Kinetic Laws of Heating Initiated Reactions for Materials in Aerospace Applications
QIAO Haitao,WANG Zhiyong,SONG Jiangpeng
AECC Beijing Institute of Aeronautical Materials,Beijing 100095
Abstract:Cure and decomposition reaction kinetics of typical organic materials in aerospace applications are introduced.From the data of dynamic differential scanning calorimetry (DSC) experiments,and based on changes of the peak temperatures (Tp) with different heating rates (β),a linear equation,Tp=T1+ΔTlnβ,has been obtained more reasonably.The above equation can be used to explain some laws of higher or lower of apparent activation energies (Ea),by which the apparent activation energy (Ea) is nearly equal to RT12/ΔT.A number of kinetic investigations of typical thermosetting resins and energetic materials in aerospace applications were chosen to validate the above equations.
Key words:thermosetting resin,energetic material,cure,thermal decomposition,reaction kinetics,aerospace application
1 INTRODUCTION
There are two important species of organic materials in aerospace applications,one of which is structural material such as high-temperature polymer matrix and adhesive used for composite structures,another is propellant which can provide the main energy for spacecraft launching.
More than 30 years ago,several adhesives including Hysol EA934NA and EA 9394 were investigated to meet the thermal requirements of the Galileo mission which flew to Jupiter.ZiLi-4 (SY-14A) was one of the earliest film adhesives for non-perforated honeycomb core bonding,developed almost at the same time by LAI Shihong,TANG Falun and ZOU Xianwu in China.SY-14A film adhesive has been used in structural bonding for the most advanced fighters in China up to now.SY-14A film adhesive also has been contributed to structural bonding of rocket fins and re-entry modules for aerospace applications.EA934NA and EA 9394 have been also used as liquid shimming adhesives or repair adhesives in bonding civil aircraft structures such as for the AS907 aero engine and Airbus A380.
Satellite structures are one of the best examples where weight saving is at an absolute premium.This means composite structure and adhesive bonding are the most important manufacturing considerations.AF-163-2 film adhesive has been used to manufacture large antennae reflectors by bonding Nomex honeycomb with Kevlar/epoxy prepregs,and also,Redux 319L (175°C curing and 180g/mareal weight) and Redux 312(120°C curing and 100g/mareal weight) have been selected to save weight in satellite structures.Bismaleimide adhesives have been used in bonding for military/civil aircraft structures,as an example,Hexcel Composites HP 655 was qualified by Airbus Industries for use in structures either in or adjacent to the Trent 900 engines,which are to power the Airbus A380.With regard to higher heat-resistance materials,composite and adhesive based on cyanate ester have started finding applications in the satellite market due to their exceptionally low water uptake and out-gassing characteristics,low dielectric constant and low loss tangent electrical properties.Typical applications of cyanate materials include manufacture of antenna reflector and telescopes in space.From the Handbookedited by Philippe Cognard,we can see more details about aerospace applications of adhesives and composite bonding.
Although thermosetting materials and energetic materials have different roles,their chemical cure or decomposition reactions have to be conducted under continuous heating to realize their different functions.A number of reaction kinetic methods developed by Kissinger,Ozawa,Crane,et al.were usually used to study both decomposition and cure reaction kinetics.The Kissinger method is one of the most popular approaches for determining kinetics by thermal analysis.The Kissinger method is based on a series of experiments in which small milligram quantities of the reacting material are heating at several heating rates (β
) while the reaction exothermic peak(T
) is recorded.Plot of ln (β/T
) versus 1/T
usually generate a straight line.The slope of the line equals -E/R
whereE
is the activation energy.Approximate 10 years later than the Kissinger method,Takeo Ozawa,whose contribution was regarded as outstandingamong many similar researches,established a simpler equation.According to the Ozawa method,the data point at fixed conversion (α
) of a series of nonisothermal rate data at differentβ
form a straight line when plotting logβ
against the reciprocal temperature.In 1973,L.W.Crane,P.J.Dynes and Kaelblederived an equation to investigate the curing kinetics of HT424 epoxy-phenolic structural adhesive.Although their equation is similar to the equation of Ozawa in form,their derivation was based on the method by Ellersteinand utilized a similar derivation method to the Kissinger method.Although isothermal experiments have been used in kinetic studies by many researchers,the results obtained by an isothermal method of investigation are often questionable when a sample undergoes considerable reaction in being raised to the temperature of interest.The advantages of evaluating reaction kinetics by nonisothermal experiments are that considerably less experimental data is required than in the isothermal method.Along with introducing a number of kinetic studies of some typical materials used in aerospace applications,this article proposes a much simpler method in order to illustrate some laws in reaction kinetics for both cure and decomposition reactions using dynamic nonisothermal differential scanning calorimetry (DSC) experiments.
