A gain-of-function mutation of OsMAPK6 leads to long grain in rice
Lina Xiong,Luin Tan,Ran Xu,Zuofeng Zhu,Xianyou Sun,Hongying Sun,*,Chuanqing Sun,*
a State Key Laboratory of Plant Physiology and Biochemistry,China Agricultural University,Beijing 100193,China
b National Center for Evaluation of Agricultural Wild Plants(Rice),Beijing Key Laboratory of Crop Genetic Improvement,Laboratory of Crop Heterosis and Utilization,Ministry of Education,Department of Plant Genetics and Breeding,China Agricultural University,Beijing 100193,China
Keywords:
A B S T R A C T Grain size is one of the most important agronomic traits controlling grain yield.Development of novel germplasm with large grains would be beneficial for crop improvement.We report the genetic identification and functional analysis of the LONG GRAIN 6(LOG6)gene,which is identical to MITOGENACTIVATED PROTEIN KINASE 6(OsMAPK6),affecting grain length of rice.Map-based cloning revealed that the long-grain phenotype of log6-D results from a glutamine(E)to lysine(K)mutation in the conserved TEY motif of OsMAPK6.In near-isogenic lines(NILs),the log6-D allele increased grain length and grain yield of Guichao 2(GC2),Teqing(TQ),and 93–11.Sequence analysis revealed 10 OsMAPK6 haplotypes,with xian(indica)and geng(japonica)harboring different haplotypes.Our findings shed light on the function of MAPKs and offer a novel dominant allele for improving the grain yield of rice.
1.Introduction
Rice(Oryza sativaL.)is one of the three major cereal crops,with half of the world’s population using it as a staple food.Grain size is an important determinant of rice grain yield.In recent years,several critical factors regulating rice grain size have been identified[1],most of which are involved in G-protein signaling[2–4],MAPK signaling[5–9],the ubiquitin–proteasome pathway[10,11],phytohormone perception and homeostasis[12–14],or transcriptional regulation[15–18].However,the molecular mechanisms that determine final rice grain size are still largely unknown.Identifying novel grain-size regulators and further studying the mechanisms controlling rice grain size would be beneficial for breeding highyielding rice cultivars.
Mitogen-activated protein kinase(MAPK)signaling is an evolutionally conserved signal transduction module in eukaryotes[19]that mediates many critical developmental and defense signals in plants[20,21].A MAPK cascade generally contains three sequentially activated kinases:a MAPK kinase kinase(MKKK),a MAPK kinase(MKK),and a MAPK.After sensing upstream signals,MKKK phosphorylates and activates MKK.The activated MKK then activates MAPK by dual phosphorylation of the conserved tripeptide TxY motif in MAPK[22,23].The activated MAPK then transduces signal to downstream factors by phosphorylation.Several studies[5–8]have shown that MAPK signaling also functions in regulating grain size.In rice,MAPK kinase kinase 10(OsMKKK10),MAPK kinase 4(OsMKK4),and OsMAPK6 act in a cascade to promote grain growth.The canonical TEY loop motif is critical for the function of MAPK6 in determining rice grain size.When the TEY sequence was changed to AEF,the resulting protein was not phosphorylated by its upstream MAPK kinase and played a dominant negative role[22,23].When TEY was changed to TEC,a constitutively active version of OsMAPK6(CA-OsMAPK6)was produced that played a dominant positive role[8].Besides the TEY motif,the C-terminal α-helix of MAPK6 affecting the formation of homodimers is also essential for its role in determining grain size.A mutant with deletion of the C-terminal α-helix displayed dwarfism and small grains[6].A dual-specificity MAPK phosphatase(OsMKP1)represses grain growth by deactivating OsMAPK6[7–9].
To identify superior novel rice germplasm and further study the molecular mechanisms of rice grain size control,we aimed to characterize a dominant long-grain mutant,long grain 6(log6-D).Our results offer a novel allele ofMAPK6for further improvement of modern crop varieties.
