Comparison of mechanisms and efficacies of five formulas for improving blood circulation and removing blood stasis
LI Jinxia, ZHOU Xiaoqing*, ZHENG Caixing, LAI Lina, LI Ling
a. School of Traditional Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan 410208, China
b. Hunan Provincial Key Laboratory for TCM Diagnostics, Changsha, Hunan 410208, China
c. School of Humanities and Management, Hunan University of Chinese Medicine, Changsha, Hunan 410208, China
Keywords Stasis syndrome Promoting circulation and removing stasis formula Network pharmacology Danshen Yin Huoluo Xiaoling Dan Shixiao San Taohong Siwu Tang Xuefu Zhuyu Tang
ABSTRACT
1 Introduction
Blood stasis, a major syndrome in cardiovascular diseases such as atherosclerosis (AS) and coronary heart disease, is the obstruction and abnormal circulation of blood, resulting in the accumulation of blood in vessels or organs. The syndrome of blood stasis manifests as body pain, lump, bleeding, purple tongue, and acerbity pulse. The blood rheology,coagulation, fibrinolysis, and microcirculation of patient with blood stasis syndrome may be abnormal[1].Over the years, blood stasis has been effectively treated with traditional Chinese medicine (TCM).The study of blood stasis syndrome is very prominent in TCM academic research, and has attracted attention both locally and internationally[2]. TCMs that promote blood circulation and remove blood stasis have been manufactured and patented. The dosage forms vary from injection to capsule and they have been widely used in cardiology and emergency care departments. After thousands of years of knowledge accumulation, TCM has led to the development of different formulas for promoting blood circulation and removing blood stasis.However, some questions regarding their efficacies and mechanisms remain unanswered. What are the differences and similarities of so many promoting blood circulation and removing blood stasis formulas? How can targeted selection of TCMs for blood stasis removal be done? These questions remain unclear and require further exploration and research.
In our preliminary work, the five most commonly used formulas for promoting circulation and removing stasis were screened by pharmacology experts,namely Danshen Yin (丹参饮, DSY), Huoluo Xiaoling Dan (活络效灵丹, HLXLD), Shixiao San (失笑散,SXS), Taohong Siwu Tang (桃红四物汤, THSWT), and Xuefu Zhuyu Tang (血府逐瘀汤, XFZYT). These five formulations simply focus on promoting blood circulation and removing blood stasis, which have horizontal comparability for the treatment of blood stasis syndrome. TCMs for promoting blood flow and removing blood stasis have varying mechanisms and efficacies. DSY focuses on gently reconciling with blood; HLXLD disperses stasis and swelling; SXS removes stasis and relieves pain; THSWT breaks blood stasis; and XFZYT breaks stasis and promotes the circulation of Qi. As these five formulations have differences in efficacy and mechanism in the treatment of blood stasis syndrome, it is necessary to conduct comparative studies to explain the connotations of different methods of promoting blood stasis. Generally, TCM exerts therapeutic effects on multiple targets and pathways of the human body through their complex active components, which cannot be accurately determined solely by conventional methods[3,4].Therefore, we employed a comprehensive strategy that includes chemoinformatics, bioinformatics, and network biology methods to unravel therapeutic targets and mechanisms of five formulas for promoting circulation and removing stasis. A rabbit model of blood stasis syndrome was also established. After oral administration of the five formulas, their effects on blood lipid and rheology were explored by comparing their mechanisms and curative effects. This study will improve the accuracy of clinical syndrome differentiation and pertinence of treatment.