2 CURE PROPERTIES OF SY-14A
In 2009,using a Q10 type TA Instrument (USA),dynamic DSC experiments were performed with SY-14A film adhesive at different heating ratesβ
of 2.5,5 and 10 K/min.The DSC curves of SY-14A at different heating rates are shown in Figure 1.Original data from DSC tests and calculated values from the data are listed in Table 1.Plot ofT
versusβ
is shown in Figure 2,which indicates a poor linear relationship.In contrast,plot ofT
versus lnβ
shows an extremely good linear correlation,as shown in Figure 3.From the linear regression equation as shown in Figure 3,T
=450.28846+10.95166lnβ
,asβ
=1K/min,T
is equal to 450.28846 K (about 177°C),which is in the scale of suggested cure temperature (178±3°C) according to the standard of SY-14A (Q/6S 104-2004).Activation energies are determined using Kissinger and Ozawa methods,from linear fitting plots which are shown in Figure 4 and Figure 5,respectively.The two linear plots have the same correlation coefficient of 0.99986 and nearly the sameE
value as listed in Table 1.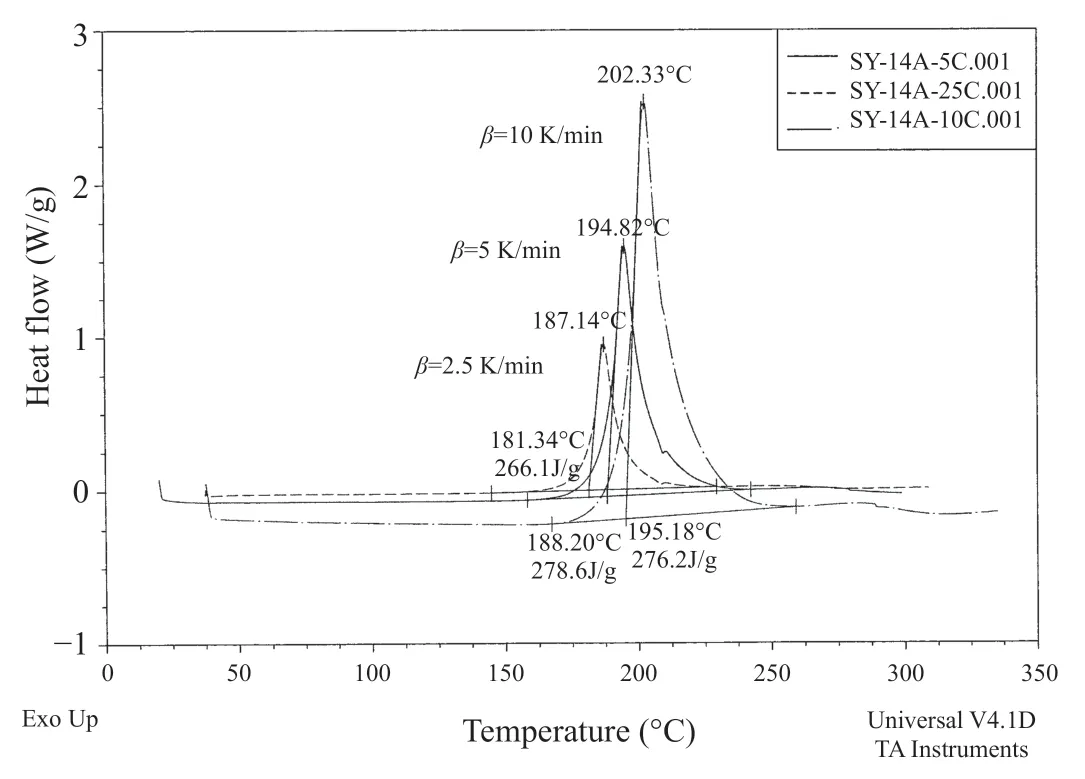
Figure 1 DSC curves of SY-14A at different heating rates
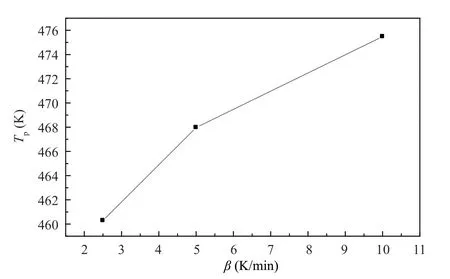
Figure 2 Plot of Tp vs lnβ for SY-14A

Figure 3 Plot of Tp vs lnβ for SY-14A
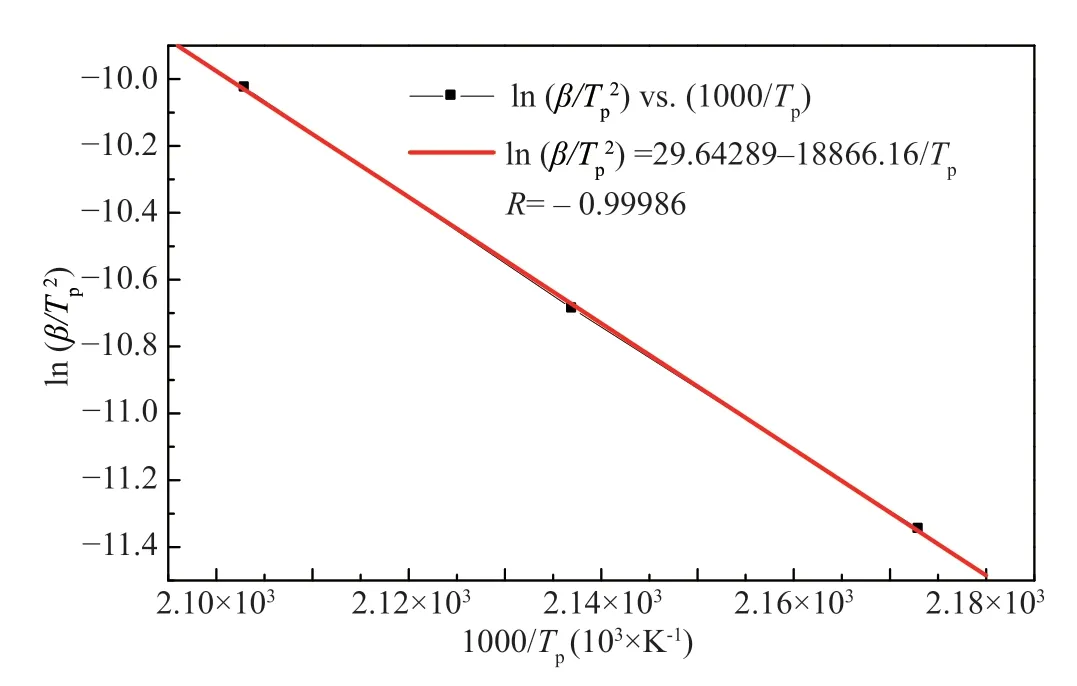
Figure 4 Plot of ln (β/Tp2) vs 1000/Tp for SY-14A

Figure 5 Plot of lnβ vs Tp for SY-14A

Table 1 DSC data and calculated results for SY-14 adhesive
3 CURE OF 5528 CYANATE RESIN AND ITS MODIFIED ADHESIVE
In our previous work,5528 cyanate ester resin modified with hydroxyl-terminated polyethersulfone (SY-CN adhesive)has a lower maximum cure peak temperature of about 20K reduction versus unmodified 5528 cyanate as shown in Table 2,but the curing activation energy increases from 80.33 kJ/mol(by Kissinger method) and 84.49 kJ/mol (by Ozawa method) of 5528 cyanate resin to 92.28 kJ/mol (by Kissinger method) and 95.49 kJ/mol (by Ozawa method) of SY-CN adhesive.From the data in Table 2,a linear equation ofT