2.Materials and methods
2.1.Plant materials
SIL40,an introgression line derived from a cross between Dongxiang wild rice and thexiancultivar GC2,was mutagenized with ethylmethane sulfonate to generate a library for genetic screening of mutants.Thelog6-Dmutant was identified in the mutant library.An F2segregating population was derived from a cross between thelog6-Dmutant and thegengcultivar Zhonghua 17(ZH17),and used for mapping a candidate causal gene oflog6-D.Thelog6-Dmutant,SIL40,and the mapping population were grown in experimental fields in Beijing and Hainan province.
2.2.Primers
Primers used in this study are listed in Tables S1 and S2.
2.3.Scanning electron microscopy
Grains of thelog6-Dmutant and SIL40 were fixed in 2.5%glutaraldehyde–phosphate buffer(0.2 mol L-1phosphate buffer,3% glutaraldehyde),and then a series of dehydration and desiccation treatments were applied as described previously[24].Dried grains were attached to the sample table,plated with gold,and observed with a Hitachi S-2460 scanning electron microscope(Olympus,Tokyo,Japan).
2.4.Genetic confirmation
The coding sequence was amplified from thelog6-Dmutant and cloned into the binary vector pCAMBIA1301,driven by the maize ubiquitin promoter.The recombinant plasmid was introduced intoAgrobacterium tumefaciensstrain EHA105 and then transformed into SIL40.The corresponding empty construct was also transformed into SIL40.
3.Results
3.1.The log6-D mutant forms long grains
A dominant mutant with long grain was identified and designated aslog6-Daccording to its long-grain phenotype(Fig.1A).The mean grain length oflog6-Dwas 10.0 mm,28.7% greater than that of SIL40(Fig.1A,D).Thelog6-Dmutant also showed narrower grain,greater grain length to width ratio,and thicker grain than SIL40(Fig.1B,C,and E–G).As a result,the grain weight oflog6-Dwas dramatically increased relative to that of SIL40(34.5%,P<0.01)(Fig.1H),and the grain number per panicle oflog6-Dwas significantly lower than that of SIL40(Fig.1I,J).Correspondingly,the numbers of primary and secondary branches oflog6-Dpanicles were significantly decreased in comparison with those of SIL40 panicles(Fig.1K,L).
To investigate how thelog6-Dmutation affected grain size at the cellular level,we employed scanning electron microscopy to observe the epidermal cells of glumes.Cell number in the longitudinal direction of thelog6-Dmutant lemmas was significantly greater than that of SIL40(Fig.1O),while the length of lemma epidermal cells of thelog6-Dmutant was similar to that of SIL40(Fig.1M,N,and P).We also detected the expression levels of cell cycle-related genes.Transcripts of the cell cycle-associated genesCYCD1,CYCD1,qCYCB2,qCYCD4,OsEXPB3,andOsEXPB4were higher inlog6-Dthan in SIL40(Fig.S1).Thus,the larger grain of thelog6-Dmutant was due to increased cell proliferation.
To better evaluate the role oflog6-Din determining final grain size,we generated three near isogenic lines(NILs)in the backgrounds of three high-yieldingxiancultivars:GC2,TQ,and 93–11(Fig.S2A–C).In comparison with GC2,TQ and 93–11,the grain length,grain thickness and thousand-grain weight increased significantly(Fig.S2D,F–G,J,L,M,P,R,and S).Although the number of grains per panicle of the three NILs decreased(Fig.S2H,N and T),the yield of the three NILs was higher than those of the recurrent parents,by 46.5%,15.7%,and 12.7%,respectively(Fig.S2I,O,and U).These results demonstrated thatlog6-Dacts in determining grain size and yield.
3.2.Map-based cloning of the log6-D mutation
Genetic analysis showed thatlog6-Dwas controlled by a single dominant gene(Fig.S3A–C).To identify the causative mutation oflog6-D,we generated a F2population by crossing thelog6-Dmutant with agengcultivar,ZH17.The mutation was first roughly mapped to the short arm of chromosome 6 between the SSR markers RM587 and RM585(Fig.S4A).Then,2098 F3plants were used for fine mapping of the mutation.It was localized to a 69-kb region between the InDel markers LN4 and LN29(Fig.S4B).In this region,there were 12 predicted open reading frames(ORFs)(Fig.S4C).Sequencing revealed only one mutation in theLOC_Os06g06090locus(Fig.S4D).In thelog6-Dmutant,a G-to-A mutation occurred in the fourth exon(at position+685)of theLOC_Os06g06090locus,resulting in an amino acid change from glutamine(E)to lysine(K)(Fig.S4E).
