive Biomedicine Online, 2015, 31(5): 638-646.
[45] WANG L, LIU GS, SHI WQ, et al. Effects of exogenous melatonin on superovulation and gonadotrophin hormones in sika deer[J]. Zhongguo Luye Jinzhan, 2011, 15(7): 12107-12118.
[46] DENG SL, CHEN SR, WANG ZP, et al. Melatonin promotes development of haploid germ cells from early developing spermatogenic cells of Suffolk sheep under in vitro condition[J]. Journal of Pineal Research, 2016, 60(4): 435-47.
[47] CASAO A, PEREZ-PE R, ABECIA JA, et al. The effect of exogenous melatonin during the non-reproductive season on the seminal plasma hormonal profile and the antioxidant defence system of Rasa Aragonesa rams[J]. Animal Reproduction Science, 2013, 138(3-4): 168-174.
[48] MURA MC, LURIDIANA S, BODANO S, et al. Influence of melatonin receptor 1A gene polymorphisms on seasonal reproduction in Sarda ewes with different body condition scores and ages[J]. Animal Reproduction Science, 2014, 149(3-4): 173-177.
[49] WANG L, ZHUO ZY, SHI WQ, et al. Melatonin promotes superovulation in sika deer (Cervus nippon)[J]. International Journal of Molecular Sciences, 2014, 15(7): 12107-12118.
[50] REKIK M, TACOUBI R, FEHRI Y, et al. Melatonin administration enhances the reproductive capacity of young rams under a southern Mediterranean environment[J]. Animal Science Journal, 2015, 86(7): 666-672.
[51] DENG SL, ZHANG Y, YU K, et al. Melatonin up-regulates the expression of the GATA-4 transcription factor and increases testosterone secretion from Leydig cells through RORα signaling in anin vitrogoat spermatogonial stem cell differentiation culture system[J]. Oncotarget, 2017, 8(66): 110592-110605.
[52] HE C, MA T, SHI J, et al. Melatonin and its receptor MT1 are involved in the downstream reaction to luteinizing hormone and participate in the regulation of luteinization in different species[J]. Journal of Pineal Research, 2016, 61(3): 279-290.
[53] WOO MM, TAI CJ, KANG SK, et al. Directaction of melatonin in human granulosa-luteal cells[J]. Journal of Clinical Endocrinology & Metabolism, 2015, 86(10): 4789-4797.
[54] VIGUI C, CARATY A, LOCATELLI a, et al. Regulation of luteinizing hormone-releasing hormone (LHRH) secretion by melatonin in the ewe. II. Changes in N-methyl-D,L-aspartic acid-induced LHRH release during the stimulation of luteinizing hormone secretion by melatonin[J]. Biology of Reproduction, 1995, 52(5): 1156-1161.
[55] BUCHANAN K L, YELLON SM. Delayed puberty in the male Djungarian hamster: Effect of short photoperiod or melatonin treatment on the GnRH neuronal system[J]. Neuroendocrinology, 1991, 54(2): 96-102.
[56] CAROLINE A, BENTSEN AH, MARIE-EMILIE S, et al. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule[J]. Endocrinology, 2012, 153(3): 1352-1363.
[57] REVEL FG, SABOUREAU M, MASSON-P VET M, et al. Kisspeptin mediates the photoperiodic control of reproduction in Hamsters[J]. Current Biology, 2006, 16(17): 1730-1735.
[58] CARNEVALI O, GIOACCHINI G, MARADONNA F, et al. Melatonin induces follicle maturation in Danio rerio[J]. Plos One,2011, 6(5): e19978.
[59] MOHSENI M, MIHANDOOST E, SHIRAZI A, et al. Melatonin may play a role in modulation of bax and bcl-2 expression levels to protect rat peripheral blood lymphocytes from gamma irradiation-induced apoptosis[J].Mutation Research/fundamental & Molecular Mechanisms of Mutagenesis,2012, 738-739(1): 19-27.
[60] TAMURA H, NAKAMURA Y, TERRON MP, et al. Melatonin and pregnancy in the human[J]. Reproductive Toxicology,2008, 25(3): 291-303.
[61] REITER RJ, TAN DX, MANCHESTER LC, et al. Melatonin and reproduction revisited[J].Biology of Reproduction,2009, 81(3): 445-456.
[62] GAO C, HAN HB, TIAN XZ, et al. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos[J]. Journal of Pineal Research, 2012, 52(3): 305-311.
[63] WANG F, TIAN XZ, ZHANG L, et al. Melatonin promotes the in vitro development of pronuclear embryos and increases the efficiency of blastocyst implantation in murine[J]. Journal of Pineal Research, 2013, 55(3): 267-274.
[64] STURMEY RG, REIS A, LEESE HJ, et al. Role of fatty acids in energy provision during oocyte maturation and early embryo development[J]. Reproduction in Domestic Animals, 2010, 44(s3): 50-58.
[65] TAMURA H, TAKASAKI A, TAKETANI T, et al. The role of melatonin as an antioxidant in the follicle[J]. Journal of Ovarian Research, 2012, 5(1): 5.
[66] LEON PMM, CAMPOS VF, CORCINI CD, et al. Cryopreservation of immature equine oocytes, comparing a solid surface vitrification process with open pulled straws and the use of a synthetic ice blocker[J]. Theriogenology, 2012, 77(1): 21-27.
[67] SARIZKAN S, BUCAK MN, TUNCER PB, et al. Effects of different extenders and centrifugation/washing on postthaw microscopic-oxidative stress parameters and fertilizing ability of Angora buck sperm[J]. Theriogenology, 2010, 73(3): 316-323.
[68] KHALIL WA, MAREI WFA, KHALID M, et al. Protective effects of antioxidants on linoleic acid-treated bovine oocytes during maturation and subsequent embryo development[J]. Theriogenology, 2013, 80(2): 161-168.
[69] HAO YH, LAI LX, MAO JD, et al. Apoptosis and in vitro development of preimplantation porcine embryos derived in vitro or by nuclear transfer[J]. Biology of Reproduction, 2003, 69(2): 501-507.
[70] RODRIGUEZ-OSORIO N, KIM IJ, WANG H, et al. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro[J]." Journal of Pineal Research, 2010, 43(3): 283-288.
[71] SAMPAIO RV, STEFANNE DBC, MIRANDA MS, et al. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro[J]. Reproductive Biology & Endocrinology, 2012, 10(1): 103.
[72] MARIA EM, MANUNTA ML, SPEZZIGU A, et al. Melatonin deprival modifies follicular and corpus luteal growth dynamics in a sheep model[J]. Reproduction, 2014, 147(6): 885-895.
[73] SHI JM, TIAN XZ, ZHOU GB, et al. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes[J]. Journal of Pineal Research, 2010, 47(4): 318-323.
[74] ABECIA JA, FORCADA F, ZIGA O, et al. The effect of melatonin on the secretion of progesterone in sheep and on the development of ovine embryos in vitro[J].Veterinary Research Communications,2002, 26(2): 151-158.
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Expression Analysis of Heat Shock Protein 70 Gene in Rice (Oryza sativa L.)
- Changes in Physiological and Biochemical Characteristics of Floral Organ
- Research Progress on Lonicera japonica Thunb. Affected by Environmental Stress
- Research Progress on Genetic Diversity of Snap Bean
- Allelopathic Effects of Cedrus deodara Needle Extracts on Seed
- Development Status and Countermeasures of Passiflora spp. Seedling Industry in Qinzhou, Guangxi