=(489.46+24.84 lnβ
)K is obtained for the case of 5528 cyanate ester resin,andT
=(473.03+19.89 lnβ
)K for the case of SY-CN adhesive.In the following section,the reason for the reduction of cure peak temperature of the modified SY-CN adhesive accompanied by increase of activation energy will be explained using the two above regression equations.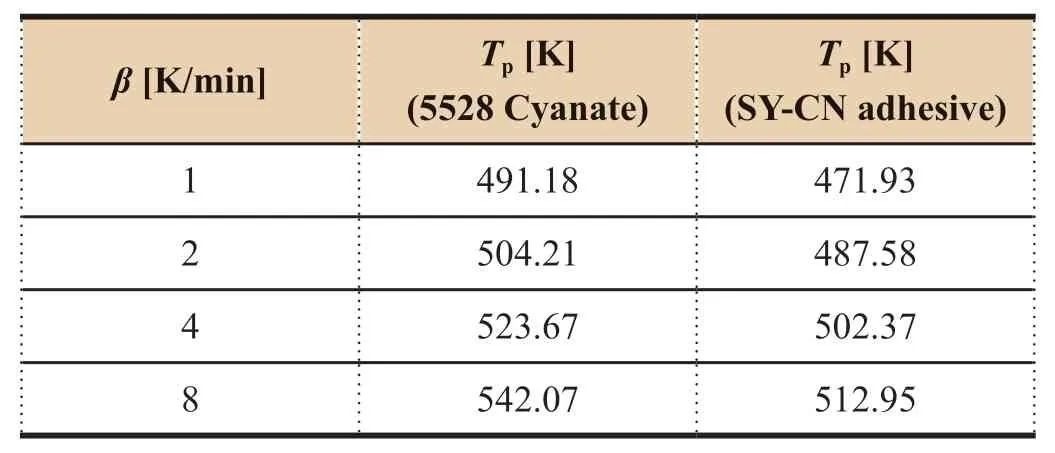
Table 2 DSC data for 5528 cyanate ester and SY-CN adhesive
4 CURE OF BMP350 POLYIMIDE RESIN
Zhou Hongfeiinvestigated the curing reaction process of BMP350 PMR type polyimide resin,DSC data and calculated energy values are shown in Table 3.From the data in Table 3,a linear regression plot ofT
versus lnβ
can be obtained as shown in Figure 6.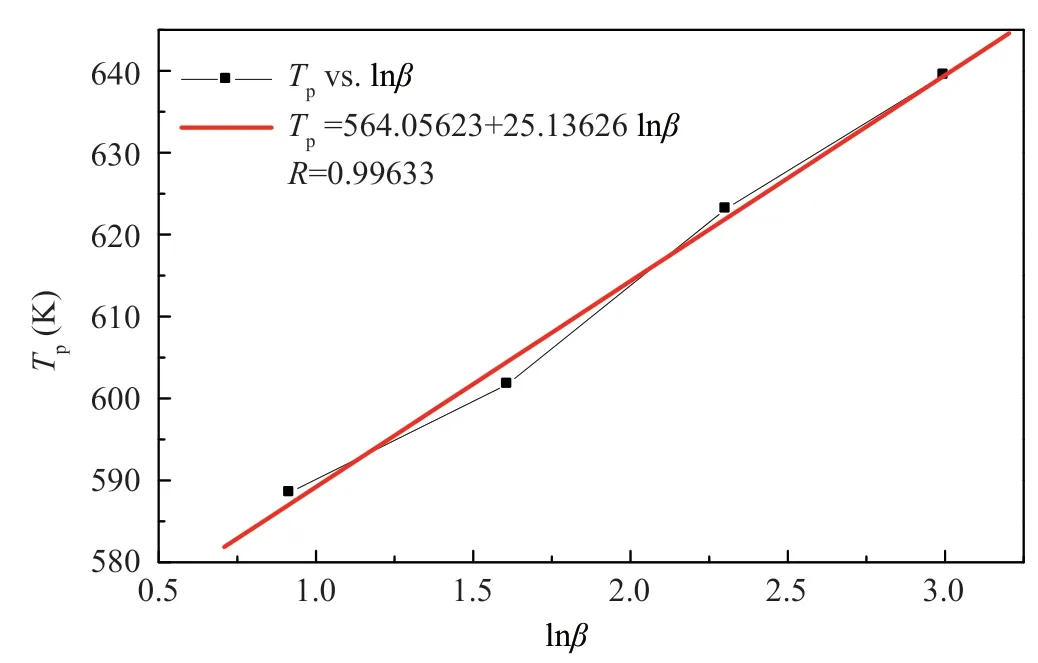
Figure 6 Plot of Tp vs lnβ for BMP350 polyimide resin