To further confirm that the single nucleotide change inLOC_Os06g06090caused the large-grain phenotype oflog6-D,we generated transgenic plants in the background of SIL40 overexpressing the full-length coding sequence ofLOC_Os06g06090(derived from thelog6-Dmutant).All nine independent transgenic plants showed phenotypes similar to those of thelog6-Dmutant.Grain length of the transgenic plants was significantly longer than that of SIL40,while the grain number per panicle of the transgenic plants was reduced relative to that of SIL40(Fig.S4F–K).We formed an RNA interference(RNAi)construct with an inverted repeat harboring a 411-bp mutation allele fragment and introduced it into thelog6-Dmutant to generate five independent transgenic plants with phenotypes similar to that of SIL40.Grain length of transgenic plants was reduced and grain width was increased relative to thelog6-Dmutant(Fig.S4L–P).Thus,theLOC_Os06g06090gene corresponded toLOG6and mutation inLOC_Os06g06090was responsible for the long-grain phenotype in thelog6-Dmutant.
3.3.LOG6 corresponds to mitogen-activated protein kinase 6
TheLOG6locus corresponds to theMITOGEN-ACTIVATED PROTEIN KINASE 6gene(OsMAPK6).A loss-of-function mutant ofOsMAPK6formed small grains[6].However,no gain-of-function allele ofOsMAPK6has been reported.That thelog6-Dmutation is dominant and leads to long grains(Fig.S3A–C)suggests that it is a gain-of-function mutation ofOsMAPK6.Transient expression experiments in rice leaf protoplasts showed that both log6-D-GFP and LOG6-GFP(green fluorescent protein)fusion proteins were localized in the cell membrane and nucleus(Fig.S5).Thus,the single nucleotide substitution did not change the cellular localization oflog6-D.
3.4.Haplotype analysis of OsMAPK6
Haplotype analysis using 3K genome data of rice[25]showed that there were 10OsMAPK6haplotypes divided into two main groups,one containing mostlyxianand the other mostlygeng(Fig.2A).Genetic differentiation analysis showed thatFstremained above 0.9 in the 560 kb interval containingOsMAPK6(Fig.2B),showing that no recombination had occurred between the two groups of haplotypes.Nucleotide polymorphism analysis ofOsMAPK6revealed marked genetic variation at the DNA level(Fig.2C).However,conservation analysis of 87 homologous protein sequences showed that OsMAPK6 was highly conserved(Fig.2D).
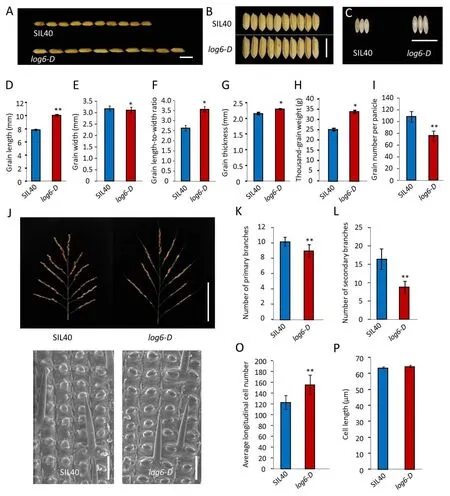
Fig.1.The log6-D mutant forms larger and fewer grains.(A–C)Grains of SIL40 and log6-D.Scale bar,1 cm.(D–I)Grain length(D),grain width(E),grain length to width ratio(F),grain thickness(G),thousand-grain weight(H)and grain number per panicle(I)of SIL40 and log6-D.(J)Panicles of SIL40 and log6-D.Scale bar,10 cm.(K,L)Number of primary branches(K)and of secondary branches(L)of SIL40 and log6-D panicles.(M,N)Scanning electron micrograph of the outer surface of SIL40 and log6-D lemmas.Bars,100 μm.(O)Number of outer epidermal cells in the longitudinal direction of SIL40 and log6-D lemmas.(P)Mean length of outer epidermal cells of SIL40 and log6-D lemmas.Student’s t-test was used to generate P values.*,P<0.05,**,P<0.01.
