Network pharmacology research and experimental verification of Huangqi (Astragalus Radix) and Jinyingzi (Rosae Laevigatae Fructus) in treating benign prostatic hyperplasia
ZHOU Hun, YANG Meng, YU Yipin, LIU Hui, QING Zhixing, CHEN Qihu*
a. Graduate School, Hunan University of Chinese Medicine, Changsha, Hunan 410208, China
b. Department of Andrology, The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan 410007, China
c. College of Veterinary Mediche, Hunan Agricultural University, Changsha, Hunan 410128, China
Keywords Huangqi (Astragalus Radix, HQ)Jinyingzi (Rosae Laevigatae Fructus, JYZ)Benign prostatic hyperplasia Network pharmacology Apoptosis Angiogenesis
ABSTRACT
1 Introduction
Benign prostatic hyperplasia (BPH) is the most common urological disease prevalent in middle-aged and older men. The incidence is more than 88% in men over 80 years. Its early manifestation includes bladder irritation, such as frequent urination, urgent urination, and increased nocturia. When bladder outlet obstruction reaches a certain level, lower urinary tract symptoms appear gradually, primarily including urinary symptoms and urinary storage symptoms, while urinary retention and incontinence appear at later stages[1,2]. BPH tends to recur and can be lingering, is difficult to heal, and may cause various complications, seriously affecting men’s urethral function and quality of life[3,4]. In recent years, several studies have found a significant correlation between prostate cancer and prostate hyperplasia[5,6]. Therefore, early diagnosis, treatment, and prevention of BPH are essential for improving patients’ quality of life and preventing progression to prostate cancer.
Based on clinical experience, our team created the Yishen Tonglong Capsule (益肾通癃胶囊), an in-hospital preparation that includes Huangqi (Astragalus Radix), Jinyingzi (Rosae Laevigatae Fructus),Gouqizi (Lycii Fructus), Buguzhi (Psoralese Fructus),Shanyao (Dioscorese Rhizoma), Shudi (Rehmanniae Radix Praeparata), Shanzhuyu (Corni Fructus), Fuling (Poria), Sanleng (Sparganii Rhizoma), Ezhu(Curcumae Rhizoma), and Gancao (Glycyrrhizae Radix et Rhizoma). This formulation has been used clinically to treat BPH for many years[7]. In the Yishen Tonglong Capsule, HQ is considered a “sovereign”medicine, as it is good at tonifying the original Qi, reinforcing the Qi of the spleen and lung, assists the Qi of congenital kidneys, and interferes with kidney deficiency pathogenesis in BPH. JYZ has a sour taste and belongs to the kidney and bladder meridians. It is good at strengthening the “essence” and reducing urine and can relieve frequent nocturnal urination symptoms. Together with HQ, they are “sovereign”and “minister” medicines that softly warm the Yang,securing “essence”, and reducing urination. Clinical observation has revealed that some prescriptions such as Modified Buzhong Yiqi Decoction (加味补中益气汤)[8], Qianlongtong Capsules (前癃通胶囊)[9],Yishen Tonglin Decoction (益肾通淋汤)[10], Huangqi Chunze Decoction (黄芪春泽汤)[11]which consider HQ as a “sovereign” medicine showed a good curative effect in BPH treatment. In addition, XU et al.[12]confirmed that HQ alone could reverse prostate tissue hyperplasia in a BPH rat model. Pharmacological studies have shown that HQ could relieve tension in the internal urethral sphincter, improve bladder smooth muscle contractility, and reverse prostatic epithelial and stromal cell hyperplasia[13]. LU et al.[14]observed that JYZ water extract could inhibit the spasmodic contraction of rat bladder smooth muscle,reduce micturition frequency, prolong micturition interval, and increase micturition volume. In this study, we used a network pharmacology approach to predict the active components and potential targets of HQ and JYZ in treating BPH, and used a BPH rat model for verification.
2 Materials and methods
2.1 Network pharmacology analysis
2.1.1 Collection of active components for HQ and JYZAll information on chemical components related to HQ and JYZ was obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP), Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM), and literature. The active components were screened based on the following criteria: oral bioavailability(OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 in TCMSP,and cutoff score > 20 in BATMAN-TCM. All software and databases used are listed in Table 1.
Table 1 Databases and related analysis platforms used in this study
2.1.2 Prediction of potential targets of HQ and JYZ
The above databases were used as platforms to screen the potential targets of the active components of HQ and JYZ. Perl software was used to standardize the related compounds and potential targets, and an Excel spreadsheet was used to remove duplicates.
2.1.3 Screening of targets related to benign prostatic hyperplasia“Benign prostatic hyperplasia” was used as a keyword to search the disease-related targets in human gene databases (GeneGards and DisGeNET). The targets retrieved from the two databases were then combined using Excel software,resulting in the construction of a BPH target gene database excluding duplicate targets.
2.1.4 Construction of HQ-JYZ-BPH target gene databaseUsing the Perl language, the data attributes were identified, and the intersection target genes were obtained. Subsequently, the “drug-disease” intersection target gene database was obtained. The“drug-composition-target gene network map” was constructed using Cytoscape 3.7.2 software. The network topology was analyzed using the “network analyzer” function in Cytoscape.
2.1.5 Construction of protein-protein interaction(PPI) map and screening of key genesThe intersection target genes of HQ, JYZ, and BPH were inputted into the STRING database (http://stringdb.org/). The species was set as “Homo sapiens”, and the lowest interaction threshold was set as the highest confidence, “highest confidence (0.900)”. The PPI network information was obtained by hiding the unrelated target genes in the PPI network, and Cytoscape was used to visualize the PPI network. The degree algorithm in the Cytohubba plug-in was used to find the core targets. In these networks, nodes represent compounds and targets, and the edges represent interactions between two nodes. The higher the degree value, the more important the node is in the network.
