Pharmacological aspects of fisetin
Lucia Dwi Antika, Rita Marleta Dewi
1Research Center for Chemistry, Indonesian Institute of Sciences, Banten, Indonesia
2Center for Research and Development for Biomedical and Basic Health Technology, National Institute of Health Research and Development, Ministry of Health, Indonesia
ABSTRACT
KEYWORDS: Fisetin; Anti-inflammatory; Anti-diabetic; Anticarcinogenic; Anti-osteoporosis; Cardioprotective activity
1. Introduction
In recent decades, natural products have been employed for the treatment of various chronic diseases. It has been reported that many countries, particularly developing countries, rely on their local natural products for medications[1]. The research of bioactivity from natural products has been on the rise due to the increased cases of chronic diseases. From the pharmaceutical point of view, plantderived compounds showed potential in regulating many processes in chronic diseases including inflammation, cancer, metabolic and degenerative diseases[2,3]. In this sense, the current review focuses on the biological properties of polyphenol fisetin in human health and the advancements of its use in the prevention and treatment of disease.
2. Fisetin
Fisetin (3,3',4',7-tetrahydroxyflavone) is a hydrophobic polyphenolic compound (molecular formula: CHO; molecular weight: 286.24 g/mol) primarily found in strawberry, and also in other fruits and vegetables including apple, blueberry, grape,persimmon, kiwi, onion, and cucumber[4]. It is predominantly present in the flesh of strawberry (160 µg/g)[5]. Fisetin was first isolated from Venetian sumach (Rhus cotinus L.) in 1833 and the chemical structure of fisetin is shown in Figure 1. Structurally,fisetin is identified to have two aromatic rings, which are linked through a three-carbon oxygenated heterocyclic ring, and is supplemented with four hydroxyl group substitutions and one oxo group[6,7]. A number of studies have reported that fisetin has a broad range of pharmacological properties such as antioxidant[8], antiinflammatory[9-12], antimicrobial[13,14], anti-osteoporotic[15], antidiabetic[10], and anti-carcinogenic[4]activities.
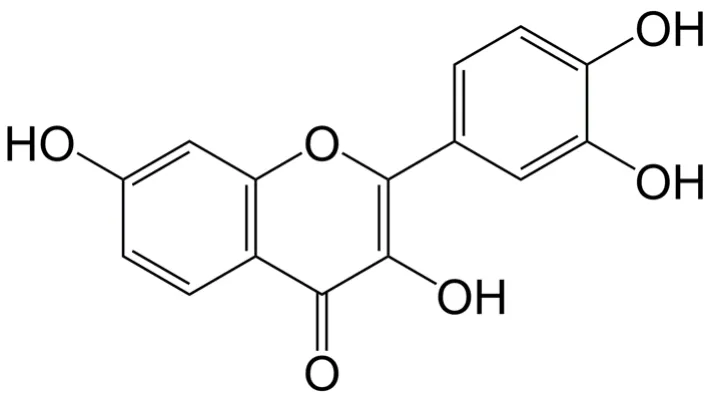
Figure 1. Chemical structure of fisetin.
3. Pharmacological activities of fisetin and its use for the prevention and treatment of disease
3.1. Antioxidant activity
Many pieces of literature revealed that diets that include strawberry and some other fruits could exert antioxidant properties[16]. Several studies reported antioxidant activity of fisetin by scavenging the free radicals 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium salt) (ABTS)[8,17]. Wang et al. reported that the ICvalues of fisetin for scavenging DPPH and ABTS were (9.69±0.53) µM and(2.43±0.14) µM, respectively[17]. The scavenging activity of fisetin was greater than positive controls butylated hydroxyanisole and Trolox, indicating that fisetin at a low concentration has the ability to scavenge free radicals and protect deoxyribonucleic acid (DNA).Reactive oxygen species (ROS) such as DPPH radical (DPPH) are converted into a stable DPPH-H molecule by taking up hydrogen donated by the carboxyl group. The capability of active compounds in donating their hydrogen atom resulted in the reduction of violetcolored DPPH solution into a yellow-colored DPPH-H molecule product[18,19]. The possibility of fisetin in stabilizing the radicals indicates antioxidant activity through ROS scavenging activities via hydrogen atom transfer mechanism[17]. In addition, a study by Basu et al. also suggested the antioxidant activity of fisetin from strawberry by elevating circulating antioxidant biomarkers, notably plasma antioxidant, plasma catalase enzyme, glutathione peroxidase and reductase enzymes, whole blood glutathione concentration and serum lipids in obese adults[20].