2 Materials and methods
2.1 Chemical composition and candidate compounds screening
The composition of the five formulas that promote circulation and remove stasis is shown in Table 1.The chemical compounds of the five formulas were obtained from the Traditonal Chinese Medicine System Pharmacology Database (TCMSP, http://lsp.nwu.edu.cn/tcmsp.php, version 2.3)[5], the Shanghai Institute of Organic Chemistry of CAS, Chemistry Database (http://www.organchem.csdb.cn), and the TCM Integrated Database (http://www.megabionet.org/tcmid/). According to the calculations by LIU et al.[6], we estimated oral bioavailability (OB), which indicates the percentage of an orally administered dose of unchanged drug that reaches the systemic circulation at prescreening , and drug-likeness (DL),which evaluates the structural similarity between compounds and the clinical drugs in the DrugBank database. OB > 30% and DL > 0.18 were used to select candidate compounds[4].

Table 1 Composition of the five formulas
2.2 Construction of drug-target network and diseasetarget network
According to the systematic drug targeting approach developed by YU et al.[7], to explore the therapeutic targets of the predicted active compounds of the five formulas, the canonical simplified molecular input line entry specification (SMEILES) codes of the chemicals were imputed in the TCMSP database, the Swiss Target Prediction database (http://www.swisstargetprediction.ch/)[8]and the STITCH database(http://stitch.embl.de/)[9]. The UniProt website(http://www.uniprot.org/) was used to convert the target protein names into official gene symbols. By searching with the keywords “blood stasis” “stagnation of blood” “stasis syndrome”, known blood stasis related targets were obtained from the PharmGKB database (http://www.pharmgkb.org/)[10], the Online Mendelian Inheritance in Man (OMIM, http://omim.org/) database, the Genetic Association Database(GAD, http://geneticassociationdb.nih.gov/), and the Therapeutic Target Database (TTD, http://bidd.nus.edu.sg/BIDD-Databases/TTD/TTD.asp). The targets of stasis syndrome in atherosclerosis were also collected from relevant literature. After collecting and sorting the data, the five drug-target networks and the disease-target network were constructed and visualized with Cytoscape (Version 3.2.1).
2.3 Identifying stasis-related target in the five formulas
Using the Cytoscape plugin Bisogenet, proteinprotein interaction (PPI) data of both the five drugtarget networks and the disease-target network were obtained in the InAct Database, Human Protein Reference Database, Molecular Interaction Database,Database of Interacting Proteins, Biological General Repository for Interaction Database, and Biomolecular Interaction Network. Thereafter, we merged the five drug-target PPI networks with the disease-target PPI network in Cytoscape to construct five interaction networks of drug-disease-target networks. The topological properties of each node in the five interaction networks were assessed by calculating six measures with a Cytoscape plugin CytoNCA: degree centrality (DC), betweenness centrality (BC), closeness centrality (CC), eigenvector centrality (EC), network centrality (NC), and local average connectivity (LAC).These parameters represent the topological importance of a node in the network; the larger the quantitative value, the more important the node in the network[11].
2.4 Gene Venn mapping and pathway enrichment analysis
To analyze the target differentiation of the five formulas, target information was imported into the Bioinformatics & Evolutionary Genomics website(http://bioinformatics.psb.ugent.be/webtools/Venn/),the drug-target Venn diagram was mapped. Then, we performed enrichment analysis of the candidate targets of the five formulas using ClueGO, a Cytoscape plugin that visualized non-redundant biological terms for large clusters of genes in a functionally grouped network. The resultant candidates were divided into two categories: biological processes and signaling pathways. The ClueGO network was created with Kappa statistics and reflected the relationship between the terms based on the similarity in their associated genes.
2.5 Preparation of the decoctions of the five formulas
The composition of the five formulas has shown before. All the medicinal plants were purchased from the First Hospital of Hunan University of Chinese Medicine. Plant authenticity was identified twice morphologically and anatomically by a pharmacist.The herbs were soaked in 2 000 mL distilled water for 1 h before heating. All the ingredients were boiled for 0.5 h, after which the plants were filtered out and heated in 500 mL water for a second extraction under the same conditions. The filtered suspensions were mixed and concentrated to a density of 1 g/mL. The aqueous formula was stored at 4 °C and heated to 37 °C in a water bath before use.