2.1.6 Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysisHQ, JYZ, and BPH genes were imported into R software, and GO and KEGG pathway enrichment analyses were performed (screening conditions:P-value cutoff < 0.05,Q-value cutoff < 0.05). GO analysis showed the top 20 annotations for biological processes (BP), cellular components (CC), and molecular function (MF). KEGG analysis revealed the top 20 significantly enriched pathways. The networks were then mapped.
2.2 Animal experiment
2.2.1 Efficacy for BPH by HQ and JYZ extract
Sixty SPF grade SD male rats (8 weeks old, 220 - 270 g;Certificate No. 4300470036219) were purchased from Hunan Silaike Jingda Experimental Animal Co., Ltd.,(Changsha, China) with a unit license of SCXK(Xiang) 2016-0002. All rats were raised in an SPF laboratory at the First Hospital of Hunan University of Chinese Medicine, with a unit license number of SCXK (Xiang) 2015-2003. Animals had free access to feed and water. The protocol used in this study was approved by the Research Ethics Committee of Hunan University of Chinese Medicine (Ethical approval number: JN.NO20170730S1001130[241]).
The rats were randomly divided into six groups:blank, sham, model, and high-, medium-, and lowdose treatment groups (10 in each group). The blank group did not receive any treatment. In the sham group, the scrotum was cut open, leaving the testicles intact. Rats were reproduced as previously described[15]in the model and treatment groups. After bilateral orchiectomy, testosterone propionate solution (4 mg/kg) was subcutaneously injected into the back for three weeks. At the end of the administration, the prostate tissues from two randomly selected rats in each group were used for pathological confirmation of model success.
To obtain HQ and JYZ extract, the crude powder of HQ and JYZ (100 g passed through a No. 3 sieve)was mixed with 75% ethanol. The mixture was heated and subjected to reflux extraction twice, adding 1 000 mL solvent each time after 2 h. The solution was then filtered, combined and concentrated under rotate reduced pressure to remove ethanol, added water to volume to 100 mL, shaken well.
The BPH model rats were administered the treatments by gavage continuously for eight weeks. The rats in the blank, sham, and model groups were administered normal saline (1 mL/100 g perday), whereas rats in the treatment groups were administered HQ and JYZ extract at low-dose 1.285 g/(kg·d), medium-dose 2.570 g/(kg·d), and high-dose 5.140 g/(kg·d).
Twenty-four hours after the last administration,the prostate tissues were removed from the rats. The wet weight of the prostate was weighed using an electronic balance, and the volume of the prostate was measured by the drainage method in a 10 mL measuring cylinder. The prostate index was determined using the following equation:
Prostate index = wet weight of prostate (mg) /body weight of rats (g).
Prostate tissue was fixed in 4% paraformaldehyde solution, embedded in paraffin, refrigerated at 4 °C,and sectioned.
Part of the prostate tissue was stained with hematoxylin-eosin (HE) and observed under a light microscope; the other part was stained by immunohistochemistry. After dewaxing, hydration, microwave antigen repair, and elimination of endogenous peroxidase using 3% H2O2, the sample was blocked using 10% sheep serum (Batch No. 16070096; Gibco,Waltham, CA, USA), after which an anti-CD31 antibody was added (Batch No. ab27364; Abcam, Cambridge, UK), and incubation was conducted overnight at 4 °C, then put in EnVision Detection Kit(Dako, Batch No. GK500705) incubating at room temperature for 1 h, DAB (Shanghai Hengfei Bio. Co.,Ltd., Batch No. DA1010) incubated, hematoxylin restained, dehydrated, and sealed. The positive standard for CD31 was a single endothelial cell or cell cluster stained brownish-yellow in the section indicating blood vessel count. According to the Weidner counting method, three areas with the densest staining of blood vessels (hot spots) were determined under a low-power light microscope. The microvessel density was then counted under a light microscope at 200 times magnification, and the mean value of three fields was taken as the microvessel density (MVD).
2.2.2 Active component targets verificationAnother 40 SD male rats (8 weeks old, 220 - 270 g; Certificate No. 4300470036219) were randomly divided into four groups: blank, sham, model, and optimal dose groups. The preparation of HQ and JYZ extracts was the same as before. The rats in the optimal dose group were administered an optimal dose of HQ and JYZ extract for eight weeks (the optimal dose was confirmed in the previously described efficacy experiment).
The prostate tissues were extracted and fixed in 4% paraformaldehyde solution, embedded in paraffin, refrigerated at 4 °C, and sectioned. After xylene dewaxing, gradient alcohol dehydration, antigen repair, and elimination of endogenous peroxidase using 3% H2O2, the sample was blocked using 10% sheep serum (Batch No. 16050096, Gibco). Primary (PBS)and secondary antibodies (VEGF, Batch No. ab1017,Abcam) and Akt1 antibody (Batch No. ab6705, Abcam,) were added and incubated at 4 °C overnight,after which DAB (Shanghai Hengfei Bio. Co., Ltd.,Batch No. DA1011) incubation, hematoxylin re-staining, and microscopic observation were conducted.The double-blind method was used to read the film;cells with an apparent brownish yellow color were positive. Ten visual fields were randomly selected,and the sum score of the staining degree and staining density were calculated. Staining degree evaluation was as follows: no staining (0 points), light yellow (1 point), yellow (2 points), and brownish yellow(3 points), and staining density was evaluated as follows: ≤5.0% (0 points), 6.0% - 10.0% (1 point), 11.0% -20.0% (2 points), 21.0% - 50.0% (3 points), and > 50.0%(4 points)[16]. If the total score was less than or equal to 5 points, the expression was negative, and if the total score was more than or equal to 6 points, the expression was positive. The positive rate was calculated.
