Sang-Yod rice bran hydrolysates alleviate hypertension, endothelial dysfunction,vascular remodeling, and oxidative stress in nitric oxide deficient hypertensive rats
Gulladawan Jan-On, Akarachai Tubsakul, Weerapon Sangartit, Poungrat Pakdeechote, Veerapol Kukongviriyapan, Ketmanee Senaphan, Chakree Thongraung, Upa Kukongviriyapan✉
1Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
2School of Medicine, Walailak University, Nakhon Si Thammarat, Thailand
3Department of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
4Division of Physiology, Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen, Thailand
5Department of Food Technology, Faculty of Agro-Industry, Prince of Songkla University, Songkla, Thailand
ABSTRACT
KEYWORDS: Sang-Yod rice bran hydrolysates; Hypertension;Endothelial dysfunction; Oxidative stress; Vascular remodeling
1. Introduction
Hypertension is one of the major health problems. It has a high prevalence and is a risk of cardiovascular diseases and chronic kidney diseases[1]. Hypertension is a leading cause of mortality and morbidity,therefore its management is of high priority.
Cumulative evidence has shown that oxidative stress plays a critical role in the pathogenesis of hypertension[2]. During the development of hypertension, the elevation of the renin-angiotensin system (RAS)causes activation of nicotinamide adenine dinucleotide phosphate(NADPH) oxidases resulting in the formation of reactive oxygen species (ROS), and the uncoupling of endothelial nitric oxide synthase(eNOS) causes the production of superoxide anion (O)[3]. Superoxide and other ROS oxidize proteins, lipids, and nucleic acids, or react with nitric oxide (NO) to create a very powerful oxidant, peroxynitrite(ONOO), leading to a diminution of NO bioavailability and further sustaining oxidative stress. Reduced NO bioavailability significantly contributes to vascular endothelial dysfunction and elevation of blood pressure[4]. Moreover, long-term changes in hemodynamics due to increased systemic vascular resistance in hypertension induce pathological changes in vascular structure so-called vascular remodeling[5]. Increased ROS production, decreased NO synthesis,and reduced activity of antioxidants have been observed in human and experimental hypertension[6]. Therefore, the therapies targeting against ROS generation or increasing NO and antioxidants may be useful in minimizing endothelial dysfunction and vascular remodeling, thereby preventing or treating hypertensive disorders.
Several classes of drugs are used in the treatment of hypertension,including diuretics, Cachannel blockers, angiotensin-converting enzyme (ACE) inhibitors, and AT-1 receptor blockers[7]. However,blood pressure is inadequately controlled in a number of patients.Multiple drug therapy is usually employed to increase efficacy,but it comes up with various adverse reactions. In addition to pharmacotherapy, lifestyle modifications such as exercise, salt reduction, and consumption of some fruits and vegetables are recognized as effective strategies for managing hypertension[8].
Rice bran is a major by-product of rice milling[9]. It is a rich source of health-promoting substances, but its value is largely untapped. Rice bran oil and rice bran hydrolysates have gained significant attention due to their rich in bioactive compounds that possess antioxidant,antiallergic, antidiabetic, antihypertensive, anti-inflammatory, and immunomodulating properties[10-15]. Pigmented rice has become increasingly popular because of its possible health benefits. Several studies indicated that rice bran obtained from different varieties of pigmented rice contains numerous nutrients and bioactive compounds,which protect the tissues against oxidative damage[16]and possess various biological activities[17]. Recently, we have shown that hydrolysates from red-pigmented rice bran, namely Sang-Yod rice bran hydrolysates (SRH), could prevent the blood pressure increase in a rat model of NO deficiency induced by N-nitro-L-arginine methyl ester(L-NAME)[18].
In this study, the antihypertensive effect of SRH was assessed in rats with hypertension induced by L-NAME. The effects of SRH on endothelial dysfunction, oxidative stress, ACE-angiotensin axis, and vascular structural changes due to hypertension were analyzed. The study also evaluated whether SRH could act in synergy with lisinopril (Lis),an ACE inhibitor, on lowering high blood pressure, decreasing oxidative stress, and preventing vascular remodeling. The supplementation with SRH may be a safe and effective strategy to control high blood pressure and vascular complications. This study may verify the beneficial effect of SRH as a nutraceutical and functional food.