2.6 Animal experiment
Seventy male New Zealand rabbits (conventional grade) weighing 2.0 - 2.5 kg were provided by the Hunan Taiping Laboratories (Changsha, China). The rabbits were housed in a conventional environment and fed with free water, room temperature (20 ± 2) °C,relative humidity 65% ± 5%, and lighting was turned on every 12 h. All procedures were approved by the Animal Experiments and Experimental Animal Welfare Committee of Hunan University of Chinese Medicine (Registration No. LLBH-202006010001).
According to the stochastic averaging principle,the rabbits were divided into seven groups, namely,control, model, DSY, XFZYT, SXS, HLXLD, and THSWT groups, with 10 rabbits in each group. The stasis syndrome model was established by improving the composite modeling method by YANG et al.[12].Except for the control group, all rabbits were fed a 90 g/d high-fat diet (containing 4.5 g cholesterol) for 60 d, and from the second day, rabbits were injected with epinephrine using a syringe and hypodermic needle, two days at a time, and a total of 15 times.Blood samples were collected after 30 days of modeling, and significantly increased blood lipid and viscosity represented an established stasis model. On the 30th day, rabbits in the DSY, XFZYT, SXS, HLXLD,and THSWT groups were orally administered the relevant decoction [DSY: 3.92 g/(kg·d), XFZYT:7.10 g/(kg·d), SXS: 1.12 g/(kg·d), HLXLD: 5.60 g/(kg·d),THSWT: 4.48 g/(kg·d)] for 30 d. Rabbits in the control group were given the same volume of normal saline instead. Blood samples were collected from the central ear artery and aortic arches were removed and fixed in 10% neutral-buffered formalin.
2.7 Blood sample examination
Blood samples were centrifuged at 1 509.3 × g at 4 °C for 15 min to isolate the serum and plasma. Rabbit whole blood viscosity (WBV, low cut, and high cut)was measured using an NEX-1 vertebral plate viscometer at a shear rate of 5/s and 120/s. The plasma viscosity (PV) was measured using a capillary viscometer in an electronic computer automatic analysis. The hematocrit value (HCT) was measured using Wynn’s method. Total cholesterol (TC),triglyceride (TG), low density lipoprotein (LDL), high density lipoprotein (HDL), apolipoprotein A1(apoA1), and apolipoprotein B100(apoB100) levels were measured by biochemical methods using a Hitachi 7150 Automatic Analyzer.
2.8 Aorta histology observation
Formalin-fixed aorta tissues were embedded in paraffin and stained with hematoxylin-eosin staining.The slices were observed and captured using a Nikon Eclipse 80i microscope at × 200 magnification. Aortic plaque grayscale and plaque area were analyzed using an Optimas microscopic image analysis system.
2.9 Statistical analysis
Statistical analysis was performed using SPSS 19.0.Continuous data are presented as the mean ±standard deviation (SD). One-way ANOVA was performed to test the differences between groups. The least significant difference method was applied when the variances were equal, whereas Tamhane’s T2 test was performed when the variances were not equal.P< 0.05 was considered to be statistically significant.
3 Results
3.1 Candidate compound screening and drug-target network generation
After the search in the various databases, we obtained 437 chemical compounds in DSY, after limiting the OB and DL values, and 76 candidate compounds were screened. Using the same method,118 of 731 chemical compounds were selected for HLXLD, 61 of 766 chemical compounds were selected for THSWT, 187 of 1 555 chemical compounds were selected for XFZYT, and 11 of 70 chemical compounds were selected for SXS. We then explored the therapeutic targets of the predicted active compounds of the five formulas. After integrating the available chemical, genomic, and pharmacological information to predict putative targets, we obtained 1 515, 1 910, 736, 2 968, and 363 targets of DSY,HLXLD, THSWT, XFZYT, and SXS, respectively. The targets in each formula show repetition to a high extent, indicating that these drugs shared targets and show synergistic effects in treating blood stasis. After removing the duplication, there were 116, 147, 137,162, and 133 targets of DSY, HLXLD, THSWT, XFZYT,and SXS, respectively. There were 82 shared targets in the five formulas. Further, to understand the complex interactions between the compounds and their corresponding targets at the system level, we constructed a network based on the candidate compounds and the potential targets of the five formulas (Figure 1).