2.3 Statistical analysis
Statistical analysis were performed using SPSS 22.0(SPSS Inc., Chicago, IL, USA). The measurement data were expressed as the mean ± standard deviation(SD). One-way ANOVA was used to compare the group differences. Statistical significance was set atP<0.05.
3 Results
3.1 Active components of HQ and JYZ and related targets
Thirty-three active components from HQ and two active components from JYZ were identified. After removing duplicates, 33 active compounds remained. The specific results are listed in Table 2. A total of 527 target genes from HQ and 382 from JYZ were predicted. Some active components act on the same target gene. After removing the replicated target genes, 772 target genes were obtained.
3.2 Common target genes of drug and BPH
BPH target genes were searched in the DisGeNET and GeneGards databases. The correlation between target genes and BPH was calculated using the Gifts algorithm. Ninety-nine target genes in GeneGards and 770 genes in DisGeNET were filtered. After removing duplicates, we obtained 817 BPH target genes. The intersection of drug target genes (772) and BPH target genes (817) was obtained using Perl software. Finally, 112 drug-BPH common target genes were identified.
3.3 Network construction of drug and BPH targets
The drug and BPH target network construction results are shown in Figure 1A. The triangles represent the 33 active components of HQ and JYZ,and the prisms represent the 112 drug-BPH common target genes. The network topology was analyzed using the “network analyzer” function in Cytoscape.The degree analysis indicated that quercetin and kaempferol were the two most active components.
3.4 PPI network construction and key gene screening results
In the PPI network shown in Figure 1B, the ellipse nodes represent single gene proteins, and the lines between nodes represent the interaction between two gene proteins. The size of the graph changed from small to large, and the color gradient of the nodes changed from yellow to red, indicating the change in degree value from small to large. AKT1,JUN, MAPK1, IL-6, TNF, ESR1, and VEGFA were the top-ranked gene proteins making up the key target genes for HQ and JYZ in the treatment of BPH (Figure 1B). These target genes were primarily associated with apoptosis, angiogenesis, and inflammation. We then selected AKT1 (apoptosis and inflammation)and VEGF (angiogenesis) as the target genes for verification.
3.5 GO enrichment analysis
GO enrichment analysis revealed 2 074 BP, 49 CC,and 106 MF. The enriched BP included response to steroid hormones, cellular response to drugs,reactive oxygen species metabolism, reactive oxygen species response, cellular response to oxidative stress, and response to nutritional level (Figure 2A).CC enriched were nuclear transcription factor complex, nuclear chromatin, RNA polymerase II transcription factor complex, vesicular lumen, transcription factor complex, membrane rafts, caveolae,among others (Figure 2B). For MF, enrichment was observed in nuclear receptor activity, steroid hormone receptor activity, steroid binding, transcription cofactor binding, RNA polymerase II transcription factor binding, adrenergic receptor activity, and G protein-coupled amine receptor activity (Figure 2C).Based on the number and significance of gene enrichment, the top 20 GO biological processes were selected, and histograms were produced.