2. Materials and methods
2.1. Chemicals
Protease G6 was purchased from Siam Victory Chemicals Co., Ltd.(Bangkok, Thailand). Amyloglucosidase, ACE, angiotensinⅡELISA kit, glutathione, glutathione reductase, L-NAME, NADPH, nitrate reductase, and phenylephrine hydrochloride were obtained from Sigma-Aldrich (MO, USA). Sodium nitroprusside and acetylcholine chloride were from Fluka (Buch, Switzerland). Pentobarbital sodium was from Ceva Animals Health (Bangkok, Thailand). Mouse antieNOS IgG was purchased from BD Transduction Laboratory (CA,USA). Rabbit anti-angiotensinⅡtype 1 receptor (ATR), mouse monoclonal anti-gp91, and anti-mouse IgG were from Santa Cruz Biotechnology (CA, USA). All other chemicals were of the highest purity commercially available.
2.2. SRH preparation
Sang-Yod rice cultivated in Phatthalung province, Thailand was used for the preparation of SRH. The preparation was two-stage enzymatic hydrolysis as previously described[18]. Briefly, Sang-Yod rice bran was firstly digested with 5% v/w amyloglucosidase and followed by 5% v/w protease G6, for 60 min each. The hydrolysis process was stopped by increasing the temperature to 95 ℃ for 10 min. After centrifugation, the supernatant was lyophilized and kept at 4 ℃. The SRH comprise a high amount of protein (42%), as well as various phenolic compounds, including the following (mg/100 g dry SRH): ferulic acid (85.9), catechin (44.7), tannic acid (40.6),quercetin (36.4), isoquercitrin (27.6), p-hydroxybenzoic acid (22.3),rutin (22.5), protocatechuic acid (21.3), caffeic acid (19.4), gallic acid (13.6) and kaempferol (2.8).
2.3. Animals and experimental groups
Adult male Sprague-Dawley rats weighing 200-220 g were obtained from Nomura Siam International (Bangkok, Thailand). The animals were kept in a temperature- and humidity-controlled room of a 12-h light/dark cycle in the Northeast Laboratory Animal Center, Khon Kaen University, Thailand. They were fed with a rodent chow diet.After one week of acclimatization, rats were assigned to the normotensive control group and the L-NAME-induced hypertensive group. Rats in the control groups received tap water, whereas rats in the hypertensive groups received the nitric oxide synthase (NOS)inhibitor L-NAME (50 mg/kg/day) in drinking water for 6 weeks.Rats were intragastrically administered daily with deionized water as the vehicle, SRH (500 mg/kg), Lis (1 mg/kg) or SRH (500 mg/kg) plus Lis (1 mg/kg) in a volume of 5 mL/kg, starting from week 4 to week 6 of the experiment. The experimental groups (n=8/group)were comprised of (1) control, (2) control+SRH, (3) L-NAME, (4)L-NAME+SRH, (5) L-NAME+Lis, and (6) L-NAME+SRH+Lis. The dosages of SRH and Lis were based on previous studies[13,18].
2.4. Hemodynamic and vascular reactivity measurements
Systolic blood pressure (SBP) in rats was monitored weekly by using tail-cuff plethysmography (IITC Life Sciences, CA, USA).The animals were trained every day for one week to accustom them to tail-cuff blood pressure measurement procedure before starting the experiment. At the end of the experiments, rats were anesthetized with pentobarbital sodium (60 mg/kg, i.p.). The left femoral artery was cannulated for monitoring of blood pressure (BP)and heart rate (HR) using the data acquisition software (BIOPAC Systems, CA, USA). Hindlimb blood flow (HBF) was measured by an electromagnetic flow probe placing around the abdominal aorta. Hindlimb vascular resistance (HVR) was calculated by mean arterial pressure (MAP) divided by HBF. After monitoring baseline hemodynamic data, vascular reactivity was evaluated by testing the vascular responses to vasoactive drugs, including endothelium-dependent vasodilator acetylcholine (3, 10, 30 nmol/kg), the endothelium-independent vasodilator sodium nitroprusside(1, 3, 10 nmol/kg), and the alpha sympathomimetic vasoconstrictor phenylephrine (0.01, 0.03, 0.1 µmol/kg). Finally, animals were sacrificed with an overdose of pentobarbital. Blood samples, carotid arteries, and aorta were collected for biochemical and protein expression analysis.
2.5. Biochemical assays
The vascular superoxide (O) formation was measured in a strip of carotid arteries using a lucigenin-enhanced chemiluminescence method as previously describe[19]. A lipid peroxidation marker,malondialdehyde (MDA) and a protein oxidation marker, protein carbonyls, were measured in plasma as previously described[20].The reduced glutathione (GSH) and the oxidized forms, glutathione disulfide (GSSG), indicators of cellular redox state were assayed in whole blood using a previously described method[10]. The plasma nitrate and nitrite, ACE activity and angiotensinⅡ(AngⅡ) were measured in plasma as previously described[18,20].