3.2 Identification of candidate targets for the five formulas

Figure 1 Construction of the drug-target network of the five formulas

Figure 2 Identification of candidate targets of the five formulas A, the five formulas’ drug-target PPI networks. B, stasis-target PPI network. C, drug-stasis-target merged networks. D, significant targets networks. E, candidate targets networks. a represents DSY, b represents HLXLD, c represents SXS, d represents THSWT, e represents XFZYT.

Table 2 Candidate targets of the five formulas
After searching the four existing databases, namely GAD, PharmGKB, OMIM, and TTD, we retrieved 471 stasis-related targets. System biology studies have shown that genes and proteins are interconnected and that the PPI networks are relevant to understanding the role of various proteins in complex diseases such as stasis[13]. Therefore, we constructed a drugtarget PPI network for the five formulas (Figure 2A,DSY: 6 136 nodes and 135 607 edges, HLXLD: 6 551 nodes and 145 451 edges, SXS: 5 328 nodes and 132 081 edges, THSWT: 6 545 nodes and 146 429 edges, XFZYT: 6 775 nodes and 151 764 edges) and a known stasis-target PPI network (Figure 2B, 6 950 nodes and 163 465 edges) using the Bisogenet plugin.Further, to unravel the pharmacological mechanisms of the five formulas, we intersected the five drugtarget PPI networks with the stasis-target PPI network(Figure 2C, DSY: 4 250 nodes and 113 031 edges,HLXLD: 4 498 nodes and 119 496 edges, SXS: 4 198 nodes and 114 238 edges, THSWT: 4 492 nodes and 120 016 edges, XFZYT: 4 637 nodes and 123 610 edges). Based on a previous study[14], we identified nodes with degrees that were more than twice the median degree of all nodes (DSY: 64, HLXLD: 64, SXS:78, THSWT: 64, XFZYT: 64) as significant targets, and constructed a network of significant targets for the five formulas against stasis (Figure 2D, DSY: 1 048 nodes and 46 887 edges, HLXLD: 1 098 nodes and 49 274 edges, SXS: 829 nodes and 37 591 edges,THSWT: 1 101 nodes and 49 432 edges, XFZYT: 1 130 nodes and 50 575 edges). We further evaluated six topological features of the five networks to identify candidate targets, namely DSY: DC > 105, BC >475.80, CC > 0.19, EC > 0.02, LAC > 16.45, NC > 23.49;HLXLD: DC > 106, BC > 415.72, CC > 0.51, EC > 0.02,LAC > 17.74, NC > 19.54; SXS: DC > 75, BC > 362.43,CC > 0.52, EC > 0.02, LAC > 20.13, NC > 22.06;THSWT: DC > 69, BC > 417.52, CC > 0.51, EC > 0.02,LAC > 17.60, NC > 19.17; XFZYT: DC > 70, BC >433.22, CC > 0.51, EC > 0.02, LAC > 17.26, NC > 18.84.Finally, we collected 269, 358, 288, 370, and 376 candidate targets of DSY, HLXLD, SXS, THSWT, and XFZYT against blood stasis, respectively (Figure 2E).
To further understand the differentiation of the candidate targets of the five formulas, we mapped a Venn diagram. The detailed targets and diagram are shown in Table 2 and Figure 3. Five formulas shared 232 candidate targets against blood stasis, indicating that most targets of the five formulas are the same.