3.6 KEGG pathway enrichment analysis
KEGG pathway enrichment analysis of the 122 common target genes was conducted using R. A total of 135 enriched pathways were identified. The most significantly enriched pathways for the effects of HQ and JYZ on BPH were the PI3K/AKT, IL-17, TNF, p53,MAPK, VEGF, JAK-STAT, and NF-κB signaling pathways (Figure 2D).
3.7 Rat model performance
In the model and treatment groups (Figure 3), the size of the prostate gland cavity differed, gland size was irregular, some glands were papillary and protruded into the lumen, acinar epithelial cells were highly columnar, hyperplasia was present in the interstitial tissue, and a large number of inflammatory cell infiltrations were observed. Thiswas consistent with the pathomorphological characteristics of BPH, indicating that the BPH model was successfully replicated.
Table 2 Active components in HQ and JYZ
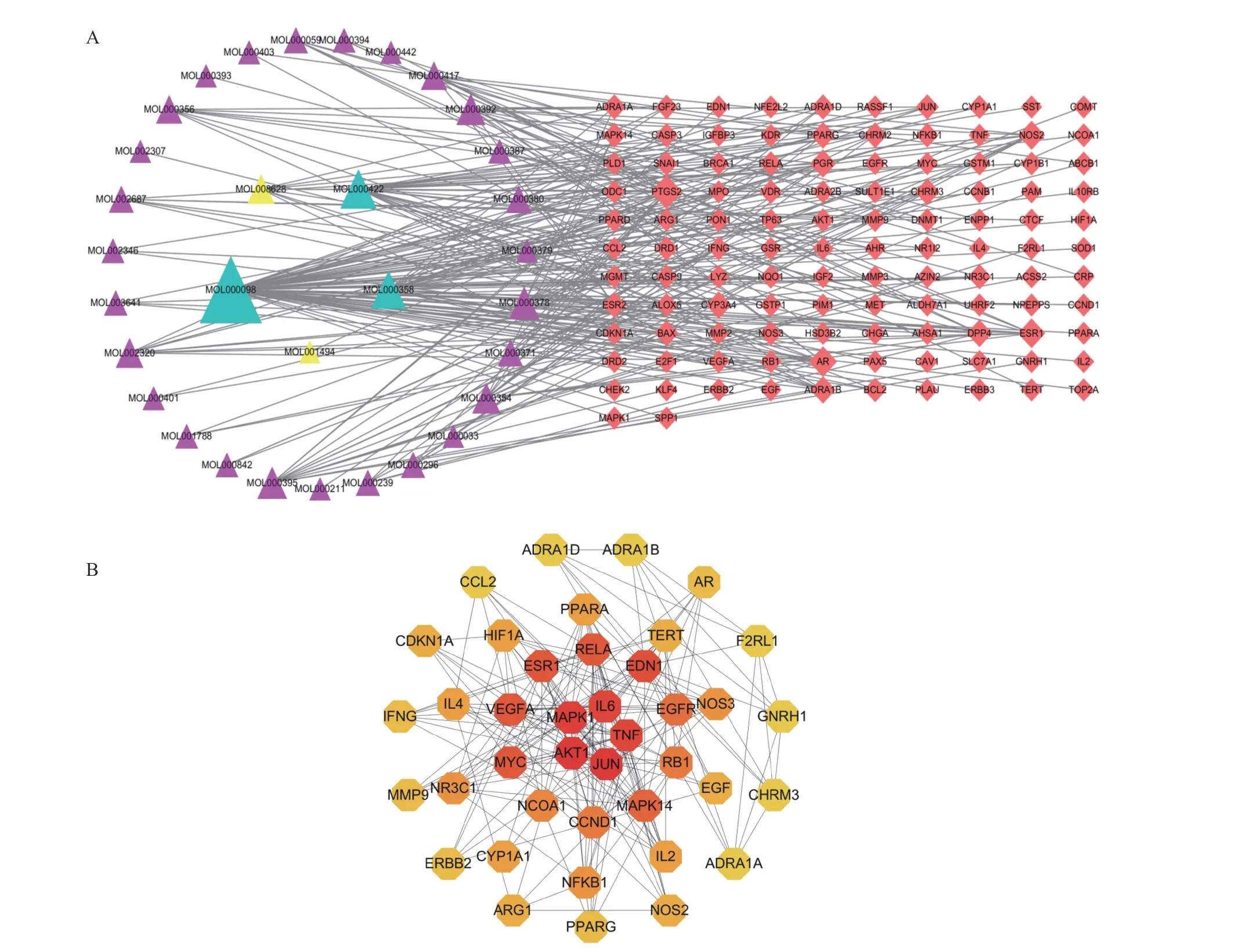
Figure 1 Network of drug-BPH target genes and key target genes A, network of drug-BPH target genes. The triangles represent the active components, and the prisms represent the genes.Yellow represents JYZ, purple represents HQ, and green represents common ownership of the two drugs. B, network of drug-BPH target genes and key target genes. The darker the color, the greater the degree value. Red represents the key genes.
3.8 Comparison of prostate volume, wet weight, and prostate index
Figure 4 shows that compared with the blank and sham groups, the prostate volume, wet weight, and prostate index in the treatment groups were significantly increased (P< 0.05). The prostate volume, wet weight, and prostate index in the high-,medium-, and low-dose groups were significantly lower than those in the model group (P< 0.05), while no statistical significance was found between the high- and medium-dose groups. The prostate volume, wet weight, and prostate index in both the high- and medium-dose groups were significantly higher than those in the low-dose group. We selected the medium dose (2.57 g/kg) as the optimal dose for the next step.
3.9 Comparison of MVD in prostate tissue
Figure 5 demonstrates that MVD did not differ significantly between the sham and blank groups,whereas significant differences were observed in the other groups (P< 0.05) relative to the sham and blank groups. Compared with the model group, the high-,medium-, and low-dose groups had significantly lower MVD (P< 0.05), while no statistical significance was found between the high- and medium-dose groups.
3.10 Comparison of histopathology in prostate tissue
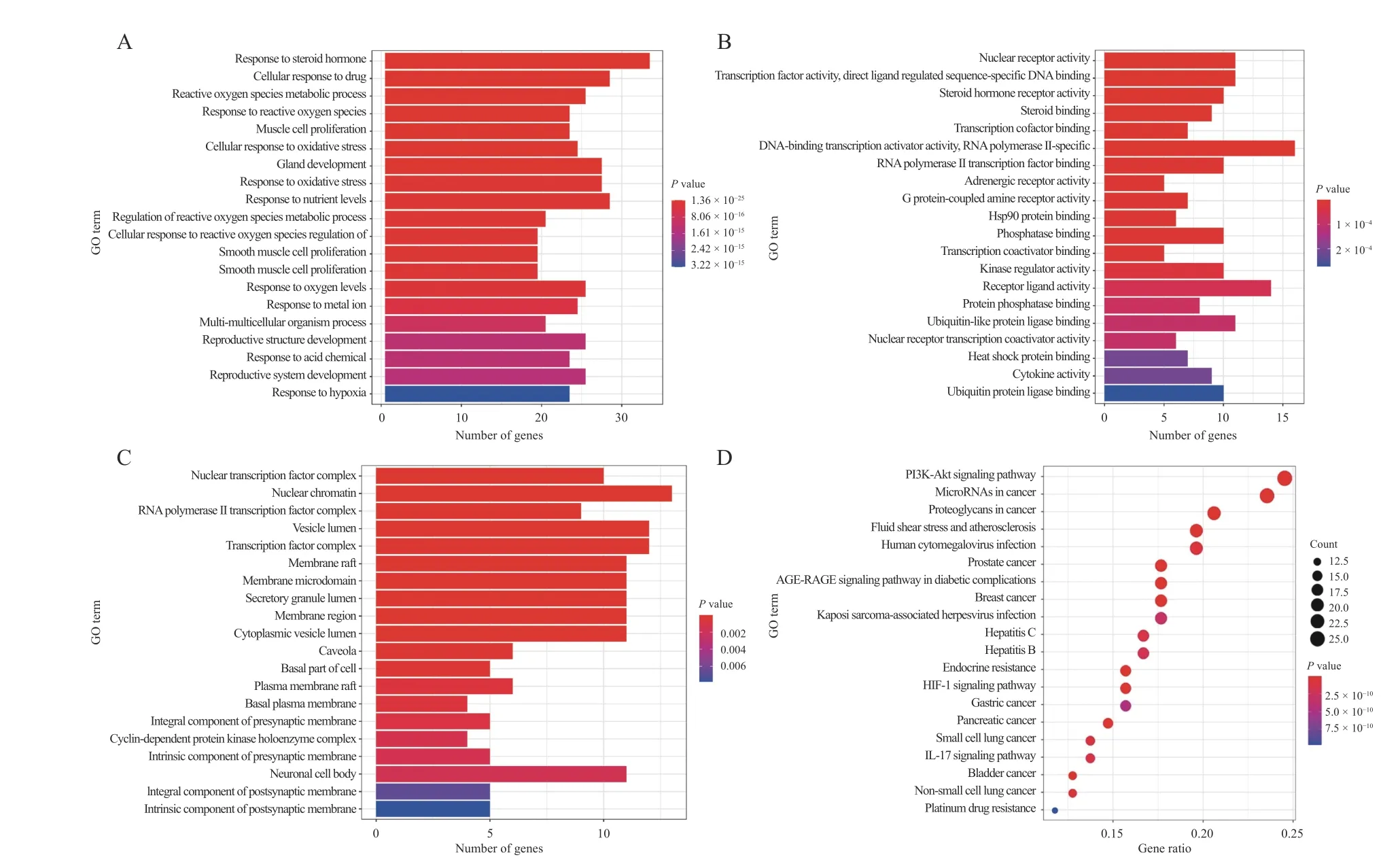
Figure 2 GO and KEGG pathway enrichment analysis of the effects HQ and JYZ on BPH A, biological processes. B, cellular components. C, molecular functions. D, main pathways of the effects HQ and JYZ on BPH.
