七鳃鳗胆道闭锁过程中胆汁酸耐受机制研究进展
杨恒,逄越,李庆伟
七鳃鳗胆道闭锁过程中胆汁酸耐受机制研究进展
杨恒1,2,逄越1,2,李庆伟1,2
1. 辽宁师范大学生命科学学院,大连 116081 2. 辽宁师范大学七鳃鳗研究中心,大连 116081
胆道闭锁(biliary atresia, BA)是一种罕见的婴幼儿肝胆疾病,其特征是纤维硬化性胆管病变,导致肝外胆管和肝内胆管阻塞或闭塞,胆汁不能向肠道排泄,胆汁酸对肝实质细胞造成严重损伤,最后导致肝硬化和肝衰竭危及生命。目前,胆道闭锁的发病机理尚不明确,临床上普遍采用“先葛西”、“后移植”的序贯性治疗方式。葛西手术(Kasai)通过建立胆汁引流通道从而延长患儿自体肝生存时间,但随着门静脉高压和原发性胆管炎等并发症的出现,最后患者仍需要肝移植来挽救生命。七鳃鳗(lamprey)是唯一在生长发育过程中胆管能够自发消失的脊椎动物,在幼体期七鳃鳗具有完整的胆道系统,但在变态过程中,七鳃鳗表现出发育性胆道闭锁,胆管及胆囊逐渐退化直至整个胆道系统完全丧失,同时肝细胞发生重排和精细结构改变。研究发现,七鳃鳗可以在变态发育过程中形成胆道闭锁症状时维持血浆正常的胆汁酸水平,从而不会发生肝硬化和肝衰竭,适应性地在胆道闭锁和胆汁淤积症中存活。为探究七鳃鳗胆汁酸耐受在胆道闭锁疾病中的应用,本文对近年来七鳃鳗发生胆道闭锁而产生对胆汁酸耐受机制的相关研究进展进行了总结,以期为人类胆道闭锁疾病的诊断和治疗提供参考。
七鳃鳗;变态发育;胆道闭锁;胆汁淤积;耐受机制
在脊椎动物肝细胞内质网中,胆固醇被转化为两亲性多功能小分子胆汁酸或胆汁醇,与牛磺酸、甘氨酸、葡萄糖醛酸或硫酸根的结合形式称为胆盐,是胆汁的主要组成成分,胆盐合成是脊椎动物肝脏的一种特有功能,在食物消化和糖脂代谢中发挥着重要作用[1]。正常情况下,胆盐随着胆汁流入肠道被重新吸收后,经门静脉返回肝脏形成胆盐肠肝循环[2]。胆道闭锁时胆汁无法流入肠道,不能有效建立肠肝循环导致肝内胆汁淤积,肝细胞胆盐转运体及核受体的表达会在疾病的不同阶段作出适应性改变[3]。在胆汁淤积早期,肝细胞会减少对胆盐的摄取及分泌来降低肝内胆盐水平及胆小管内胆汁压力,从而保护肝细胞及胆管细胞免受损伤;在胆汁淤积终末期,机体为代偿肝细胞内高胆盐浓度,通过肝细胞胆管膜及基底膜双向排出胆盐,细胞基底膜胆盐转运体高表达以最大限度将胆盐排入循环系统,引起血浆中胆盐浓度升高对肝脏细胞造成危害[4]。
目前胆道闭锁的诊断与治疗都面临着一定的困难,患儿的早期诊断通常需要多种诊断方式相结合,具有不稳定性,并且极易与肝炎综合征等肝脏疾病发生混淆,从而错过最佳的治疗时期[5]。葛西手术(Kasai)可实现胆汁引流是BA的首选手术方法,但Kasai手术是一种姑息手术且成功率得不到保证,患儿最后仍需要肝移植挽回生命,肝源的严重缺乏成为了首要难题,针对胆道闭锁的特异性药物研发愈发显得重要。
七鳃鳗(lamprey)是现存的无颌类脊椎动物代表之一[6],幼体期具有完整的胆道系统,在变态过程中七鳃鳗胆管和胆囊会逐渐退化[7],变态完成后整个胆道系统完全消失(图1)。在七鳃鳗变态过程中类似的形态学变化最终可能与胆汁分泌减少相一致,七鳃鳗幼体的肝脏由一个分支小管网络组成,每个分支小管由4~6个锥形扁平细胞围绕一个管腔组成[8]。在变态早期观察到胆管变性的最初征象是胆管细胞微绒毛的断裂和明显的基底膜折叠,这种特征在胆道闭锁和胆汁性肝硬化患者的胆管中都有报道[9]。随着变态的进行,胆管细胞微绒毛的断裂更加严重,胆小管逐渐退化,膜碎片和可变电子密度的囊泡聚集在管腔内,这些残留物或被肝细胞吞噬,或与胆汁汇聚在一起,类似的现象也发生在哺乳动物的胆汁淤积期间[10]。变态中期肝细胞粗面内质网解聚,遍及肝脏的管腔变窄,胆管逐渐萎缩,胆管残余物完全被胶原纤维取代[9]。