3.3 Enrichment analysis of candidate targets of the five formulas
To confirm the candidate targets of the five formulas involved in various biological processes and molecular functions, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis using a Cytoscape plugin, ClueGO. The detailed information is shown in Figure 4 and Table 3.The results indicate that fluid shear stress and atherosclerosis are the common biological processes by which the five formulas prevent stasis, indicating that their blood stasis curing effects depends on adjusting the fluid shear stress. The five formulas are used in the treatment of AS based on this mechanism.With the exception of XFZYT, all the formulas regulated lipolysis in adipocytes. Moreover, except for DSY, all the formulas regulated AGE-RAGE signaling pathway and complement and coagulation cascades, reducing the formation of lipid plaque and blood clots. The results of differentiation showed that HLXLD, SXS, and XFZYT regulated the HIF-1 signaling pathway, providing feedback to hypoxia.DSY regulated the cGMP-PKG signaling pathway,HLXLD reduced platelet activation, SXS regulated the calcium signaling pathway, and XFZYT regulated the PPAR signaling pathway, all of which play important roles in preventing atherosclerosis and thrombosis.
3.4 Effects of the five formulas on body weight
In this study, we conducted an animal experiment to explore the differentiation of the five formulas. As shown in Figure 5, rabbit body weight in the control group increased gradually from day 0 to day 60th. In contrast, body weight in the model group was lower than that of the control group throughout the study.The DSY, XFZYT, SXS, HLXLD, and THSWT values on day 30th and day 60th were higher than those of the model group but lower than those of the control group. However, the differences were not statistically significant (P> 0.05).
3.5 Effects of the five formulas on serum lipid and apolipoprotein

Figure 4 Enrichment analysis of candidate targets of the five formulas A, DSY. B, HLXLD. C, SXS. D, THSWT. E, XFZYT. *P < 0.05, **P < 0.01.

Table 3 Enrichment analysis of candidate targets of the five formulas

Figure 5 Effects of the five formulas on body weight
As shown in Figure 6A - 6D, after 30 days of high-fat feeding + epinephrine injection, the values of TC, TG,HDL, and LDL in the model group were significantly higher than those in the control group. On day 60th,the values continued to increase and the differences were statistically significant (P< 0.01). At day 30th,the values of TC, TG, HDL, and LDL in the DSY, SXS,HLXLD, THSWT, and XFZYT groups showed no differences compared with the values of the model group as the treatments were not initiated. On day 60th, the values of TC, TG, and LDL in each group decreased, among which the ones of XFZYT, HLXLD,and SXS groups were statistically significant (P< 0.05).XFZYT had the lowest value, followed by HLXLD and SXS. The values of HDL in each treatment group showed no differences with the model group. We calculated the TC/HDL and TG/HDL, as shown in Figure 6E and 6F. The results showed that XFZYT,HLXLD, and SXS decreased the HDL values, among which XFZYT had the best effect (P< 0.01).
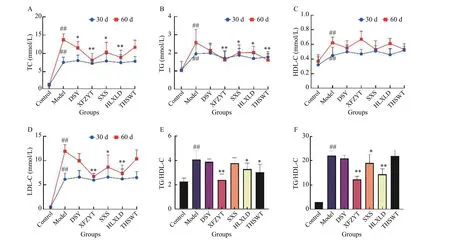
Figure 6 Effects of the five formulas on serum lipid A, TC. B, TG. C, HDL. D, LDL. E, TC/HDL. F, TG/HDL. ##P < 0.01, compared with the control group. *P < 0.05, **P < 0.01, compared with the model group.
As shown in Figure 7, on day 30th and 60th, the values of apoB100in the model group significantly increased and apoA1/apoB100decreased compared with the control group (P< 0.01). On day 60th, in the XFZYT and HLXLD groups, apoB100decreased and apoA1/apoB100increased compared with the model group (P< 0.05).