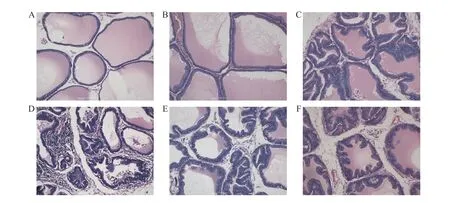
Figure 3 Comparison of histopathology in prostate tissue (HE staining, 200 × )A, blank group. B, sham group. C, model group. D, high-dose group. E, medium-dose group. F, low-dose group.
The histopathological findings for prostate tissue are shown in Figure 3. In the blank (Figure 3A) and the sham groups (Figure 3B), the prostate gland cavity was remarkably regular and of similar size, the gland was organized evenly and clearly, the acinar epithelial cells were monolayer columnar, and there was no hyperplasia and inflammatory cell infiltration in the interstitial tissue. In the model group (Figure 3C), the size of the prostate gland cavity was larger, the gland was irregularly shaped, some glands were papillary and protruded into the lumen, the acinar epithelial cells were highly columnar, hyperplasia was apparent in the interstitial tissue, and a high inflammatory cell infiltration was seen. In the high-(Figure 3D), medium- (Figure 3E), and low-dose(Figure 3F) groups, the size of the prostate gland cavity was relatively regular, the arrangement of glands was still clear, most acinar epithelial cells were single columnar, and a few were highly columnar, low hyperplasia and inflammatory cell infiltration could be seen in the interstitial tissue.
3.11 Target verification
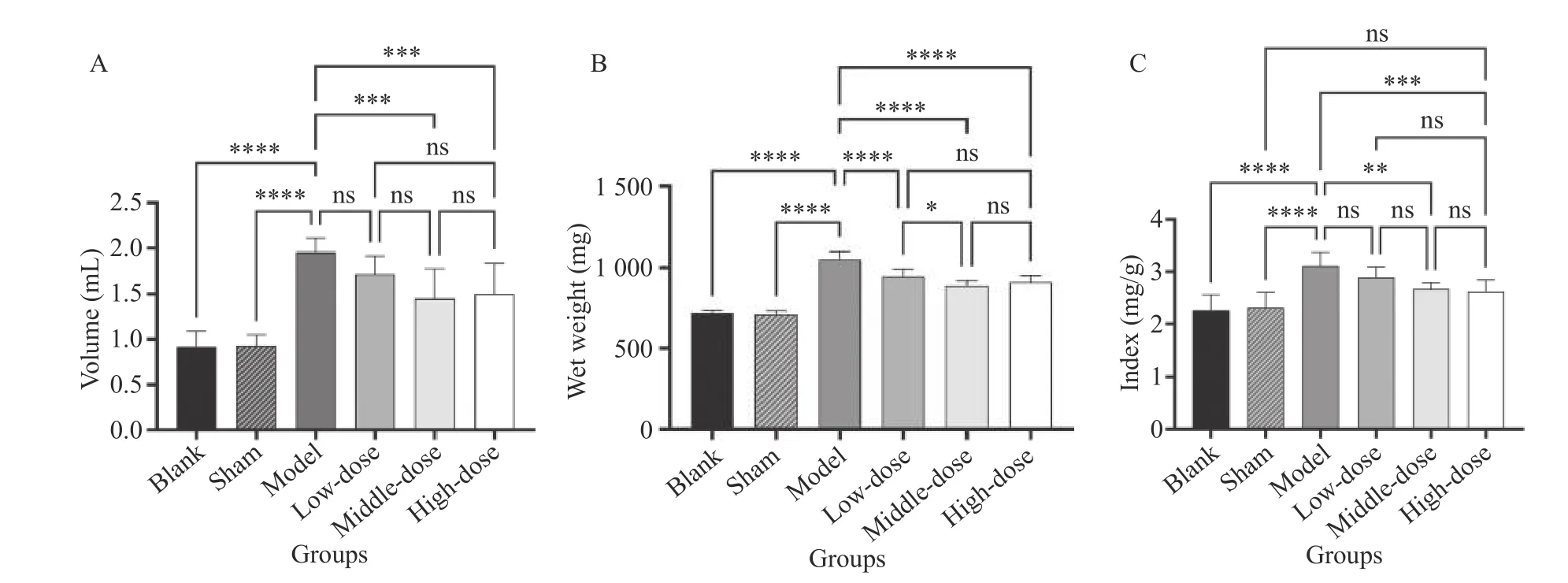
Figure 4 Comparison of prostate volume, wet weight, and prostate index A, comparison of prostate volume. B, comparison of wet weight. C, comparison of prostate index. *P < 0.05, **P < 0.01,***P < 0.0001, ****P < 0.00001, ns represents no significance.