2.6. Western blot analysis
Homogenates from aorta were prepared for analysis of protein expression of gp91, AngⅡtype 1 receptor (ATR), and eNOS by previously described method[10,14]. The densities of target proteins were visualized using ImageQuant400 imager (GE Healthcare,PA, USA), where β-actin was used as a protein loading control.
2.7. Morphometric analysis of the vascular wall
In a separate set of experiments, arteries from six animals per group were used for histology. Briefly, rats were sacrificed using an overdose of the anesthetic drug; the thoracic aortas were fixed with 4% phosphate-buffered paraformaldehyde. The vessels were processed using standard histological methods. Sections of 5µm thickness were stained with hematoxylin and eosin (H&E),Picrosirius Red, and Miller's elastic staining for determination of the number of vascular smooth muscle cells (VSMCs) and the area fractions of collagen and elastin in the aortic medial layer, respectively. The stained sections were examined by light microscopy and the images were captured using a digital microscope camera (Nikon DS-Ri1 Camera). The aortic wall thickness, medial cross-sectional area (CSA), and media to lumen ratio (M/L) were analyzed as previously described[21]. The number of VSMCs was evaluated by counting the number of nuclei in the H&E staining sections. The area fractions of collagen and elastin within the aortic medial layer were assessed by automatically counting threshold pixels stained with Picrosirius Red and Miller's elastic staining and dividing by the total number of medial pixels.
2.8. Statistical analysis
Data are expressed as mean ± standard deviation (SD).Comparisons among groups were analyzed by one-way analysis of variance (ANOVA) followed by post-hoc Student Newman-Keuls test. Statistical significance was preset at P-value of less than 0.05.
2.9. Ethical statement
The handling of animals followed the guideline of the National Research Institutes; Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of Khon Kaen University approved the animal study protocols (ACUC-KKU-40/2559).
3. Results
3.1. Effect of SRH on hemodynamic parameters and vascular reactivity
There was a rapid increase in SBP in L-NAME-treated rats compared with the normotensive controls (P<0.05) (Figure 1).L-NAME caused significant and sustained increases in blood pressure throughout 6 weeks of the experimental period. SRH or Lis prevented the rise in SBP, and the combination of SRH with Lis significantly reduced SBP toward the normal values (P<0.05)(Figure 1). SRH had no effect on SBP in normotensive rats.
Table 1 shows the effect of SRH on hemodynamic status of rats in all experimental groups. Persistent severe hypertension was found in rats-treated with L-NAME for 6 weeks. Apart from a marked increase in blood pressure (systolic, diastolic, and mean arterial pressure), HBF was significantly decreased, while HVR was increased in L-NAME-treated rats compared with those in normotensive controls (P<0.05) (Table 1). Treatment with SRH, Lis,or SRH+Lis significantly improved the hemodynamics, especially in the SRH+Lis group (Table 1). Moreover, SRH or Lis also decreased HR compared with L-NAME hypertensive rats (P<0.05) (Table 1). SRH had no effect on hemodynamics in normotensive control groups.
The vascular reactivity to pharmacological drugs was assessed as shown in Figure 2. L-NAME significantly blunted the vasodilator responses to acetylcholine, whereas the vasoconstrictor responses to phenylephrine were potentiated (P<0.05) (Figure 2B and C).However, the vasodilator responses to sodium nitroprusside remained unchanged (Figure 2A). The data suggest that L-NAME impaired vascular responsiveness. Administration of SRH, Lis, or SRH+Lis significantly improved the vascular responsiveness by increasing the endothelium-dependent vasodilation to acetylcholine and decreasing the vasoconstriction response to phenylephrine (P<0.05) (Figure 2).In normotensive control rats, vascular responses to all vasoactive drugs were unchanged.
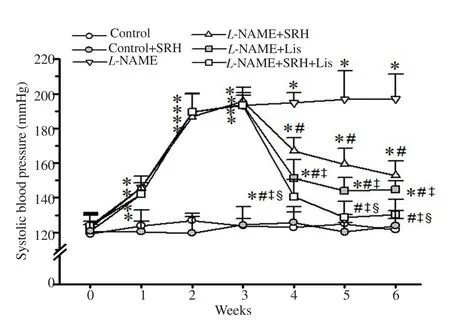
Figure 1. Effect of SRH on systolic blood pressure during the experimental period measured by tail-cuff plethysmography. Data are mean ± SD of 6-8 rats. *P<0.05 versus the normal control group, #P<0.05 versus the L-NAME group, ‡P<0.05 versus the L-NAME + SRH group, §P<0.05 versus the L-NAME + Lis group. Lis, lisinopril; L-NAME, Nω-nitro-L-arginine methyl ester; SRH, Sang-Yod rice bran hydrolysates.