3.6 Effects of the five formulas on hemorheology
As shown in Figure 8, on day 60th, HCT, PV, high and low shear rate, and WBV all significantly increased in the model group compared with the control group(P< 0.01). XFZYT distinctly decreased the values of HCT, PV, high and low shear rate and WBV versus model group (P< 0.05); HLXLD decreased HCT and low shear rate and WBV (P< 0.05); SXS decreased HCT (P< 0.05); DSY and THSWT decreased high and low shear rate and WBV (P< 0.05). The results suggest that different formulae act on different aspects of hemorheology. XFZYT had the best effect.
3.7 Effects of the five formulas on pathological changes of aorta

Figure 7 Effects of the five formulas on apolipoprotein A, apoA1. B, apoB100. C, apoA1/apoB100. ##P < 0.01, compared with the control group. *P < 0.05, **P < 0.01, compared with the model group.

Figure 8 Effects of the five formulas on hemorheology A, HCT. B, PV. C, WBV high shear rate. D, WBV low shear rate. #P < 0.05, ##P < 0.01, compared with the control group. *P < 0.05,**P < 0.01, compared with the model group.

Figure 9 Effects of the five formulas on pathological changes in aorta A, Control. B, Model. C, XFZYT. D, HLXLD. E, SXS. F, DSY. G, THSWT.
We conducted HE staining of rabbit aorta slices, as shown in Figure 9. The control group showed no abnormal change; in the model group, the vascular wall was uplifted, the endangium thickened,atheromatous plaque formed and the lumen became smaller, a large number of xanthoma cells infiltrated,cholesterol clefts could be seen, smooth muscle cells were disorderly arranged; in the XFZYT group, the vascular wall was partly lifted, the endangium slightly thickened, and the xanthoma cell infiltration was to a lesser extent than in the model group. In the HLXLD group, the range of endangium thickening was wider than that in the XFZYT group but smaller than that in the model group, there was inflammatory cell infiltration and smooth muscle cell proliferation; SXS and DSY group endangium thickened and the lumen became narrowed, with large numbers of foam cells infiltration; THSWT group showed no significant difference compared with the model group.
We analyzed the OD value and the area of atheromatous plaque in each group, as shown in Figure 10.The results showed that, compared with the model group, the five formulas decreased the value of OD and plaque area, out of which XFZYT and HLXLD showed statistical significance (P< 0.05).
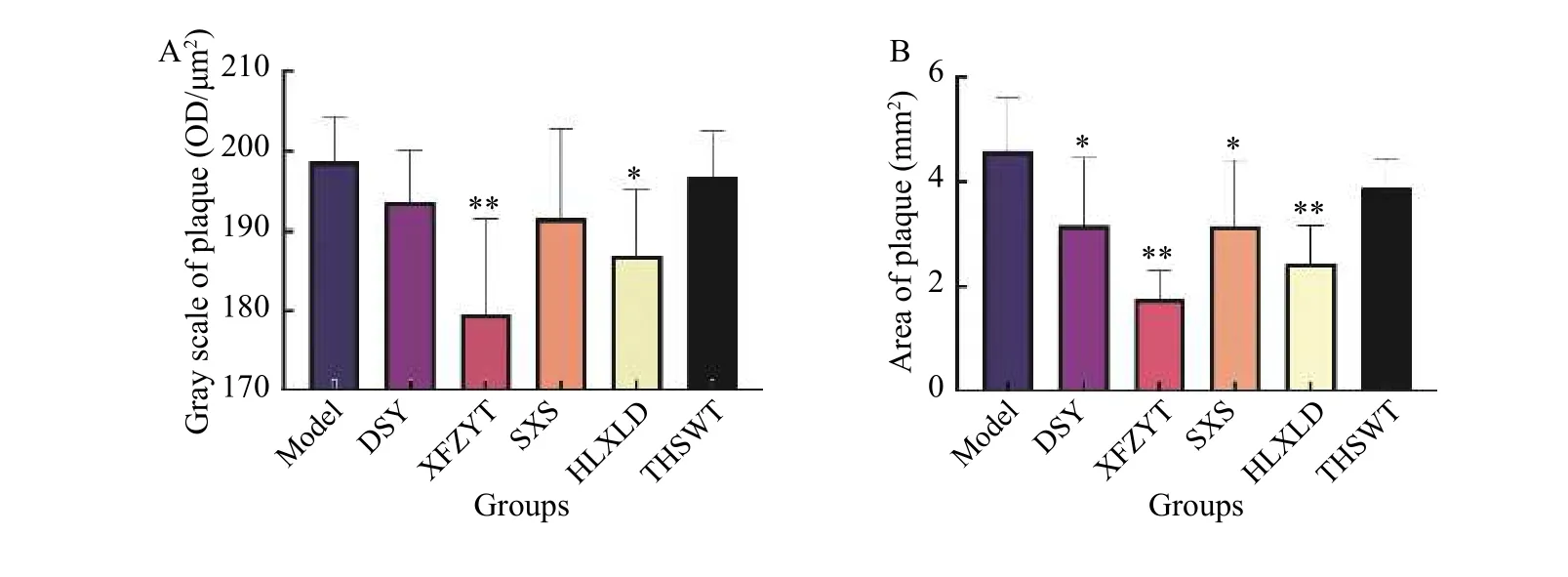