此外,胆管细胞内的包涵体(致密小体和自噬空泡)逐渐积累,研究表明这些包涵体的大小和数量反映了单个导管细胞的退化状态,在人类胆道闭锁和胆汁性肝硬化患者的胆管细胞内也观察到了类似的包涵体成分[11]。在变态末期,胶原纤维被肝细胞索所取代,幼体肝脏典型的分支小管被重建成由3个或更多同心排列的肝细胞组成的实心索,人类肝脏从早期胚胎发育到早期婴儿期也发生了类似的从小管到索状的转变过程[12]。七鳃鳗肝细胞在变态早期停止胆汁酸合成,经历细胞结构重组,最终在变态晚期恢复胆汁酸合成,并增殖以填补曾经由胆道系统占据的空间,退化的胆管上皮内没有游动的吞噬细胞,表明退化主要通过自溶和自噬作用进行,处于自溶晚期的细胞像人类胆道闭锁一样,脱落到胆管管腔内[13]。
七鳃鳗胆管退化过程中所表现出的病理特征与人类胆道闭锁十分相似:包括胆管细胞增生、巨噬细胞增加、胆管周炎、胆管闭塞、门脉周围淋巴细胞浸润、基底膜增厚和胶原原纤维的存在[14],七鳃鳗会出现胆汁淤积特征但不会发生肝硬化和肝衰竭[15]。七鳃鳗是如何耐受胆汁淤积,维持胆盐稳态并成功适应胆囊、肝内和肝外胆管的程序性消失的呢?有研究表明,七鳃鳗胆道闭锁的适应机制包括以下几个方面:(1)成熟期七鳃鳗通过鳃中胆汁酸修饰基因的特异性表达降低胆汁酸细胞毒性并将其排出体外[16];(2)改变成年七鳃鳗肝脏与血浆胆盐池组成,上调肾脏中有机阴离子和胆盐转运体的同源基因表达,加快七鳃鳗体内毒性胆盐的肾脏排泄[17];(3)下调肝脏胆汁酸合成能力,通过肠道合成来满足机体对胆汁酸的需要[18]。七鳃鳗通过以上多器官的相互协作适应性地耐受胆道闭锁(图2)。
七鳃鳗作为一种胆道闭锁的天然动物模型[20],可以观察到胆道闭锁整个发生发展过程,为临床上胆道闭锁的早期诊断提供理论依据。七鳃鳗胆道闭锁是一种与人类致病性胆道闭锁在许多细胞特征上相似的发育过程,但又不同于人类胆道闭锁的病理适应机制。七鳃鳗作为一种原始的无颌类脊椎动物已经进化出一种独特的适应途径来耐受发育性胆道闭锁带来的危害,了解这些机制的分子基础可能有助于阐明胆盐合成的进化和婴儿胆道闭锁的潜在治疗方法。

图1 七鳃鳗胆道闭锁前后肝脏HE染色对比
A:七鳃鳗胆道闭锁前肝脏HE染色(标尺:200 μm)和放大图(标尺:50 μm)。GB:胆囊;箭头所指为七鳃鳗胆管。B:七鳃鳗胆道闭锁后肝脏HE染色(标尺:200 μm)和放大图(标尺:50 μm)。
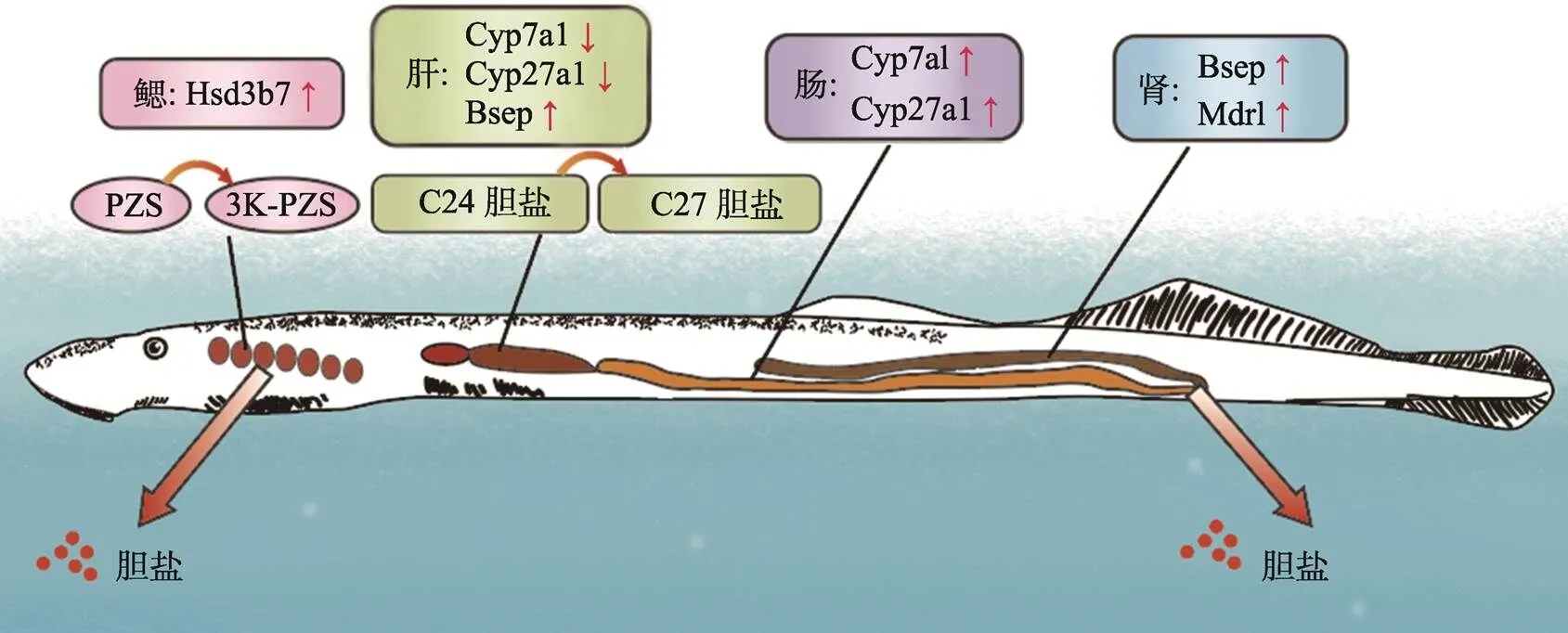
图2 七鳃鳗胆道闭锁期间胆汁酸耐受机制
在七鳃鳗胆道闭锁期间:鳃组织中基因表达上调催化胆盐PZS转化成毒性较小的3k-PZS;编码胆盐合成限速酶基因和在肝脏表达下调,在肠道表达上调代偿胆汁酸合成;肾脏中胆盐转运基因和表达上调,促进胆盐排泄到体外。根据参考文献[19]修改绘制。
1 七鳃鳗通过胆盐合成与转运基因特异性表达来适应胆道闭锁
七鳃鳗与哺乳动物有着不同的胆盐池组成,它能合成独特的5种α-胆汁酸(表1)。从已知胆盐的结构变化判断,七鳃鳗的胆汁酸合成途径似乎比哺乳动物更少受限[26],例如C3、C5、C7、C12和C24位点的修饰似乎是可变的,这表明七鳃鳗的胆盐合成酶可能在底物选择方面更为多样[27,28]。研究发现七鳃鳗在不同的发育阶段胆盐池的组成有所差异,可能与七鳃鳗胆盐合成关键酶在不同时期的表达模式不同有关,例如七鳃鳗幼体不能产生3,12-diketo- 4,6-petromyzonene-24- sulfate (dkPES),可能是因为缺乏3β-羟基-Δ5-C27-甾体脱氢酶(HSD3B7)活性[29]。另一方面,大部分成年七鳃鳗不会产生石油甾醇二硫酸盐(petromyzosterol disulfate, PSDS),可能是由于C3特异性磺基转移酶的表达抑制[30],这些胆盐合成酶在不同发育阶段产生不同的胆盐混合物中发挥着重要作用。