3.2. Effect of SRH on oxidative stress and NO production
A marked increase in vascular Oproduction was found in rats after 6 weeks of L-NAME treatment (P<0.05) (Table 2). This was confirmed by the upregulation of gp91subunit of NADPH oxidase (P<0.05) (Figure 3A). Treatment with SRH or Lis reduced Oformation and downregulated gp91protein expression(P<0.05) (Table 2 and Figure 3A). The levels of MDA and protein carbonyl in plasma were increased 2- to 3-fold, indicating that L-NAME induced lipid peroxidation and protein oxidation (P<0.05)(Table 2). Moreover, L-NAME also induced depletion of the antioxidant defense system, since the blood GSH and the redoxratio of GSH/GSSG were markedly decreased (P<0.05) (Table 2).Treatment with SRH, Lis, or SRH+Lis alleviated oxidative stress and restored the redox homeostasis (P<0.05) (Table 2). It is notable that the combination of SRH with Lis was more effective than monotherapy.
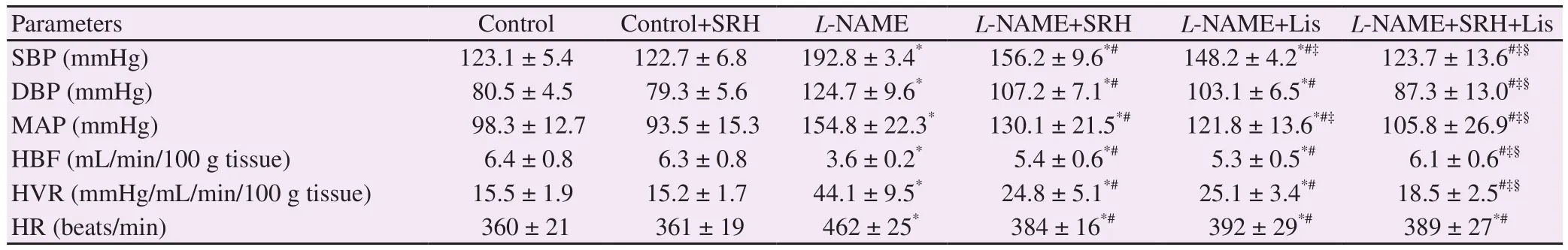
Table 1. Effect of SRH on hemodynamic status in control and nitric oxide deficient hypertensive rats.
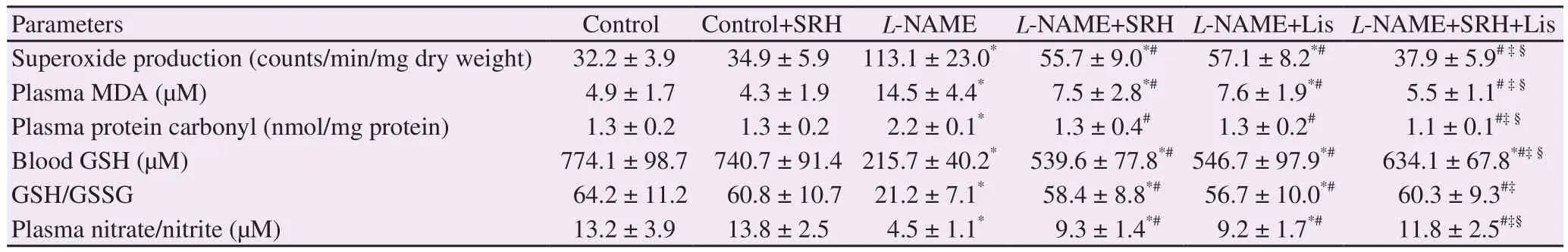
Table 2. Effect of SRH on oxidative stress in control and nitric oxide deficient hypertensive rats.