Figure 10 Effects of the five formulas on plaque A, gray scale of plaque. B, area of plaque. *P < 0.05, **P < 0.01, compared with the model group.
4 Discussion
Cardiovascular disease is a major non-communicable disease worldwide, and it is the leading cause of death in China[15]. Blood stasis is a major cardiovascular disease syndrome[16]. Promoting blood circulation and removing stasis are two ways of treating stasis syndrome; however, formulas for promoting circulation and removing stasis vary in their mechanisms and efficacies. This leads to difficulty in selecting the most appropriate formula for stasis syndrome.
TCM formulas are characterized by multiple compounds, multiple targets and multiple pathways.The efficacy of TCM formulas in the treatment of complex diseases depends on the synergistic effects of the multiple compounds and their targets[17]. Different formulae may act on different aspects of the complex network to cure the same disease. Therefore, to determine the compounds and targets of each formula for treating stasis syndrome, five commonly used formulas were selected, and a network pharmacology method was used. In this study, we obtained 269, 358, 288, 370, and 376 candidate targets of DSY,HLXLD, SXS, THSWT, and XFZYT against blood stasis, respectively, and there were 232 shared candidate targets, indicating that most targets of the five formulas for treating blood stasis were the same. Furthermore, the results indicate that fluid shear stress and atherosclerosis were common biological processes that were targeted by the five formulae. Shear stress represents the frictional force exerted by the flow of blood at the endothelial surface of the vessel wall and plays a central role in vascular biology and contributes to the progression of atherosclerosis[18]. Scholars believe that the “vessel” of TCM is closely related to endothelial cells (EC), and the injury of EC can cause blood stasis, which is the major mechanism of atherosclerosis[19]. Sustained laminar flow with high shear stress upregulates the expression of EC genes and proteins that protect against blood stasis[20]. The results indicate that all the five formulas target KLF2 and Nrf2, which are key shear stress-induced transcription factors that control the EC genes[21], and NFKB1, VCAM1, JUN, and AP1, which reflect EC injury in disturbed flow[22]. Therefore, regulating the expression of KLF2, Nrf2, NFKB1, VCAM1, JUN, and AP1, adjusting fluid shear stress, and alleviating injury of ECs, might be the common mechanism by which the five formulas promote circulation and remove blood stasis.