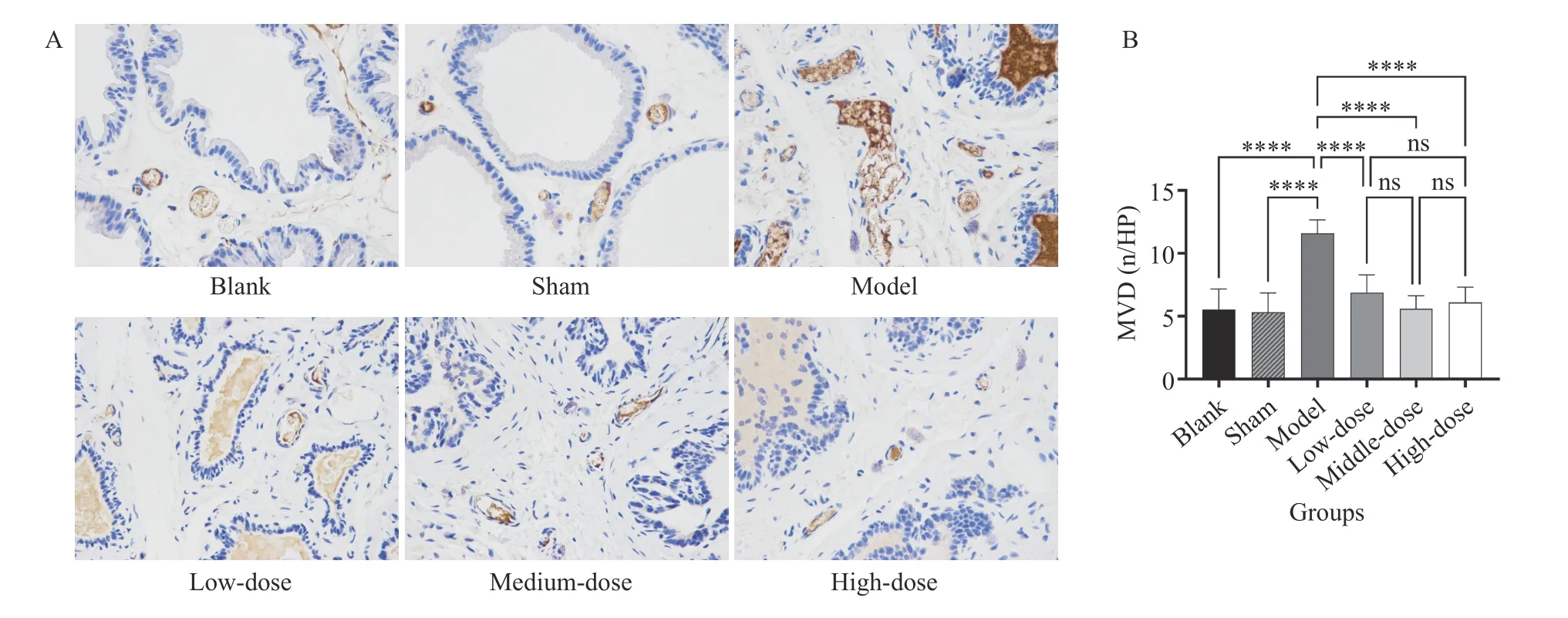
Figure 5 Comparison of MVD in prostate tissue (400 × )A, comparison of histopathology results. B, statistical analysis. ****P < 0.00001, ns represents no significance.
The predicted targets were mostly related to apoptosis and angiogenesis, and AKT1 (apoptosis)was the most significant protein according to the PPI and KEGG results. We selected AKT1 and VEGF (the most established factor for angiogenesis) as targets for verification.3.11.1 Comparison of AKT1 expression in prostate tissueFigure 6 shows that compared with the blank and the sham groups, AKT1 expression in the model and optimal dose groups was significantly increased(P< 0.05). The optimal group had significantly lower AKT1 expression than the model group (P< 0.05).Therefore, the HQ and JYZ extract significantly inhibited AKT1 expression in the prostate tissue of rats.3.11.2 Comparison of VEGF expression in prostate tissueIt can be seen from Figure 7 that compared with the blank and sham groups, VEGF expression in the model and optimal dose groups was significantly increased (P< 0.05). The optimal dose group had significantly lower VEGF expression than the model group (P< 0.05). Therefore, the HQ and JYZ extract significantly inhibited VEGF expression in the prostate tissue of rats.
4 Discussion
According to the clinical s ymptoms of BPH, it belongs to the “Longbi” and “Jinglong” categories in TCM.The primary pathogenesis of BPH is a deficiency in origin and an excess in superficiality. A deficiency in origin primarily means deficiency of Qi and Yang in the spleen and kidney, while excess in superficiality refers to turbid phlegm, congestion, and damp heat.From clinical experience, our team recognized the importance of kidney deficiency pathogenesis, and formulated Yishen Tonglong Capsules, with HQ as the “sovereign” medicine, which is beneficial for vital energy, nourishes the middle earth, and assists the triple energizers to gasify. Within this formulation,JYZ is the “minister” medicine, which can astringe the empty Qi to enhance the efficacy of the“sovereign” medicine. Combining the two medicines tonifies the kidneys and Qi, strengthens the essence,and reduces urine. Accordingly, in the present study,we sought to clarify the mechanism of action of HQ and JYZ using a network pharmacology approach.