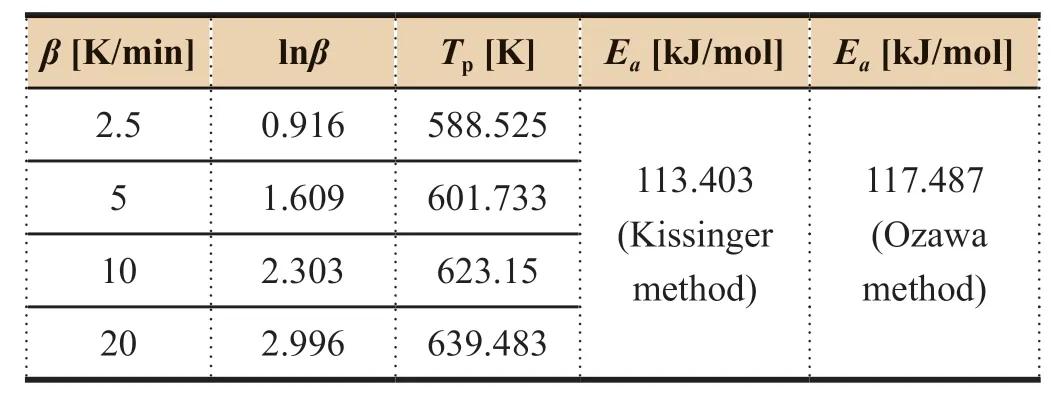
Table 3 DSC data and cure activation energy results for BMP350 PMR type polyimide resin
5 DECOMPOSITION OF ENERGETIC MATERIALS
Two investigations on thermal decomposition of hexanitrohexaazaisowurtzitane (CL-20) were conducted by XU Jinxiang,SONG Naimeng,ZHANG Tong1ai,et al.Peak temperatures tested by different thermal analysis instruments and activation energies calculated were different between the above two studies,as shown in Table 4.Linear regression plots ofT
versus lnβ
can also be obtained as shown in Figures 7 and 8,which shows slightly different extents of linear correlation.Obviously different calculatedE
values by the two investigations may be due to the difference in purity,crystalline form or particle size.CalculatedE
values by XU Jinxiangare also different by different methods and at different heating rates as shown in Table 5.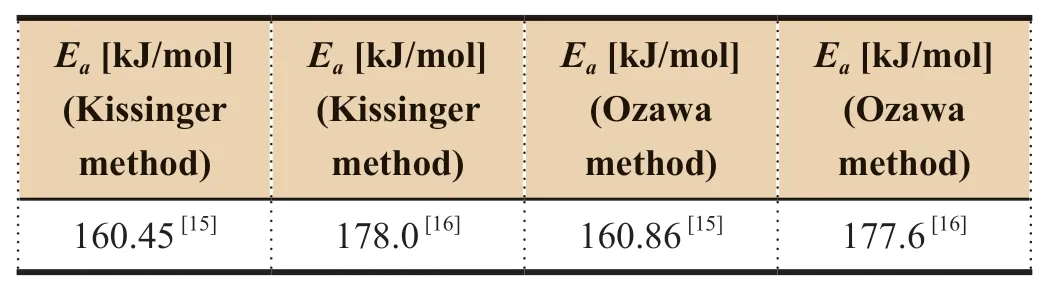