在哺乳动物中,胆汁酸的合成受到多种反馈抑制机制的调节,胆汁酸鹅去氧胆酸(chenodeoxycholic acid, CDCA)和白介素1β (interleukin-1beta, IL-1β)可以通过诱导原癌基因c-Jun的表达抑制肝细胞核因子4α (hepatocyte nuclear factor 4-alpha, HNF4α)激活,使得胆汁酸生物合成限速酶CYP7A1 (胆固醇7-羟化酶)的基因表达受到抑制[31,32]。因此,JNK/c-Jun信号通路可抑制胆汁酸的合成,保护肝细胞免受胆汁酸的毒性作用[33]。七鳃鳗特有胆盐3k-ACA和3k-PZS在成熟期七鳃鳗的鳃中由酶HSD3B7催化生成,会改变JNK/c-Jun的表达[34],它们可能通过类似的JNK/c-Jun机制来抑制胆盐合成。
与成年雄性七鳃鳗肝脏组织相比,胆汁酸修饰相关的基因、和在鳃组织中的表达显著增加,和编码的酶分别催化类固醇激素和胆汁酸与硫酸盐结合[19]。LC-MS/MS分析和基因表达数据表明PZS在鳃上皮转化为毒性较低的3k-PZS,成年雄性七鳃鳗通过上述酶催化的脱氢作用和硫化作用降低胆汁酸的细胞毒性、增加这些化合物的溶解度,促进其分泌到体外,同时它对排卵期的雌性七鳃鳗有很强的信息素引诱作用[35]。在胆盐排出过程中,胆盐转运体发挥了重要作用:基因编码ATP依赖的胆盐输出泵,该泵可以从肝细胞中分泌胆盐[36],在成年雄性七鳃鳗肝脏中保持高表达,基因编码一种胆盐共转运蛋白,负责从血液中摄取胆盐到肝细胞,从幼体到成年的雄性七鳃鳗肝脏基因表达量下降[37],通过和两种胆盐转运基因正反向的共同作用,减少肝脏对胆盐摄取的同时增加了胆盐的排出。有趣的是,编码的共转运蛋白主要负责小肠管腔内的顶端细胞对胆盐的吸收[38],在成年雄性七鳃鳗中表达量很高,转运蛋白SLC10A2已被证明与5α-胆汁醇(例如七鳃鳗中的3k-PZS)有很高的亲和力,这种Na+/胆盐共转运蛋白可能在七鳃鳗从循环中摄取胆盐这一过程中发挥作用。
胆盐转运基因的组织特异性表达表明,这些转运基因在七鳃鳗的胆盐排泄过程中发挥重要作用。进一步确认和在转运5α-胆汁醇中的功能,检查转运蛋白(包括SLC10A2)在肝脏和鳃中的具体细胞位置对于理解七鳃鳗的胆盐排泄十分有必要。七鳃鳗可同时生成C24和C27胆汁醇,产生C24胆汁醇时裂解胆固醇侧链的酶机制可能不同于人类和啮齿类中产生C24胆盐的多种过氧化物酶体酶[39],上述问题一旦确定,将为揭示胆盐生物合成和排泄的复杂机制提供证据。

表1 七鳃鳗特有的胆汁酸
2 七鳃鳗通过改变肝脏胆盐池组成和加快胆盐肾脏排泄来适应胆道闭锁
胆汁酸通过与调节基因表达的核受体和G蛋白偶联受体相互作用,参与一系列的信号转导,调控胆盐稳态、葡萄糖代谢和心血管功能[40];然而胆汁酸也有几种病理作用,例如致癌性和细胞毒性[41]。通过肝肠代谢和循环维持胆盐的稳态,这对其生理功能和解毒至关重要[42]。七鳃鳗在变态过程中肝肠循环被破坏[43],成年七鳃鳗是如何耐受胆汁酸的细胞毒性适应胆汁淤积引起的肝损伤,并维持这种独特的胆盐稳态呢?以下的适应机制可能解释了这一现象:
(1)成年七鳃鳗下调了胆盐合成限速酶CYP7A1的肝脏表达,减少肝脏内源性胆盐的产生[18];(2)成年七鳃鳗将幼体期血浆和肝脏的主要胆盐C24胆盐转化为C27胆盐(胆盐成分的改变在胆汁淤积的啮齿类动物中也有描述[44]),与此同时将细胞毒性较大的胆盐PZS转化为3k-PZS[17];(3)成年七鳃鳗的肝脏中含有高浓度的胆红素和胆绿素,胆红素和胆绿素都具有抗氧化的特性,成年七鳃鳗可能通过下调肝脏中胆绿素还原酶的表达来提高胆绿素水平[45],这可以提高对肝脏的保护作用[46,47]有研究显示,胆绿素能够降低人肝细胞的胆盐细胞毒性,在胆绿素还原酶活性较低的细胞中观察到的保护作用最好[48]。七鳃鳗肾脏在变态过程中经历了相当大的结构重塑,成年七鳃鳗通过肾脏排泄有效地清除胆盐和有机阴离子,此外研究还发现成年七鳃鳗肾脏中胆盐输出泵BSEP和多药耐药相关蛋白1 (multidrug resistance associated protein 1, MDR1)mRNA表达显著增加,BSEP、MDR1是哺乳动物中关键的胆盐转运体,因此这两种转运蛋白在肾脏中的表达上调可能是成年七鳃鳗在肾脏排泄胆汁产物的原因[17]。
综上所述,七鳃鳗通过减少肝脏胆盐的合成和增加胆盐的肾脏排泄,降低胆汁淤积程度;改变肝脏胆盐池组成的同时在肝脏中积累高水平的胆绿素来防止肝组织损伤。因此,在肝脏中积累胆绿素并进一步诱导肾中胆盐和有机阴离子转运体的表达可能是治疗胆道闭锁和其他慢性胆汁淤积性肝病的新方法。
3 七鳃鳗通过肠道合成和分泌胆盐来适应胆道闭锁
对所有脊椎动物来说,胆盐合成是肝脏的一项独特而重要的功能,胆盐被排泄到肠腔中溶解脂肪酸,从而促进脂溶性维生素的吸收[49]。在胆道闭锁期间,七鳃鳗肝脏基因表达与胆盐浓度显著下调,的表达下调与人类胆道闭锁或其他胆汁淤积性疾病的现象惊人地相似[50]。然而,在许多哺乳动物模型和人类胆道闭锁模型中,的下调并不能阻止对肝脏的胆汁淤积性损伤。出现胆汁淤积的患者会发生肝纤维化和肝硬化,七鳃鳗尽管出现发育性胆道闭锁,却仍能维持正常的血浆胆盐水平防止肝损伤。研究发现,除了已知的肝脏中胆盐合成减少等机制外,七鳃鳗还通过肠道内从头合成和分泌胆盐的独特机制来适应胆道闭锁。在发育性胆道闭锁后,七鳃鳗不再保持从肝脏到肠道的直接胆汁流动,分泌物进入体循环是运送肝脏胆盐的唯一替代途径。基因编码合成胆盐初始酶,在发育性胆道闭锁之后,肠道基因的表达增加了100倍以上,而肝脏的表达减少了同样的幅度,与此同时这两个器官的胆盐池发生了相似模式和大小的改变,C24胆汁醇硫酸盐逐渐转变为牛磺酸结合的C24胆汁酸,牛磺酸结合的胆盐形式可能更有利于寄生期七鳃鳗消化脂类物质[18]。在寄生期肠道中还检测到胆盐合成中间体7α羟基胆固醇等,并且寄生期七鳃鳗的肠细胞周转率(9~14天)比哺乳动物(2~3天)慢[51],这可能有利于肠道内胆盐的合成。这些数据表明,寄生期七鳃鳗肠道中存在胆盐的从头合成,七鳃鳗独特的生活方式和胆盐的利用是由两个器官(肝脏和肠道)在整个发育周期合成不同的共轭胆盐来适应。有趣的是,肝脏和肠道中的脂肪分解和脂肪酸合成在不同的发育阶段之间存在显著差异[52]。七鳃鳗肝和肠都支持胆盐的合成来耐受发育性胆道闭锁,在动物进化过程中随着消化系统变得更加复杂,胆盐合成的位置似乎已经从消化道转移到肝脏。
七鳃鳗在变态期间,肝胆、肠道和肾脏经历了重塑和重组,以胆道系统的变化最为剧烈,关于七鳃鳗胆管退化的原因至今仍没有定论。有研究者认为寄生七鳃鳗的食物中可能含有一些物质不需要胆汁就能被肠道吸收,随着饮食方式的改变,七鳃鳗的胆管开始自然退化[13]。另一种假说认为,终生自由游泳生活的七鳃鳗也存在类似的胆管退化过程,可能反映了所有七鳃鳗物种的相似起源,代表着这类七鳃鳗对环境和生活方式的适应性进化[53]。对于胆管退化的原因,我们认为变态完成后进入寄生期,七鳃鳗快速进食为机体储存能量与营养,以肝肠循环为主的胆汁酸代谢模式已经无法满足机体需要,随着肝胆退化,七鳃鳗可能已经进化出了一套效率更高的胆汁酸代谢途径。对于七鳃鳗胆管退化的真正原因,还需要进一步比较寄生与非寄生七鳃鳗胆管退化过程中生理生化特征以及基因表达等方面的差异才能进一步确定。