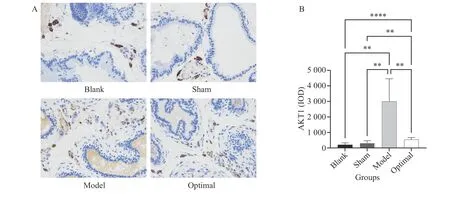
Figure 6 Comparison of AKT1 expression in prostate tissue (400 × )A, comparison of histopathology results. B, statistical analysis. **P < 0.01, ****P < 0.00001, ns represents no significance.
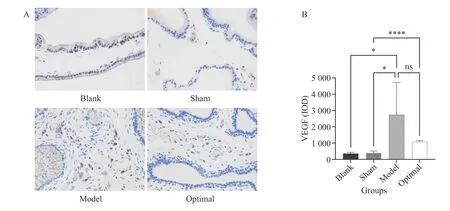
Figure 7 Comparison of VEGF expression in prostate tissue (400 × )A, comparison of histopathology results. B, statistical analysis. *P < 0.05, ****P < 0.00001, ns represents no significance.
Our results showed that 33 active components and 772 target genes were identified from HQ and JYZ, along with 817 BPH target genes and 112 drug-BPH common target genes. Based on the degree value, quercetin and kaempferol were the key active HQ and JYZ components in BPH treatment. Quercetin is a natural flavonoid with anti-inflammatory and antioxidant functions[17,18]. Related researches showed that inflammation and oxidative stress play essential roles in the pathogenesis and development of BPH[19,20]. On this basis, we speculated that quercetin might play an anti-inflammatory role in BPH treatment. Quercetin can inhibit prostatitis by improving antioxidant capacity and suppressing the phosphorylation of NF-κB and MAPKs, thus ameliorating bladder irritation and other symptoms[21].Furthermore, quercetin can improve prostate pathology and pain sensitivity in rats with type III chronic prostatitis/pelvic pain syndrome, and its mechanism is related to a reduction in the expression of various inflammatory factors[22]. Kaempferol is similar to quercetin in efficacy and also has anti-tumor effects,inhibiting the growth of colon cancer[23], breast cancer[24], and prostate cancer[25]. Currently, it is used as a cancer treatment method[26,27]. For example, kaempferol can significantly inhibit IL-6 and TNF-αlevels in the serum of mice with prostate cancer and effectively inhibit prostate cancer growth by interfering with the MAPK/ERK signaling pathway[28].
In recent years, studies have shown that the occurrence and development of BPH are closely related to angiogenesis, apoptosis, and inflammation.MOSTAFA et al.[29]indicated that the passive expansion of peripheral blood vessels enables tissues to obtain enough oxygen and nutrients, promotes prostate cell proliferation, and induces BPH. LLOYD et al.[30]reported that inflammation is a common finding in tissues associated with BPH. We identified 10 key target genes by constructing a PPI network, including the top-ranked AKT1, JUN, MAPK1, IL-6,TNF, ESR1, and VEGFA genes. These target genes are primarily related to apoptosis, angiogenesis, and inflammation. KEGG pathway enrichment analysis revealed 135 signaling pathways involved in the treatment of BPH with HQ and JYZ, including the significantly enriched PI3K/AKT, IL-17, TNF, p53, MAPK,VEGF, JAK-STAT, and NF-κB signaling pathways. The PI3K/AKT signaling pathway is related to cell proliferation, differentiation, apoptosis, and autophagy.AKT is a serine/threonine-protein kinase that plays a crucial role in a series of cellular functions, including cell growth, proliferation, migration, protein synthesis, inflammation, and angiogenesis. The downstream targets of AKT are numerous due to multiple interactions with other substrates[31]. Among the AKT isoforms, AKT1 is involved in cell growth and survival and is expressed in many tissues[32]. Membrane phosphatidylinositol-(3,4,5)-P3 (a substrate of PI3K),upstream of AKT1, activates AKT1 by stimulating Ca2+.After Ca2+/CaMKII activation, AKT1 directly phosphorylates its downstream protein, mTOR. Finally, it inhibits autophagy and regulates cell survival and apoptosis[33]. Both AKT1 and mTOR can promote the activation of the inhibitorκB (IκB) kinase complex(IKK) to allow the transduction of NF-κB. NF-κB is considered the central mediator of the inflammatory process and innate immunity and is involved in the inflammatory response and tumor formation[34]. In addition, AKT1 can phosphorylate FoxO, promote FoxO nucleation, inhibit FoxO transcription, and induce apoptosis through exogenous and endogenous mitochondrial-dependent pathways. The exogenous apoptotic pathway refers to Fas-L → Caspase-8 →Caspase-6/Caspase-7 → Caspase-3/PARP-1, while the endogenous mitochondrial-dependent pathway refers to Caspase-8 → Bax/Bak → Cytochrome C/AIF →Smac/DIABLO → Caspase-9 → Caspase-3, which eventually leads to apoptosis. VEGF is a highly specific bladder cell mitogenic factor closely related to the formation and maintenance of prostate neovascularization[35,36]. It is also downstream of AKT. Activated AKT phosphorylates eNOS to produce NO, promoting VEGF-induced endothelial cell migration, vasodilation, blood flow increase, and angiogenesis(Figure 8)[37]. KULKARNI et al.[38]revealed that the PI3K/AKT signaling pathway is one of the main molecular pathways involved in transforming normal prostate epithelial cells into the malignant form.Meanwhile, studies have shown a significant difference in the expression of VEGF in BPH and PCa[39,40].VEGF can induce proliferation and migration of vascular endothelial cells. It is an essential growth factor in the physiological and pathological processes of angiogenesis[41]. The present study predicts that the active components of HQ and JYZ might play a role in treating BPH by regulating the above-mentioned target genes and signaling pathways, providing direction for further research.