4.Discussion
In this study,we showed that thelog6-Dmutant as a gain-offunction mutant ofOsMAPK6increased grain length by 28.7%(Fig.1A,D).Introducing thelog6-Dgene into the elite cultivars such as GC2,TQ and 93–11 could significantly increase grain length and yield(Fig.S2).The chalkiness degree oflog6-Dwas also increased(Figs.1C and S2C),suggesting thatOsMAPK6not only regulates grain size but also affects grain appearance quality.We speculate that the reason for the increase of chalkiness oflog6-Dis that the mutation in the conserved TEY motif increased grain length and size,without correspondingly increasing the synthesis and transport of photosynthetic product.
MAPK was activated by dual phosphorylation of its TxY motif by the upstream MAPK kinase(MKK),while MAPK also was deactivated by dual-specific MAPK phosphatase(MKP)[26].Thelog6-Dmutation occurred coincidentally in the conserved TEY motif of OsMAPK6,leading to the change of TEY to TKY.We do not know how this E–K mutation affects the function of OsMAPK6.OsMAPK6 could be activated by OsMKK4 and deactivated by OsMKP1.We speculate that the E–K mutation in the conserved TEY motif of OsMAPK6 led to the change of OsMAPK6 activity by affecting the function of OsMKP1 on OsMAPK6.The E–K mutation oflog6-Dcould change the key motif from acid to alkaline,and the phosphatases in organisms can be divided into acid and alkaline phosphatases.The acid-to-alkaline change of environmental may decrease the enzyme activity of OsMKP1,resulting in the continuous activation of MAPK6.The increase of pH value may also affect the protein kinase activity of MKK4 or MAPK6 itself.How the E–K mutation in TEY motif affects the function of OsMAPK6 in grainsize regulation awaits further study.

Fig.2.Haplotype analysis of OsMAPK6.(A)Ten OsMAPK6 haplotypes.XI represents xian(indica),GJ represents geng(japonica),Bas represents Basmati,Aus represents aus rice,ad represents admixed rice.(B)Genetic differentiation analysis of OsMAPK6.Gray area represents the range of 560 kb,dotted red line represents OsMAPK6.(C)Genetic variation at the DNA level in OsMAPK6.Dotted red line represents OsMAPK6.(D)Conservation analysis of OsMAPK6.
Mutated versions of MAPKs with altered activity are generally employed for studying the function of MAPKs.For example,dominant-negative versions of MAPKs have been widely used in studying the function of MAPKs[22,23].These versions are generated by changing the TEY motifs of MAPKs to AEF.They cannot be phosphorylated by upstream MKKs,and thus play a dominant negative role.However,a convenient and robust method for producing gain-of-function version of MAPKs is still lacking.The TKY described here may serve as such a method.Thus,our study has supplied a potential approach to producing gain-of-function versions of MAPKs and studying the function of other plant MAPKs.
CRediT authorship contribution statement
Chuanqing Sun and Hongying Sunsupervised the experiments and drafted the manuscript.Lina Xiongperformed most of the experiments and drafted the manuscript.Xianyou Sunmaintained plant materials.Lubin Tan,Zuofeng Zhu,and Ran Xuanalyzed the data.All authors read and approved the final manuscript.
Declaration of competing nterest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China(91935302,31971870).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.03.022.
- The Crop Journal的其它文章
- WheatGene:A genomics database for common wheat and its related species
- Genetic dissection of rice appearance quality and cooked rice elongation by genome-wide association study
- The importance of aboveground and belowground interspecific interactions in determining crop growth and advantages ofpeanut/maize intercropping
- Mining favorable alleles for five agronomic traits from the elite rapeseed cultivar Zhongshuang 11 by QTL mapping and integration
- Genetic gains with genomic versus phenotypic selection for drought and waterlogging tolerance in tropical maize(Zea mays L.)
- BSA-seq-based identification of a major additive plant height QTL with an effect equivalent to that of Semi-dwarf 1 in a large rice F2 population