七鳃鳗胆道系统消失的遗传编程可能对理解人类胚胎BA潜在机制具有重要作用,有研究表明转化生长因子β (beta-和热休克蛋白90 (heat shock protein 90, HSP90)可能在七鳃鳗胆道系统退化过程中发挥重要作用。Chung-Davidson等[54]在七鳃鳗胆道闭锁期间通过干扰-信号通路,导致独特的胆管退化模式和表型,说明-信号通路在胆管退化过程中发挥重要作用;通过变态各时期转录本的分析以及基因敲低等方法验证了HSP90参与肝胆转化的多个方面,包括肝细胞再生、胆管变性和胆汁酸合成的改变[55]。以上结果提示了发育性BA的可能机制,为推断人类胚胎性BA的病因提供了可检验的假说。目前认为,婴幼儿先天性胆道闭锁是环境因素、遗传突变以及母亲围产期病毒感染等导致,如果从七鳃鳗胆道闭锁的适应性进化观点来看,我们认为婴幼儿先天性胆道闭锁也可能是一种人类的返祖现象,并非后天因素影响所致。
4 结语与展望
七鳃鳗在发育性胆道闭锁期间,通过上调基因在鳃的特异性表达降低胆盐细胞毒性并通过鳃排泄;下调肝脏中胆盐合成关键基因表达并将肝内的主要胆盐C27胆盐转化为C24胆盐降低肝内胆汁淤积程度,由肠道代偿胆汁酸合成满足机体代谢需要;上调肾脏中胆盐转运基因表达加快毒性胆盐排泄等机制耐受胆道闭锁带来的危害,这些分子机制将会为治疗人类胆道闭锁提供思路。七鳃鳗具有独特胆盐,但其合成、转运与调控的关键酶仍未阐明,这些关键分子的找寻将为阐释七鳃鳗独特的胆汁酸耐受机制提供更多分子层面的证据。
综合以上观点以及胆道闭锁的最新研究进展,现提出以下几种看法:(1)七鳃鳗的肝脏中可能含有其他抗胆汁淤积分子,在阐明其机制后,可用于开发药物治疗胆道闭锁患儿,如果能够预防晚期纤维化,将改善这些患者的预后;(2)通过药物干预加强胆盐和其他有机溶质的肾脏排泄,则可能会减缓或阻止肝损伤的进展;(3)开发出专门针对胆盐生物转化和运输的适应性途径激动剂,可能通过法尼酯X受体(recombinant farnesoid X receptor, FXR)和孕烷X受体(pregnane X receptor, PXR)等核受体发挥作用;(4)在减少肝脏中胆盐合成的同时通过基因工程等方法将胆盐合成和分泌转移到肠道来适应胆道闭锁;(5)高迁移率蛋白1 (high mobility group proteins B1, HMGB1)等细胞因子在胆道闭锁的发病机制中发挥重要作用,可能成为治疗胆道闭锁的特异性靶点[56];(6)胆管“类器官”的成功培育有望缓解器官移植所面临的困境,这种新的细胞疗法可能会成为胆道闭锁疾病治疗新途径[57]。
[1] Morita SY, Ikeda Y, Tsuji T, Terada T. Molecular mechanisms for protection of hepatocytes against bile salt cytotoxicity., 2019, 67(4): 333–340.
[2] Nie YF, Hu J, Yan XH. Cross-talk between bile acids and intestinal microbiota in host metabolism and health., 2015, 16(6): 436–446.
[3] Thompson MD, Moghe A, Cornuet P, Marino R, Tian JM, Wang PC, Ma XC, Abrams M, Locker J, Monga SP, Nejak-Bowen K. β-Catenin regulation of farnesoid X receptor signaling and bile acid metabolism during murine cholestasis., 2018, 67(3): 955–971.