Table 4 Different activation energy results for CL-20 in two references
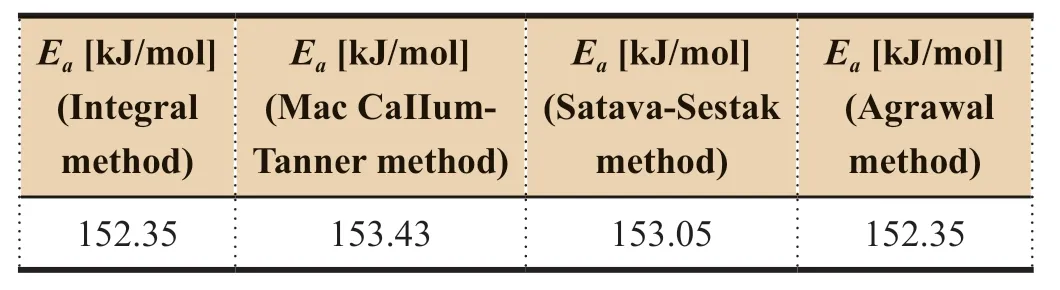
Table 5 Activation energy results determined by different methods at heating rate of 10 K/min [15]
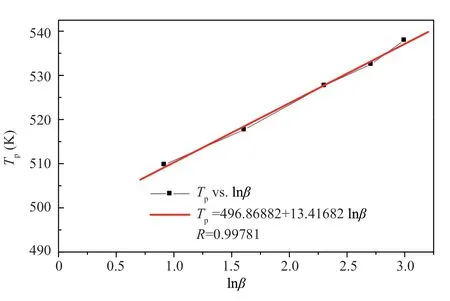
Figure 7 Plot of Tp vs lnβ for CL-20 from reference [15]
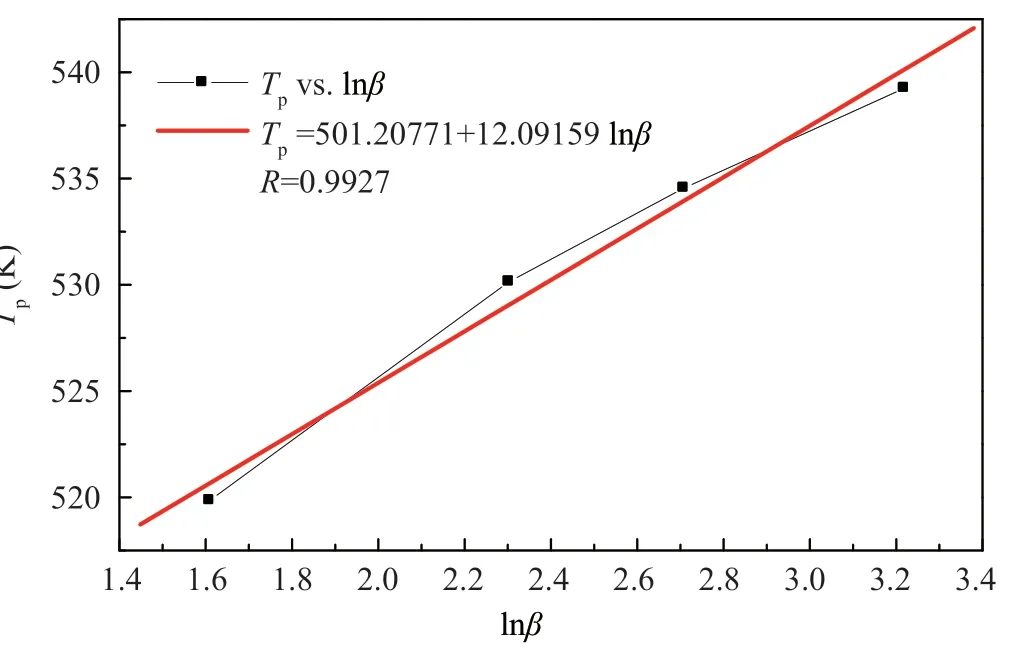
Figure 8 Plot of Tp vs lnβ for CL-20 from reference [16]
JIA Haonan et alinvestigated the thermal decomposition behaviors and thermal hazard of a composite modified double base (CMDB) propellant with higher RDX content using differential scanning calorimetry (DSC) experiments.The DSC curves of decomposition show two peak temperatures,of which two different activation energy values were obtained (as shown in Table 6).From the data in Table 6,plots of the two different stage peak temperatures with respect to lnβ
also have good linear relationships as shown in Figure 9 and Figure 10.