An in vivo study revealed that fisetin was found to have great potential by affecting antioxidant enzymes in a rat model with hepatocellular carcinoma. Male Charles Foster rats aged 18-20 weeks were induced with 1.0 mg/kg BW aflatoxin-B1 through intraperitoneal (i.p.) injection and orally administered with 20 mg/kg BW fisetin. Fisetin declined the expression of hepatic antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase. Superoxide dismutase catalyzes dismutation of Ointo a less toxic HO. Degradation of those antioxidant enzymes prevents oxidative stress-induced tumorigenesis. Fisetin also increased ROS production in hepatocellular carcinoma induced rats[21].Another study by Raygude et al. also disclosed that fisetin at the concentration of ≥ 10 mg/kg significantly reduced brain gamma aminobutyric acid along with nitric oxide and xanthine oxide in pentylenetetrazole and strychnine induced mice[22]. Intraperitoneal injection of pentylenetetrazole and strychnine resulted in tonic-clonic convulsions followed by mortality in mice. However, fisetin treated mice significantly delayed convulsions and decreased mortality in mice.
3.2. Anti-inflammatory activity
Polyphenols have been widely known to have potent antiinflammatory activities. Fisetin was reported to significantly decrease pro-inflammatory cytokines interleukin-1 beta (IL-1β) and IL-6 production in tumor necrosis factor-alpha (TNF-α) induced HaCaT cells. Fisetin was also found to block inducible nitric oxide synthase and cyclooxygenase-2 expressions, as well as NF-κB signal transduction activation in a dose-dependent manner[23]. NF-κB activation is widely known to be linked with inflammation; therefore,the ability of natural compounds to downregulate NF-κB related gene expression shows their potential as an anti-inflammatory agent.Another study by Park et al. found that inhibition of NF-κB signaling reduced the cytokine expression of IL-4, but not IL-8 in human mast cells (HMC-1)[12], suggesting that the anti-inflammatory activity of fisetin was mediated through regulation of NF-κB signaling[12,23].Furthermore, a report by Seo and Jeong also exhibited the potential of fisetin in increasing heme oxygenase-1 (HO-1) expression which plays an important role in cytoprotection, while inhibiting nitrite production.HO-1 is a cytoprotective protein regulated by the nuclear factor erythroid 2-related factor 2 (Nrf2)[23]. Nrf2 also regulates the cellular defence mechanism in human keratinocytes. In addition, fisetin also induced the translocation of Nrf2 to the nucleus and several transcription genes via Nrf-2/HO-1 pathway[23].
An in vitro study disclosed that fisetin has the potential to alleviate airway inflammation in ovalbumin (OVA)-induced asthmatic mice. A number of studies explained the potential effect of fisetin on airway inflammation and oxidative stress in asthmatic mice.Several studies reported that OVA-induced mice had more than 2-fold increase in total number of inflammatory cells including macrophage, neutrophil, and lymphocyte[24,25]. Hussain et al. also revealed the antioxidant and anti-inflammatory potential of fisetin against cigarette smoke-induced oxidative stress and inflammation in rat lungs[26]. Fisetin markedly altered secretion of inflammatory cytokines such as IL-4, IL-8, and TNF-α. In addition, fisetin reversed asthmatic mice and cigarette smoke-induced increases in inflammatory cell counts such as neutrophils, monocytes, especially eosinophils and white blood cells in bronchoalveolar lavage liquid[24,26].
Protective effects of fisetin against a systemic response to a severe infection, sepsis, were studied both in vitro and in vivo in primary human umbilical vein endothelial cells (HUVECs) and Male C57BL/6 mice, respectively. Fisetin showed a significant downregulation of high mobility group box-1 (HMGB1)-mediated membrane disruption in HUVECs in a dose dependent manner and the in vitro experiment further confirmed the results of in vivo study. Yoo et al. showed that fisetin induced a significant inhibition of the peritoneal leakage of dye in HMGB1 protein-induced sepsis model[27]. Moreover, fisetin also retarded HMFB1-mediated hyperpermeability and leucocyte migration in the septic model. It is believed that fisetin plays a role in regulating mitogen-activated protein kinase pathway, mainly by inhibition of p38 expression[27].