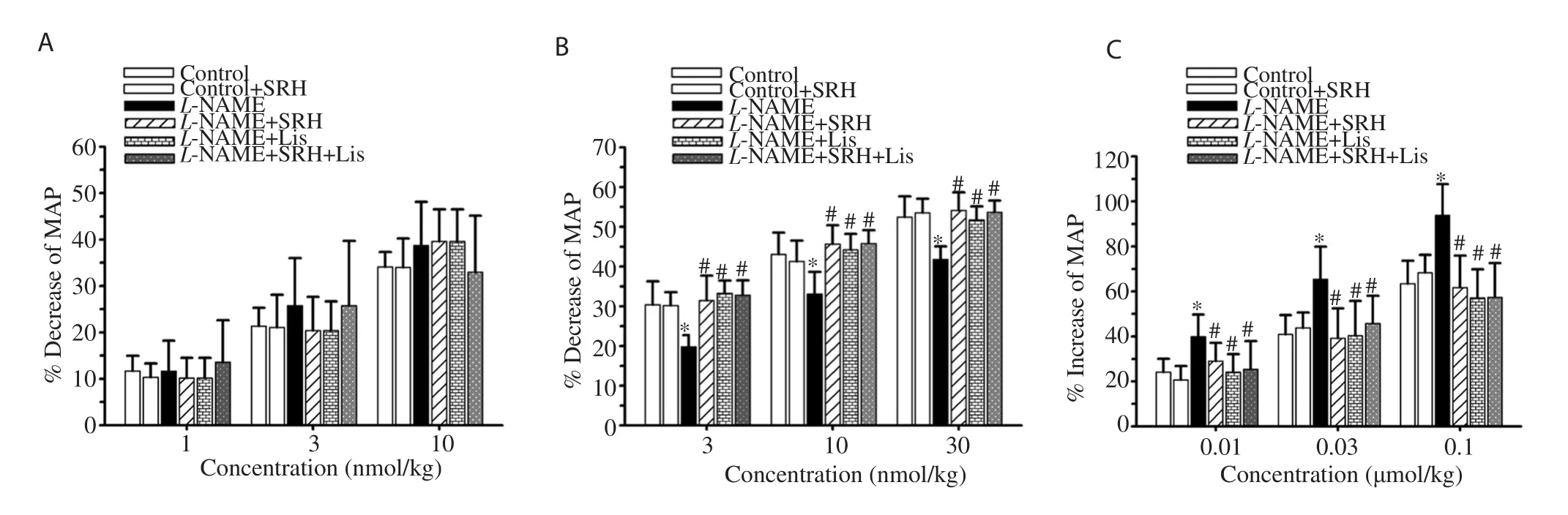
Figure 2. Effect of SRH on vascular responses to vasoactive drugs in control and nitric oxide deficient hypertensive rats. Data are mean ± SD of 6-8 rats.*P<0.05 versus the normal control group, #P<0.05 versus the L-NAME group. MAP, mean arterial pressure. A: sodium nitroprusside; B: acetylcholine; C:phenylephrine.
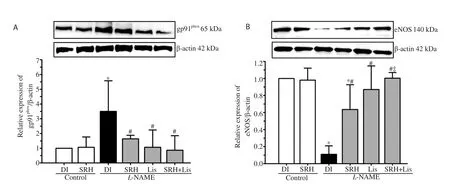
Figure 3. Effect of SRH on gp91phox (A) and eNOS (B) protein expression in aortas of control and nitric oxide deficient hypertensive rats. Data are mean ±SD of 4 rats. *P<0.05 versus the normal control group, #P<0.05 versus the L-NAME group, ‡P<0.05 versus the L-NAME + SRH group. DI, deionized water;eNOS, endothelial nitric oxide synthase.
L-NAME inhibited NO production by suppressing eNOS expression in aortic tissue (Figure 3B) and reducing plasma nitrate/nitrite level(Table 2). Treatment with SRH, Lis, or SRH+Lis significantly upregulated eNOS protein and elevated plasma nitrate/nitrite levels(P<0.05) (Figure 3B and Table 2).
3.3. Effect of SRH on the renin-angiotensin system
RAS plays an important role in the regulation of blood pressure. A dramatic increase in plasma ACE and AngⅡlevels and upregulation of ATR protein expression in the aorta were found in L-NAME hypertensive rats (P<0.05) (Figure 4). The data suggest a link between NO deficiency and RAS activation. Interestingly, SRH,Lis, or SRH+Lis markedly suppressed RAS activation in L-NAME hypertensive rats (P<0.05) (Figure 4).
3.4. Effect of SRH on vascular structural changes and remodeling
As shown in Figure 5, there were increases in the medial CSA, wall thickness, and M/L ratio of the aortas in the L-NAME-treated rats,when compared to those in the control groups (P<0.05) (Figure 5B,C and D). Additionally, L-NAME increased the number of VSMCs,decreased elastin, and increased collagen in the aortic wall (P<0.05)(Figure 5F, Figure 6C and D). The data indicate hypertrophic remodeling in this hypertension model. Treatment with SRH, Lis, or SRH+Lis significantly ameliorated the vascular remodeling seen in L-NAME hypertensive rats (P<0.05) (Figure 5 and Figure 6). There was no significant effect with respect to hypertrophic remodeling in the control rats treated with vehicle, SRH, or Lis.