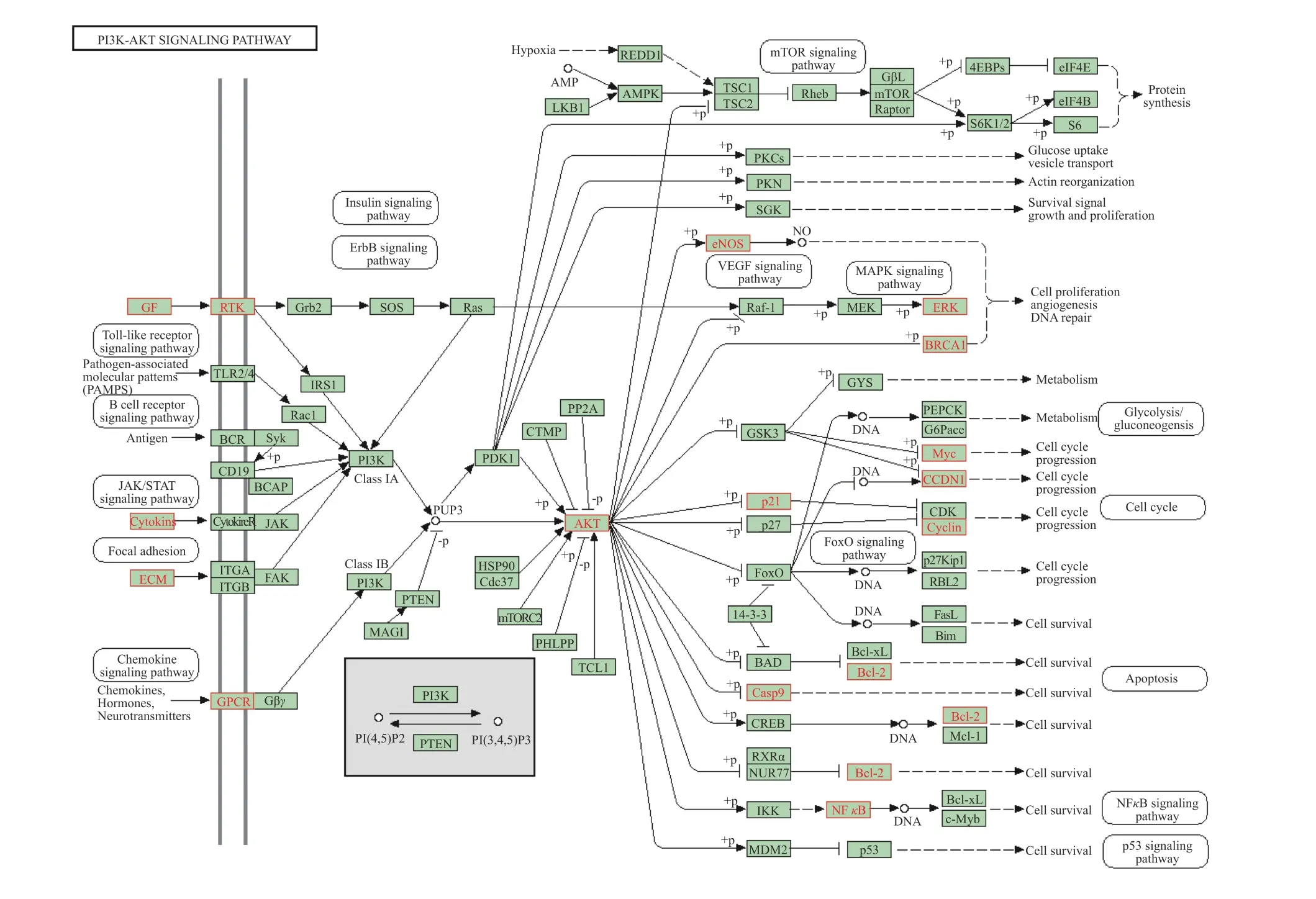
Figure 8 PI3K/AKT signaling pathway
In order to verify the above prediction results, we set up a BPH rat model and evaluated AKT1 and VEGF expression in the prostate tissue of rats to confirm the role of HQ and JYZ in BPH treatment by intervening in cell proliferation, differentiation, inflammation, and angiogenesis pathways. The results showed that the HQ and JYZ extract could downregulate the expression of AKT1 and VEGF, promote apoptosis of epithelial cells, and inhibit angiogenesis and inflammation, verifying the network pharmacology results.
In conclusion, the primary active components of HQ and JYZ are quercetin and kaempferol. The therapeutic effect of HQ and JYZ on BPH might be through the regulation of cell inflammation, angiogenesis, apoptosis, and other signaling pathways,acting on AKT1, VEGF, and other key targets.However, this study only considered two major medicines in the Yishen Tonglong Capsule. In the future,the network pharmacology approach should be used to predict the targets of the other Yishen Tonglong Capsule constituents to comprehensively clarify its mechanism of action. Furthermore, experimental verification should be conducted throughin vivoandin vitrotrials and metabolomics to provide practical guidance for the diagnosis and treatment of BPH.
Acknowledgements
We thank for the funding support from the Hunan Provincial Science and Technology Department(No. 2020JJ4068 and No. 2018SK4012).
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年2期
Digital Chinese Medicine2021年2期
- Digital Chinese Medicine的其它文章
- Comparison of mechanisms and efficacies of five formulas for improving blood circulation and removing blood stasis
- Evaluation of Baishao (Paeoniae Radix Alba) and Chishao(Paeoniae Radix Rubra) from different origins based on characteristic spectra of amino acids
- Quality 4.0 technologies to enhance traditional Chinese medicine for overcoming healthcare challenges during COVID-19
- Novel pyrimidine-benzimidazole hybrids with antibacterial and antifungal properties and potential inhibition of SARS-CoV-2 main protease and spike glycoprotein
- Research on classification diagnosis model of psoriasis based on deep residual network
- Analysis of the hotspots and trends in traditional Chinese medicine immunomodulation research based on bibliometrics