3.3. Antimicrobial activity
Many studies provide a strategy for developing antimicrobial agents from natural products, notably from dietary polyphenolic compounds that have been suggested to possess robust antimicrobial activity. A number of studies reported antimicrobial activity of fisetin. Fisetin is able to inhibit Gram-positive bacteria particularly Listeria monocytogenes (L. monocytogenes) ATCC 19115 and Streptococcus suis[28,29]. L. monocytogenes, a deadly foodborne pathogen, employs various surface proteins and virulence factors to subvert host cell functions. Listeriolysin O (LLO) is one of the most important virulence factors in L. monocytogenes that mediates the disruption of the vacuolar membrane in host cells, leading to membrane lysis[30]. Dependent on molecular modeling, fisetin was revealed to directly engage loop 2 and loop 3 of LLO, leading to the blockage of cholesterol binding. Additionally, fisetin blocked LLO oligomerization and inhibited hemolytic activity of L. monocytogenes.A previous study conducted by Wang et al. also reported that fisetin at a concentration of 1.51 µM inhibited 50% of LLO expression which was 5-times lower than cholesterol, a natural inhibitor of LLO[27,28,31].
The previous study disclosed that fisetin protected mice against L.monocytogenes infection. A total of 75% of mice that were infected with 2 × 10L. monocytogenes through intravenous inoculation were killed within 60 h. However, fisetin-treated mice survived longer than untreated mice with 43.33% of them that were dead after 84 h. Furthermore, observation of mice organs revealed that numerous spotty necroses were spotted in the liver tissue of untreated mice.Accumulation of bacteria was also found in the section of liver specimens from untreated mice. Whereas, tissues and organs of fisetin-treated mice only showed mild inflammatory lesions and the levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α were lowered in livers and spleens of treated mice[28].
Fisetin showed its antimicrobial potential in inhibition of haemolytic activity of suilysin, an essential virulence factor of a serious zoonotic pathogen Streptococcus suis serotype 2[29,32].Suilysin works in the same mechanism with LLO which bypasses through epithelial barriers and invades various organs, causing the necrosis of tissues or organs[20,33]. Fisetin at the concentration of ≥32 µg/mL attenuated Streptococcus suis strain SC19 proliferation in J774A.1 and Raw 264.7 macrophages. In addition, the number of bacteria found in the blood tissues and organs (brains, livers, and spleens) in fisetin-treated female BALB/c mice was significantly lower than that of untreated mice. These findings indicate that fisetin may be used as an antimicrobial agent by inhibiting the hemolytic activity of bacterial virulence factors[29].
3.4. Anti-carcinogenic activity
Despite advancements in modern therapeutic approach for diagnosis and treatment, cancer still remains the leading cause of mortality worldwide and its prevalence is still growing continuously in both developed and developing countries[34,35]. The use of polyphenolic compounds from plant-derived products has been reported to be therapeutically effective against malignancies, and the modulation of cancer signaling pathways by the compounds has been identified. Various studies have revealed the anti-cancer roles of fisetin against different types of cancer cells in experiments, which include inducing endoplasmic reticulum and mitochondrial stress[36],promoting autophagy and apoptosis in cancer cells[36-38], regulating cell growth and proliferation[39,40], inhibiting the epithelial to mesenchymal transition (EMT)[40], and remodeling of extracellular matrix[41].