[4] Chen HL, Liu YJ, Chen HL, Wu SH, Ni YH, Ho MC, Lai HS, Hsu WM, Hsu HY, Tseng HC, Jeng YM, Chang MH. Expression of hepatocyte transporters and nuclear receptors in children with early and late-stage biliary atresia., 2008, 63(6): 667–673.
[5] Wang L, Yang Y, Chen Y, Zhan JH. Early differential diagnosis methods of biliary atresia: a meta-analysis., 2018, 34(4): 363–380.
[6] Liang J, Liu X, Wu FF, Li QW. Progress of adaptive immunity system of agnathan vertebrates., 2009, 31(10): 969–976.
梁佼, 刘欣, 吴芬芳, 李庆伟. 无颌类脊椎动物适应性免疫系统的研究进展. 遗传, 2009, 31(10): 969–976.
[7] Zhu YG, Li J, Pang Y, Li QW. Lamprey: an important animal model of evolution and disease research., 2020, 42(9): 847–857.
朱医高, 李军, 逄越, 李庆伟. 七鳃鳗:生物进化和疾病研究的重要模式动物. 遗传, 2020, 42(9): 847–857.
[8] Sidon EW, Youson JH. Morphological changes in the liver of the sea lamprey,L., during metamorphosis. II. Canalicular degeneration and transformation of the hepatocytes., 1983, 178(3): 225–246.
[9] Boomer LA, Bellister SA, Stephenson LL, Hillyard SD, Khoury JD, Youson JH, Gosche JR. Cholangiocyte apoptosis is an early event during induced metamorphosis in the sea lamprey,L., 2010, 45(1): 114–120.
[10] Sidon EW, Youson JH. Morphological changes in the liver of the sea lamprey,L., during metamorphosis: I. Atresia of the bile ducts., 1983, 177(1): 109–124.
[11] Nikitina N, Bronner-Fraser M, Sauka-Spengler T. The sea lamprey:a model for evolutionary and developmental biology., 2009, 2009(1): pdb.emo113.
[12] Tetlock A, Yost CK, Stavrinides J, Manzon RG. Changes in the gut microbiome of the sea lamprey during metamorphosis., 2012, 78(21): 7638–7644.
[13] Suchy FJ. Biliary atresia in sea lampreys. What can it tell us about the disorder in human infants?, 2013, 57(6): 2114–2116.
[14] Chung-Davidson YW, Yeh CY, Li WM. The sea lamprey as an etiological model for biliary atresia., 2015, 2015: 832943.
[15] Davenport M, Gonde C, Redkar R, Koukoulis G, Tredger M, Mieli-Vergani G, Portmann B, Howard ER. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia., 2001, 36(7): 1017–1025.
[16] Johnson NS, Yun SS, Li WM. Investigations of novel unsaturated bile salts of male sea lamprey as potential chemical cues., 2014, 40(10): 1152–1160.
[17] Cai SY, Lionarons DA, Hagey L, Soroka CJ, Mennone A, Boyer JL. Adult sea lamprey tolerates biliary atresia by altering bile salt composition and renal excretion., 2013, 57(6): 2418–2426.
[18] Yeh CY, Chung-Davidson YW, Wang HY, Li K, Li WM. Intestinal synthesis and secretion of bile salts as an adaptation to developmental biliary atresia in the sea lamprey., 2012, 109(28): 11419–11424.
[19] Chung-Davidson YW, Wang HY, Siefkes MJ, Bryan MB, Wu H, Johnson NS, Li WM. Pheromonal bile acid 3-ketopetromyzonol sulfate primes the neuroendocrine system in sea lamprey., 2013, 14: 11.
[20] Liu CJ, Huang HF, Ma F, Liu X, Li QW. Evolution of adaptive immune system in guinea-free vertebrates., 2008, 30(1): 13–19.
刘岑杰, 黄惠芳, 马飞, 刘欣, 李庆伟. 无颌类脊椎动物适应性免疫系统的进化. 遗传, 2008, 30(1): 13–19.
[21] Yun SS, Scott AP, Li W. Pheromones of the male sea lamprey,L.: structural studies on a new compound, 3-keto allocholic acid, and 3-keto petromyzonol sulfate., 2003, 68(3): 297–304.
[22] Haslewood GA, Tökés L. Comparative studies of bile salts. Bile salts of the lampreyL.., 1969, 114(2): 179–184.
[23] Li WM, Scott AP, Siefkes MJ, Yan HG, Liu Q, Yun SS, Gage DA. Bile acid secreted by male sea lamprey that acts as a sex pheromone., 2002, 296(5565): 138– 141.
[24] Hoye TR, Dvornikovs V, Fine JM, Anderson KR, Jeffrey CS, Muddiman DC, Shao F, Sorensen PW, Wang JZ. Details of the structure determination of the sulfated steroids PSDS and PADS: new components of the sea lamprey () migratory pheromone., 2007, 72(20): 7544–7550.
[25] Li K, Wang HY, Brant CO, Ahn SC, Li WM. Multiplex quantification of lamprey specific bile acid derivatives in environmental water using UHPLC-MS/MS., 2011, 879(32): 3879– 8386.