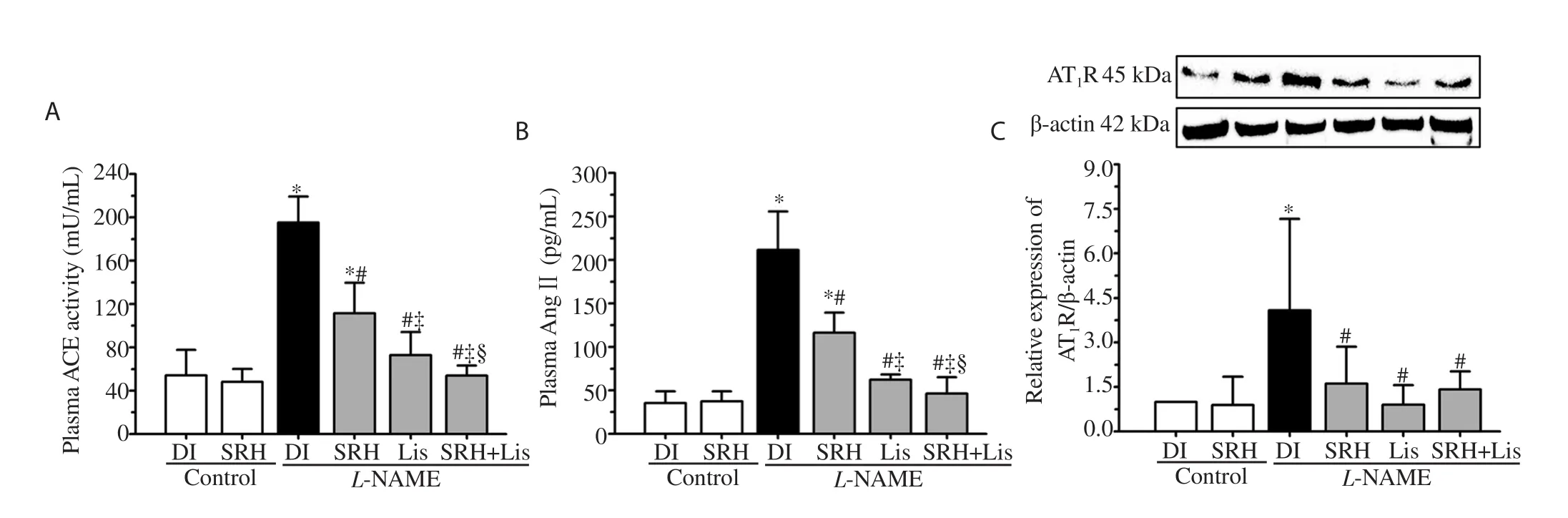
Figure 4. Effect of SRH on plasma ACE activity (A), plasma AngⅡconcentration (B) and AT1R expression (C) in aortas of control and nitric oxide deficient hypertensive rats. Data are mean ± SD of 4-6 rats. *P<0.05 versus the normal control group, #P<0.05 versus the L-NAME group, ‡P<0.05 versus the L-NAME+ SRH group, §P<0.05 versus the L-NAME + Lis group. ACE, angiotensin-converting enzyme; AngⅡ,angiotensinⅡ; AT1R, angiotensinⅡtype 1 receptor.
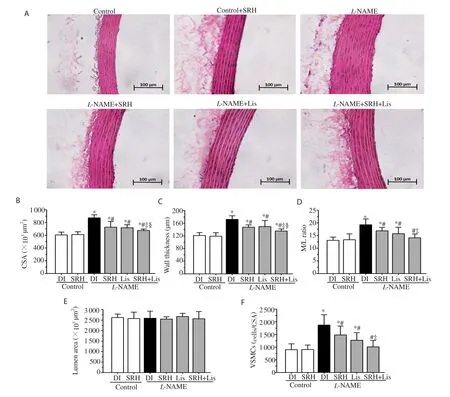
Figure 5. Effect of SRH on vascular structural changes in the aortic wall of control and nitric oxide deficient hypertensive rats. Representative photomicrographs of an aortic section (×200) stained with H&E (A) are shown together with histomorphometric data of the cross-sectional area (CSA) of aortic media (B), wall thickness (C), media to lumen ratio (M/L) (D), lumen area (E) and the number of vascular smooth muscle cells (VSMCs) per unit cross-sectional area (F). Scale bar 100 µm. Data are mean ± SD of 6 rats. *P<0.05 versus the normal control group, #P<0.05 versus the L-NAME group,‡P<0.05 versus the L-NAME + SRH group,§P<0.05 versus the L-NAME + Lis group.