A number of studies have reported that fisetin not only inhibits the growth and metastasis of cancer cells but also induces its apoptosis through multiple signaling pathways. Kang et al. showed that fisetin causes apoptosis in human non-small cell lung cancer cell line (NCI-H460) through the mitochondrial-dependent pathway[38].The mitochondrial pathway is activated by intracellular signals under cellular stress conditions. Mitochondrial outer membrane permeabilization is a critical step of apoptosis which triggers the activation of caspases and cytochrome C release[42]. Nuclear staining with Hoechst 33342 dye showed that treatment with 75 µg/mL of fisetin induced DNA fragmentation on NCI-H460 cells which is a characterization of apoptotic cells. Additionally, Western blotting analysis of pro-apoptotic proteome displayed fisetin markedly increased the protein level of Bax with a subsequent decrease in the level of anti-apoptotic Bcl-2 protein[38]. Another group of study found potential anti-carcinogenic activity of fisetin on benzo(a)pyrene (B(a)P)-induced lung carcinogenic mice. B(a)P is a group of polycyclic aromatic hydrocarbons, which is contained in tobacco,and is able to induce DNA mutation and cancer[43,44]. Six to eight weeks old of male Swiss albino mice were orally administrated with 50 mg/kg BW B(a)P twice a week for sixteen weeks to induce lung cancer. Consequently, treatment with 25 mg/kg BW of fisetin in B(a)P-induced mice resulted in increased expression of Bax with a decrease in the expression of Bcl-2. Fisetin also markedly increased the expression of cytochrome c, active (cleaved) pro-apoptotic caspase-3 and -9, which was decreased due to the continuous exposure to B(a)P[45].
A recent study by Guo et al. discovered that fisetin at the concentration of 10 µM displayed chemopreventive activity by downregulating overexpressed human epidermal growth factor receptor 2 (HER2) protein via PI3K/Akt and ERK signaling pathways in MDA-MB0453 breast cancer cell line[46]. HER2 is a proto-oncogenic tyrosine kinase receptor encoded by HER2/new gene and its overexpression induces carcinogenesis. Treatment with fisetin also induced DNA fragmentation, whereas it decreased Akt activity which is correlated with the survival of breast cell cancer observed by AO/EtBr and MTT assay, respectively[46]. Another study by Sun et al. revealed that fisetin exhibited anti-tumour potency against breast cancer cells by reducing the number of viable breast cancer cells (4T1, MCF-7, and MDA-MB-231)[47]. Western blotting analysis displayed that fisetin markedly reduced the overexpression of Akt, mammalian target of rapamycin (mTOR), PI3K, and BclxL in 4T1 cells. mTOR is a downstream protein regulated by Akt that regulates cell proliferation and translation in response to growth factors[48,49]. This result is consistent with that found in the study of Guo et al. about the capability of fisetin in regulating tumour cell proliferation, differentiation, and apoptosis through PI3K/Akt/mTOR signaling pathway[46,47]. The PI3K/Akt/mTOR signaling cascade is found to express excessively in around 70% of breast cancer[50],as well as other types of cancer such as lung, prostate, myeloma,melanoma, and colon cancer[51]. Thus, signaling pathway is considered as a targeted therapy for cancer diagnosis and treatment.Ninety percent of cancer-related mortalities are attributable to the spread of cancer cells (metastasis) instead of primary tumour growth. Cancer metastasis may be contributed by the EMT as many of EMT marker genes are found to be connected to metastasis[52].EMT lets the tumour cells lose their epithelial characteristics and gain a mesenchymal phenotype[53]. This condition allows the cancer cells to detach from primary site, migrate into the surrounding normal tissues (local invasion), and invade into lymphatic and blood system, thus form secondary tumours at a distant site[53,54]. Li et al. showed that fisetin is a promising candidate treatment against cancer due to its activity in attenuating EMT, which can block cancer cell invasion[40]. Fisetin inhibits the markers of EMT, such as MDR1, in WM35, A375, and 451Lu melanoma cells in a dosedependent manner through disturbing YB-1/RSK signaling[55]. The p90 ribosomal S6 kinase (RSK) is a downstream process of MAPK pathway that is correlated with various cellular processes, including cell growth and motility[55].
Moreover, EMT is also driven by various transcription factor genes,such as Snail, Twist1, Slug, and ZEB1. Those transcription factors are correlated with cadherin expression, a malignancy parameter for cancer progression and metastasis, and are regulated by a number of signaling pathways, including MAPK and PI3K[56,57]. Activation of MAPK and PI3K pathway activates the downstream transcription factors Snail and Slug to promote EMT, however, fisetin at the concentration of 100 mg/kg effectively inhibits the expression of those mentioned transcription factors in human triple-negative breast cancer cell lines MDA-MB-231 and BT549[40]. Fisetin treatment in human melanoma cells (A375, RPMI-7951, and Hs294T) also displays great inhibition against mesenchymal marker proteins such as Snail, vimentin, and N-cadherin. Therefore, EMT inhibition may become an alternative strategy for cancer treatment[58].