[26] Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms., 2004, 40(3): 539–551.
[27] Midzak A, Papadopoulos V. Binding domain-driven intracellular trafficking of sterols for synthesis of steroid hormones, bile acids and oxysterols., 2014, 15(9): 895–914.
[28] Björkheim I, Danielsson H, Einarsson K, Johansson G. Formation of bile acids in man: conversion of cholesterol into 5-beta-cholestane-3-alpha,7-alpha,12-alpha-triol in liver homogenates., 1968, 47(7): 1573–1582.
[29] Shea HC, Head DD, Setchell KDR, Russell DW. Analysis of HSD3B7 knockout mice reveals that a 3alpha-hydroxyl stereochemistry is required for bile acid function., 2007, 104(28): 11526–11533.
[30] Venkatachalam KV, Llanos DE, Karami KJ, Malinovskii VA. Isolation, partial purification, and characterization of a novel petromyzonol sulfotransferase from(lamprey) larval liver., 2004, 45(3): 486–495.
[31] Abu-Hayyeh S, Papacleovoulou G, Williamson C. Nuclear receptors, bile acids and cholesterol homeostasis series-bile acids and pregnancy., 2013, 368(1–2): 120–128.
[32] Liu X, Wang Y. An overview of bile acid synthesis and its physiological and pathological functions., 2019, 41(5): 365–374.
刘笑, 王琰. 胆汁酸的合成调控及其在生理与病理中的功能机制. 遗传, 2019, 41(5): 365–374.
[33] Li TG, Jahan A, Chiang JYL. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells., 2006, 43(6): 1202–1210.
[34] Siefkes MJ, Scott AP, Zielinski B, Yun SS, Li WM. Male sealampreys,L., excrete a sex pheromone from gill epithelia., 2003, 69(1): 125–132.
[35] Chung-Davidson YW, Bussy U, Fissette SD, Scott AM, Li WM. Bile acid production is life-stage and sex-dependent and affected by primer pheromones in the sea lamprey., 2021, 224(9): jeb229476.
[36] Sohail MI, Dönmez-Cakil Y, Szöllősi D, Stockner T, Chiba P. The bile salt export pump: molecular structure, study models and small-molecule drugs for the treatment of inherited BSEP deficiencies., 2021, 22(2): 784.
[37] Tran QH, Nguyen VG, Tran CM, Nguyen MN. Down- regulation of solute carrier family 10 member 1 is associated with early recurrence and poorer prognosis of hepatocellular carcinoma., 2021, 7(3): e06463.
[38] Wang LN, Zhou Y, Wang XH, Zhang GW, Guo B, Hou XM, Ran JT, Zhang QN, Li CC, Zhao XS, Geng YC, Feng SW. Mechanism of Asbt (Slc10a2)-related bile acid malabsorption in diarrhea after pelvic radiation., 2020, 96(4): 510–519.
[39] Hagey LR, Møller PR, Hofmann AF, Krasowski MD. Diversity of bile salts in fish and amphibians: evolution of a complex biochemical pathway., 2010, 83(2): 308–321.
[40] Han SY, Song HK, Cha JJ, Han JY, Kang YS, Cha DR. Farnesoid X receptor (FXR) agonist ameliorates systemic insulin resistance, dysregulation of lipid metabolism, and alterations of various organs in a type 2 diabetic kidney animal model., 2021, 8(4): 495–503.
[41] Wang RX, Sheps JA, Ling V. ABC transporters, bile acids, and inflammatory stress in liver cancer., 2011, 12(4): 636–646.
[42] Slizgi JR, Lu Y, Brouwer KR, St Claire RL, Freeman KM, Pan M, Brock WJ, Brouwer KL. Inhibition of human hepatic bile acid transporters by tolvaptan and metabolites: contributing factors to drug-induced liver injury?, 2016, 149(1): 237–250.
[43] Sidon EW, Youson JH. Morphological changes in the liver of the sea lamprey,L., during metamorphosis. II. Canalicular degeneration and transformation of the hepatocytes., 1983, 178(3): 225– 246.
[44] Zhang YC, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver., 2012, 32(1): 58–69.
[45] Makino I, Shinozaki K, Nakagawa S, Mashimo K. Measurement of sulfated and nonsulfated bile acids in human serum and urine., 1974, 15(2): 132–138.
[46] Makos BK, Youson JH. Tissue levels of bilirubin and biliverdin in the sea lamprey,L., before and after biliary atresia., 1988, 91(4): 701–710.
[47] Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, Knorr M, Karbach S, Schuhmacher S, Wenzel P, Münzel T, Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin., 2010, 49(2): 186–195.
[48] Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles., 2009, 106(13): 5171–5176.
[49] Bathena SPR, Thakare R, Gautam N, Mukherjee S, Olivera M, Meza J, Alnouti Y. Urinary bile acids as biomarkers for liver diseases II. Signature profiles in patients., 2015, 143(2): 308–318.
[50] Faiz Kabir Uddin Ahmed A, Ohtani H, Nio M, Funaki N, Iwami D, Kumagai S, Sato E, Nagura H, Ohi R.expression of fibrogenic growth factors and their receptors in biliary atresia: comparison between early and late stages., 2000, 192(1): 73–80.