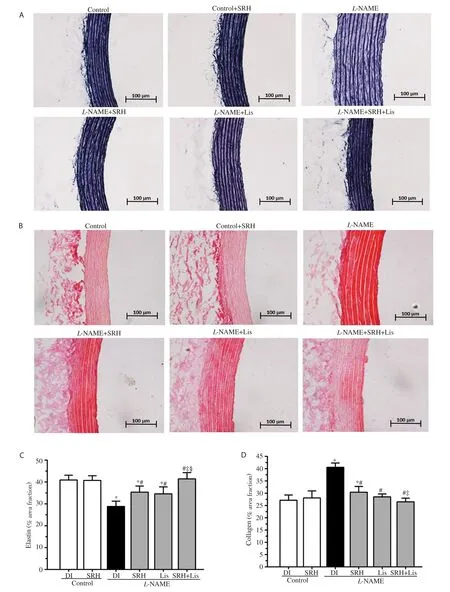
Figure 6. Effect of SRH on elastin and collagen in the aortas of control and nitric oxide deficient hypertensive rats. The representative photomicrographs of the aortic samples (×200) were stained with Miller's elastin (A) and picrosirius red (B) for assessment of area fractions of the elastin (C) and collagen (D)in the aortic medial layer. Scale bar 100 µm. Data are mean ± SD of 6 rats. *P<0.05 versus the normal control group, #P<0.05 versus the L-NAME group,‡P<0.05 versus the L-NAME + SRH group, §P<0.05 versus L-NAME + Lis group.
4. Discussion
In this study, we confirmed that chronic blockade of NOS by L-NAME in Sprague-Dawley rats increased arterial pressure,elevated peripheral vascular resistance, decreased endotheliumdependent vasodilation response to acetylcholine, increased the vasoconstriction response to phenylephrine, and caused vascular remodeling. The present study demonstrated that rice bran hydrolysates from Sang-Yod, pigmented rice (SRH), and ACE inhibitor, Lis, improved the hemodynamic status and vascular responsiveness and prevented the vascular remodeling in hypertensive rats. We found that the therapeutic effect of SRH or Lis is associated with an increase in NO bioavailability, alleviation of oxidative stress, and suppression of RAS activation. The combination therapy of SRH and Lis provides greater efficacy than either SRH or Lis alone.
Several lines of evidence suggest that the consumption of healthy foods and nutraceuticals with disease-preventing properties can play a major role in a national health strategy. Consequently,there has been growing interest in bioactive food components,including food-derived protein hydrolysates and peptides that have beneficial effects on human health[22]. In recent years, several pigmented rice varieties have become attractive, since they possess various bioactive compounds such as phenolics, flavonoids, and anthocyanin[17]. Although the bioactive peptides in SRH have not been separated and identified, we found that SRH consists of high protein and many types of polyphenols, including caffeic acid, catechin, ferulic acid, gallic acid, p-hydroxybenzoic acid,isoquercitrin, kaemferol, protocatechuic acid, quercetin, rutin, and tannic acid. Additionally, a recent study by our group found that SRH possessed antioxidant and free radical scavenging activities,as assessed by chemical-based assays including DPPH and FRAP assays[18]. Phenolic compounds possess several pharmacological activities including ROS scavenger and induction of cellular antioxidant systems[23]. Therefore, the antioxidative effect of SRH could be attributed to its phytochemical constituents. Interestingly,subchronic toxicity test of SRH showed no signs of toxicity or mortality in animals[18]. These data suggest the use of SRH for long-term treatment of hypertension.
Inhibition of NO synthesis and/or reduced NO bioavailability are important risk factors for both hypertension and cardiovascular disease. Therefore, L-NAME rats are used as the model of hypertension. In accordance with previous studies[24,25], we found that chronic blockade of NOS by L-NAME (50 mg/kg/day) for 6 weeks caused NO deficit and induced severe hypertension,endothelial dysfunction, and vascular remodeling. Previous studies reported that chronic L-NAME treatment led to high blood pressure and altered vascular reactivity to vasoactive agents as a result of decreased NO bioavailability[19,26]. Consistent with our findings,we found that the vascular response to endothelium-dependent vasodilator acetylcholine was attenuated while the response to vasoconstrictor phenylephrine was potentiated. Infusion of endothelium-independent vasodilator sodium nitroprusside, a NO donor, did not change the vascular reactivity; this is due to increases in NO bioactivity by nitroprusside itself. SRH and/or Lis improved hemodynamics by lowering blood pressure and heart rate and decreasing vascular resistance. Moreover, SRH and Lis restored endothelial function and vascular reactivity, and these effects are related to the increase in NO synthesis via upregulating eNOS and increasing plasma nitrate/nitrite level in L-NAME hypertensive rats. The data obtained from this study are in line with previous studies, which reported that rice bran hydrolysates from non-pigmented rice[10]and from pigmented rice bran[18]improved hemodynamics and increased NO bioavailability in 2K-1C and L-NAME hypertensive rats, respectively. Regarding the effect of ACE inhibitor Lis on the improvement of L-NAME-induced hypertension, our study is consistent with previous research, which reported that Lis and enalapril effectively reduced SBP, reversed the endothelial vasodilator dysfunction on norepinephrineprecontracted aortic rings, restored expression of eNOS mRNA in aortic tissue, and increased plasma nitrate/nitrite in L-NAME rats[27,28]. It is very interesting to demonstrate that a combination of SRH and Lis produced larger beneficial effects on hemodynamics and endothelial function. Considering that arginine is a substrate for both arginase and NOS, arginase activity may be a critical factor in NO bioavailability. Akinyemi et al. demonstrated that arginase activity was increased in L-NAME-induced hypertensive rats, and pre-treatment with ginger and turmeric rhizomes that are rich in phenolic compounds caused significant decreases in ACE and arginase activities with a concomitant increase in NO level[29].In this study, increased NO bioavailability and decreased ACE activity were found in L-NAME hypertensive rats after treatment with SRH. The effect of SRH might be involved with arginase inhibition.