Table 6 Parameters obtained from DSC curves at different heating rates and calculated Ea values [17]

Table 7 DSC results and Ea values calculated for CL-20 and phloroglueinol-Fe modified CL-20 [16]
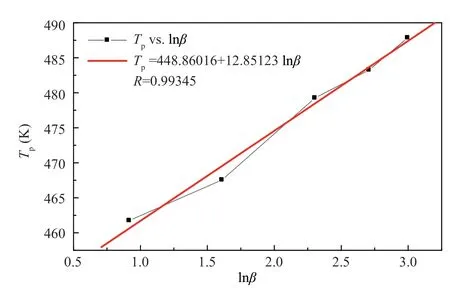
Figure 9 Plot of Tp (Stage 1) vs lnβ

Figure 10 Plot of Tp (Stage 2) vs lnβ
6 KINETIC LAWS SUITABLE FOR BOTH CURE AND DECOMPOSITION REACTIONS
From the above in-depth analysis in the most cases of cure and decomposition reactions investigated by DSC experiments,there usually exists a linear relationship between peak temperature (T
)and lnβ
,as expressed in Equation (1),which can be used to determine the curing process for thermosetting resins,also to evaluate the trend of decomposition temperature.The equation had been first proposed in 2002 for study of SY-H2 paste adhesive,and was adopted for calculation of aspect activation energy in 2019.A concise equation for assess of aspect activation energy is proposed as expressed in Equation (2).
T
is the peak temperature asβ
is equal to 1 K/min.Based on the above analysis,validations of Equation (2) will be carried out as follows.In the study mentioned above,SONG Naimeng,ZHANG Tong1ai,GAO Hongxu,et almodified CL-20 using sub-micron spherical phloroglucinol-Fe.Particle size was in the range from 500 nm to 1000 nm.The product can obviously promote the decomposition performance of CL-20 with the first decomposition peak temperature shifted about 10°C up,while the activation energy of CL-20/ phloroglucinol-Fe is increased by 81.4 kJ/mol compared with that of CL-20.Similar behavior had been also found in a modified cyanate study,which resulted in maximum cure peak temperature of about 20°C reduction compared with unmodified cyanate and more than 10 kJ/mol increase in the activation energy.This conflicting phenomenacan be explained by using Equation (2).
With regard to modification of CL-20,as listed in Table 7,plots ofT
versus lnβ
show a good linear relationship as shown in Figure 11.From the linear regression result in Figure 11,T
=(496.83504+8.19797lnβ
)K,compared with the result in Figure 8,T
=(501.20771+12.09159lnβ
)K,calculatingE
according to Equation (2),E
values of 250.339 kJ/mol and 172.728 kJ/mol are gained for modified CL-20 and CL-20 respectively.The results are listed in Table 7,which indicate that a significant reduction of ΔT
for the modified CL-20 results in a notable increase of the calculated activation energy.
Figure 11 Plot of Tp vs lnβ for CL-20/phloroglueinol-Fe

T
versus lnβ
has better linear relationship.In order to validate Equation 2,activation energy calculations for the above mentioned materials were performed.Calculation results are listed in Table 8,9,10 and 11,respectively.Compared theE
calculation results of SY-14A in Table 8 with that of BMP350 in Table 9,peak temperature of BMP350is above 100K higher than that of SY-14A,but theE
value of BMP350 calculated by all methods is lower about 40 kJ/mol,which is due to significantly higherΔT
of BMP350.TheE
value of SY-14A is very close to those of CL-20 in Table 10 and CMDB propellant in Table 10 (Stage 2).Compared with theE
calculation results of CL-20 in Table 10,theE
value from Equation 2 is about 8 kJ/mol lower than that obtained by using the Kissinger and Ozawa methods,while it is very close to the results obtained by other methods shown in Table 5 as heating rate is 10 K/min.From the above analysis results,higher or lowerE
values are mainly determined byT
andΔT
,i.e.,E
≈RT
/ΔT
.