[51] Youson JH, Langille RM. Proliferation and renewal of the epithelium in the intestine of young adult anadromous sea lampreys,L., 1981, 59(12): 2341–2349.
[52] Kao Y, Manzon RG, Sheridan MA, Youson JH. Study of the relationship between thyroid hormones and lipid metabolism during KClO4-induced metamorphosis of landlocked lamprey,., 1999, 122(3): 363–373.
[53] Morii M, Mezaki Y, Yamaguchi N, Yoshikawa K, Miura M, Imai K, Yoshino H, Hebiguchi T, Hebiguchi T, Senoo H. Onset of apoptosis in the cystic duct during metamorphosis of a Japanese lamprey,., 2010, 293(7): 1155–66.
[54] Chung-Davidson YW, Ren JF, Yeh CY, Bussy U, Huerta B, Davidson PJ, Whyard S, Li WM. TGF-β Signaling plays a pivotal role during developmental biliary atresia in sea lamprey ()., 2019, 4(2): 219–234.
[55] Chung-Davidson YW, Yeh CY, Bussy U, Li K, Davidson PJ, Nanlohy KG, Brown CT, Whyard S, Li WM. Hsp90 and hepatobiliary transformation during sea lamprey metamorphosis., 2015, 15: 47.
[56] Mohanty SK, Donnelly B, Temple H, Ortiz-Perez A, Mowery S, Lobeck I, Dupree P, Poling HM, McNeal M, Mourya R, Jenkins T, Bansal R, Bezerra J, Tiao G. High mobility group box 1 release by cholangiocytes governs biliary atresia pathogenesis and correlates with increases in afflicted infants., 2021, 74(2): 864–878.
[57] Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, Upponi SS, Brevini T, Wesley BT, Garcia-Bernardo J, Mahbubani K, Canu G, Gieseck R 3rd, Berntsen NL, Mulcahy VL, Crick K, Fear C, Robinson S, Swift L, Gambardella L, Bargehr J, Ortmann D, Brown SE, Osnato A, Murphy MP, Corbett G, Gelson WTH, Mells GF, Humphreys P, Davies SE, Amin I, Gibbs P, Sinha S, Teichmann SA, Butler AJ, See TC, Melum E, Watson CJE, Saeb-Parsy K, Vallier L. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver., 2021, 371(6531): 839–846.
Research progress of bile acids tolerance mechanism in lamprey biliary atresia
Heng Yang1,2, Yue Pang1,2, Qingwei Li1,2
Biliary atresia (BA) is a rare biliary disease in infants and young children, which is characterized by fibrosclerotic bile duct disease, leading to extrahepatic and intrahepatic bile duct obstruction or occlusion. The bile acids cannot be excreted to the intestinal tract, which causes serious damage to the liver parenchyma cells, and eventually leads to life-threatening cirrhosis and chronic liver failure. At present, the pathogenesis of biliary atresia is unknown. The sequential treatment of “first Gexi” and “later transplantation” is nowadays widely adopted in the clinic. Gossi operation (Kasai) can prolong the survival time of autologous liver by the establishment of bile drainage channel. However, it is necessary for patients to perform liver transplantation with the emergence of complications such as portal hypertension and primary cholangitis. Lampreys are the only vertebrates whose bile duct can disappear spontaneously in the process of growth and development. Lampreys have complete biliary system at the larval stage, but in the process of metamorphosis, lampreys show developmental biliary atresia, bile duct and gallbladder gradually degenerate until the whole biliary system is completely lost, and hepatocytes undergo rearrangement and fine structural changes at the same time. It has been found that lampreys can maintain normal plasma bile acid levels when the symptoms of biliary atresia are formed in during metamorphosis, so that liver cirrhosis and hepatic failure do not occur, thereby adapting to and surviving biliary atresia and cholestasis. To explore the application of bile acids tolerance in biliary atresia, we summarize the recent research progress on the mechanism of bile acids tolerance caused by biliary atresia in lampreys, and further provide a reference for the development of diagnosisandtreatments of human biliary atresia.
lampreys; metamorphosis; biliary atresia; cholestasis; tolerance mechanism
2021-08-11;
2021-09-22;
2021-11-24
国家自然科学基金项目(编号:31772884,32070518 ),辽宁省兴辽英才计划领军人才项目(编号:XLYC2002093),辽宁省科技项目(编号:2019-MS-218),辽宁省教育厅项目(编号:LJ2020012)和大连市科技创新基金项目(编号:2018J12SN079) 资助 [Supported by the National Natural Science Foundation of China(Nos.31772884, 32070518), Liaoning Climbing Scholar, the Distinguished Professor of Liaoning (No.XLYC2002093), the Program of Science and Technology of Liaoning Province (No.2019-MS-218), the Project of the Educational Department of Liaoning Province(No.LJ2020012), and the Science and Technology Innovation Fund Research Project of Dalian City (No. 2018J12SN079)]
杨恒,在读硕士研究生,专业方向:细胞生物学。E-mail: yangheng199811@163.com
逄越,博士,教授,研究方向:分子免疫进化。E-mail: pangyue01@163.com
李庆伟,博士,教授,研究方向:细胞遗传学。E-mail: liqw@263.net
10.16288/j.yczz.21-295
(责任编委: 姜长涛)