In the present study, we found that increases of ACE and Ang-Ⅱin L-NAME rats may be due to L-NAME inducing renal vasoconstriction, vascular hypertrophy, and activation of the RAS[30,31]. A reduction in renal perfusion pressure secondary to renal arteriolar vascular constriction stimulates renin release and increases ACE and AngⅡin cortical tissues[32,33]. This supports RAS activation in the L-NAME hypertension model and SRH suppressed the activation RAS axis.
It has been reported that RAS activation induces vascular oxidative stress[34]. Inhibition of NO synthesis with L-NAME activated ACE activity and induced oxidative stress[35], and the ACE inhibitor Lis reduced oxidative injury in L-NAME hypertensive rats[28]. In this study, we found that SRH and/or Lis decreased RAS activation by reducing plasma ACE activity and AngⅡlevels. Furthermore, SRH and/or Lis decrease oxidative stress by reducing Oproduction, suppressing lipid and protein oxidation, increasing antioxidant GSH, and maintaining the normal redox state. The ameliorative effects of SRH and Lis are associated with the downregulations of gp91and ATR protein expressions in the aortic tissues. Again, combined therapy with SRH and Lis is more effective than monotherapy.
Vascular remodeling is a common feature in clinical and experimental hypertension. The alterations of the arterial wall structure may be an adaptive response to disturbance of hemodynamics. However, the sustained changes compromise the organ function, leading to cardiovascular complications of hypertension[36]. In this study, L-NAME hypertension produced arterial structural alterations and vascular remodeling in these hypertensive animals. Pathological changes in vascular remodeling lead to arterial wall thickening, as shown by increases in medial CSA and M/L ratio of the aortic wall. We also noticed the vascular wall hypertrophy, VSMC hyperplasia, increased collagen,and decreased elastin depositions in the aortas of L-NAME hypertensive rats, and these alterations are partially restored after treatment with SRH or Lis. Importantly, combination treatment with SRH and Lis completely restored vascular remodeling,suggesting the synergistic effects of the two compounds. Previous studies suggested that L-NAME-induced hypertension caused a depletion of NO formation, enhanced oxidative stress, and RAS activation, which in turn contributed to vascular remodeling[2,28,35].Therefore, we postulate that increased NO bioavailability, reduced oxidative stress, and suppressed RAS activation are potential mechanisms by which SRH and/or Lis reduce hypertension and vascular remodeling.
In conclusion, our study provides evidence to support that SRH is a potential antihypertensive and antioxidant agent for the therapy of hypertension. SRH exerts effects against high blood pressure,oxidative stress, endothelial dysfunction, and vascular remodeling in NO deficient hypertension. Moreover, SRH in combination with antihypertensive drugs are a promising therapeutics in the management of hypertension. Therefore, SRH may be used as an adjuvant in the treatment of hypertension.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgments
We would like to acknowledge Dr. Justin Thomas Reese for editing the manuscript via Publication Clinic KKU, Thailand. We are grateful to Dr. Sirithorn Siriamornpun for analysis of some phenolic compounds.
 Asian Pacific Journal of Tropical Biomedicine2021年1期
Asian Pacific Journal of Tropical Biomedicine2021年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Pharmacological aspects of fisetin
- Immunostimulatory role of rBmHSP60 from filarial parasite Brugia malayi
- Network pharmacology-based analysis of effective components and mechanism of Rhizoma coptidis in treating diabetes
- Identification of climatic and environmental factors associated with incidence of cutaneous leishmaniasis in Central Iran using satellite imagery