In contrast, there was also differentiation of the targets of the five formulas, which may provide guidance for clinical drug selection. HLXLD, SXS,DSY, and THSWT acted on the regulation of lipolysis in adipocytes, indicating that these four formulations improve circulation while regulating lipids. They may be suitable for the treatment of blood stasis caused by hyperlipidemia. The use of SXS[23], DSY[24], and THSWT[25]in treating hyperlipidemia have been verified previously. XFZYT, HLXLD, SXS, and THSWT regulated the AGE-RAGE signaling pathway and complement and coagulation cascades. Researchers have found that the AGE-RAGE signaling pathway plays an important role in diabetes-mediated vascular calcification[26], and the coagulation cascade exacerbates the formation of calcified plaques; hence,these four formulas may be used to treat diabetes-related blood stasis syndrome. Research has reported the effectiveness of XFZYT[27], HLXLD[28], and THSWT[29]in promoting circulation in diabetes complications. HLXLD, SXS, and XFZYT regulated the HIF-1 signaling pathway and provided feedback to hypoxia, which indicates that these three formulas may treat blood stasis in ischemic diseases such as cerebral infarction and myocardial ischemia. This hypothesis is consistent with the findings of previous studies[30,31]. In addition, DSY regulated the cGMPPKG signaling pathway, HLXLD reduced platelet activation, SXS regulated the calcium signaling pathway,and XFZYT regulated the PPAR signaling pathway.These pathways play crucial roles in blood stasis, and their effects need to be verified by further studies.
The network pharmacology results revealed the differentiation of the targets and mechanisms of the five formulas. However, there were differences in their curative effects. Therefore, we exposed a rabbit blood stasis syndrome model to high-fat feeding +epinephrine injection. Serum lipid and blood rheology were analyzed to evaluate the curative effects of the five formulas for improving blood circulation.After 30 days of modeling, the values of PV, HCT,WBV (high and low shear rate), TG, TC, LDL, HDL,apoB100, and apoA1/apoB100significantly increased compared with the control group, indicating that the blood in rabbits became more viscous and the state of stasis was therefore established. Moreover, atherosclerotic plaque and vascular lumen stenosis were also observed. After the five formulas were orally administered, the values of TC, TG, LDL, TC/HDL, and TG/HDL in each group decreased, among which XFZYT had the best effect, followed by HLXLD and SXS. XFZYT and HLXLD decreased apoB100and increased apoA1/apoB100. With regard to blood rheology, XFZYT led to a reduction in the values of HCT,PV, and WBV; HLXLD, DSY and SXS affected HCT,DSY and THSWT regulated WBV, and the results suggested that each formula improved different aspects of hemorheology among which XFZYT showed the best effect. Referring to the pathological changes, all the five formulas decreased the values of OD and area of plaque, among which XFZYT and HLXLD showed statistical significance. From the above results, we conclude that by regulating different indices,the five formulas can improve blood lipid and hemorheology, improve the state of blood stasis, and decrease the degree of aortic plaque in stasis model rabbits. Among the five formulas studied, XFZYT and HLXLD showed better effects than DSY, THSWT and SXS.
Acknowledgements
We thank for the funding support from the Special Project of Central Government Guiding Local Science and Technology Development (No. 2019XF5062),the 211 Project of Chinese Medicine Diagnostics(No. 80019992), and the Open Fund of National Key Discipline of Chinese Medicine Diagnostics of Hunan University of Chinese Medicine (No. ZZKF201501 and No. 2015ZYZD01).
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年2期
Digital Chinese Medicine2021年2期
- Digital Chinese Medicine的其它文章
- Network pharmacology research and experimental verification of Huangqi (Astragalus Radix) and Jinyingzi (Rosae Laevigatae Fructus) in treating benign prostatic hyperplasia
- Evaluation of Baishao (Paeoniae Radix Alba) and Chishao(Paeoniae Radix Rubra) from different origins based on characteristic spectra of amino acids
- Quality 4.0 technologies to enhance traditional Chinese medicine for overcoming healthcare challenges during COVID-19
- Novel pyrimidine-benzimidazole hybrids with antibacterial and antifungal properties and potential inhibition of SARS-CoV-2 main protease and spike glycoprotein
- Research on classification diagnosis model of psoriasis based on deep residual network
- Analysis of the hotspots and trends in traditional Chinese medicine immunomodulation research based on bibliometrics