Table 8 Ea calculations for SY-14A
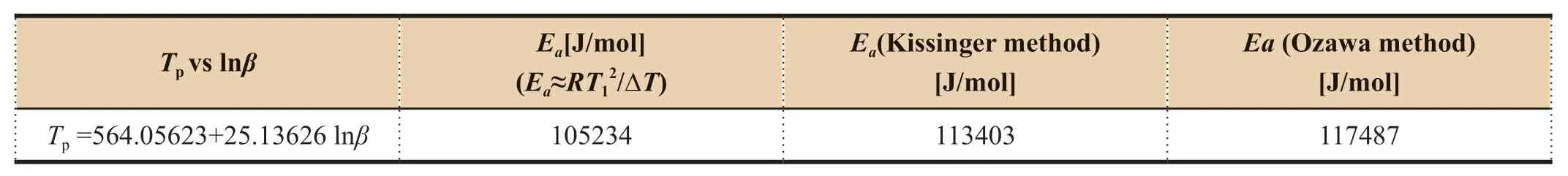
Table 9 Ea calculations for BMP350 polyimide resin [14]
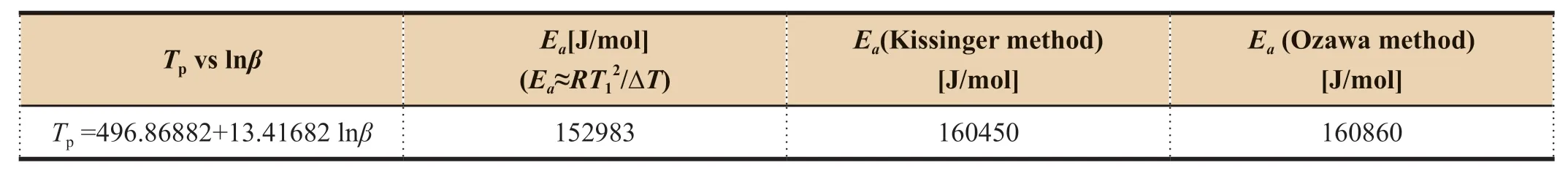
Table 10 Ea calculations for CL-20 [15]

Table 11 Ea calculations for modified double base (CMDB) propellant with higher RDX content [17]
Finally,according to the study by L.W.Crane,P.J.Dynes and Kaelble,DSC data and activation energy results determined using the Kissinger and Ozawa methods for HT424 epoxy phenolic adhesive are illustrated in Table 12.It should be noted that two peak temperature values were tested for each heating rate in the original paper and the averageT
value was taken for the calculation in Table 12.From plot ofT
versus lnβ
for all the data in Table 12,a linear equation,T
=(386.91708+20.55642 lnβ
)K (the fitting quality parameter R=0.99198),has been obtained,and according to Equation 2,E
≈RT
/ΔT
≈60548J/mol.While for the case of the data at heating rates of 5,10 and 20 K/min,the linear equation isT
=(394.98523+16.9433 lnβ
)K (R=0.99934),andE
≈RT
/ΔT
≈76555J/mol,which is close to the results in Table 12.Different selections of DSC data influence the calculatedE
values notably.
Table 12 DSC data [8]and activation energy results for HT424 epoxy phenolic adhesive
7 CONCLUSIONS
Both cure and decomposition reaction kinetics of typical organic materials in aerospace applications are usually investigated using the Kissinger and Ozawa methods.Although,a lot of dynamic differential scanning calorimetry (DSC) experiments for thermosetting resins and energetic materials,as studying on changes of the peak temperatures (T
) with different heating rates (β
),plots ofT
versus lnβ
rather than those ofT
versusβ
usually show good linear relationship,and a linear equation can be obtained,i.e.,T
=T
+ΔT
lnβ
.The equation obtained from DSC experiments can be used to extrapolate the changing trend ofT
withβ
more reasonably.A number of kinetic investigations of typical materials in aerospace applications were chosen to support the above equation.It is very interesting to note that the above equation can be used to explain some laws of higher or lower apparent activation energies (E
) of different species of materials,by which the apparent activation energy (E
) is nearly equal toRT
/ΔT
.The equation,E
≈RT
/ΔT
,is suitable to estimate the apparent activation energy (E
) for either cure or decomposition reactions.Although the concise equation is reasonable for the materials introduced in this paper and a number of DSC experiment results not mentioned here,further research is necessary to validate the above mentioned method,and also to identify exceptions.- Aerospace China的其它文章
- Artist’s Concept of Tianwen 1’s Brake and Captured by Mars
- Acoustic and Vibration Environment Prediction Technology of Instrument Cabin Based on Multi-Source Data
- Design and Kinematics Analysis of Support Structure for Multi-Configuration Rigid-Flexible Coupled Modular Deployable Antenna
- Design of Optical System for Small Long-Life Star Sensor
- In-situ Lunar Penetrating Radar Experiments on the Moon of CE-3 and CE-4 Missions
- Principle Prototype of a Recovery Launch Vehicle with Vertical Take-Off and Landing