Pal et al. found that fisetin was able to reduce the expression of overexpressed matrix metalloproteinase-2 and -9 (MMP2 and MMP-9) in human malignant melanoma cells (A375)[59]. MMPs play an important role in remodeling extracellular matrix and its dysregulation leading to tumour metastasis[60]. Other studies also reported potential activity of fisetin in treating cancer metastasis by inhibiting mRNA and protein expression levels of MMPs in PC-3 prostate cancer cells[61], human fibrosarcoma HT-1080 cells[62], breast cancer cell line (4T1 and JC cells)[63], and human colon carcinoma cell lines (HCT116 and HT29)[64]. Furthermore,a recent clinical study by Farsad-Naeimi et al. also revealed that fisetin supplementation suppressed MMP-7 level, but not MMP-9 expression in colorectal cancer[65].
3.5. Osteoprotective activity
Bone loss diseases, including osteoporosis, have become the major health problem both in developed and developing countries. It is associated with the imbalance of bone remodeling process favouring osteoclastic bone resorption over osteoblastic bone formation,which leads to bone loss[66]. Bone loss diseases are considered as a worldwide concern as it causes more than 8.9 million fractures every year[67].
The studies on the role of fisetin in bone loss may provide an excellent alternative strategy to overcome bone loss related diseases.Kim et al. documented the osteoprotective potential of fisetin[15].Fisetin was found to disturb osteoclast cytoskeletal organization by inhibiting actin ring formation in receptor activator of nuclear factorkappaB ligand (RANKL)-induced osteoclast[15]. RANKL enables osteoclasts to be differentiated from their precursor macrophages.The interaction between RANKL and RANK on osteoclast activates the formation of multinucleated osteoclasts[68,69]. Treatment with fisetin at the concentration of ≥ 5 µM elicited osteoprotective activity through retarding osteoclast activation and inhibiting gelsolin expression in RANKL-treated macrophages[15]. Gelsolin is an actin-binding protein that plays a pivotal role in actin assembly and disassembly[70]. Fisetin also markedly suppressed the induction of carbonic anhydrase Ⅱ (CA Ⅱ) and β3 integrin cellular level. CA Ⅱand β3 integrin are known to responsible for acidification of lacuna and sealing zone formation that are essential for bone resorption activity of osteoclast. Failure of acidification and sealing zone formation as a result of fisetin treatment led to a visible reduction of osteoclast maturation and bone-resorbing activity[71]. In addition,fisetin significantly reduced the upregulated downstream signal of TNF receptor-associated factor 6 (TRAF6) in OVA-induced mice which finally resulted in inhibition of pro-inflammatory cytokine releases[24]. TRAF6 is a crucial signaling molecule that regulates a wide range of biological processes, including tissue development,immunity system, and bone metabolism[72]. It is suggested that polyphenol fisetin abrogated RANKL induction by disturbing cell fusion and cytoskeletal organization of mature osteoclast through RANK-TRAF-6 pathway leading to failure in bone resorption[15].
Cell fusion is essential in the maturation of osteoclast. Hence the cells can perform the specific function of bone resorption activity[73,74]. In addition, osteoclast degrades bone matrix by releasing acid and proteolytic enzyme Cathepsin K[75,76]. The secretion level of Cathepsin K was increased in RANKL-induced macrophages. The expression of Cathepsin K in cellular level was also regulated by transcription factors, including nuclear factor of activated T cells 1 (NAFTc1) and p38, which were down-regulated in the presence of ≥ 5 µM of fisetin. These findings indicate fisetin inhibits mature osteoclast formation by regulating NF-κB activity via down-regulation of p38-c-fos-NFATc1 signaling pathway[77,78].
3.6. Anti-diabetic activity
In recent decades, researchers have proved that natural compounds isolated from medicinal plants have potential as anti-diabetic drugs.Flavonol groups including quercetin, naringenin, and hesperidin have been reported to involve in glycolysis and gluconeogenesis[79-81]. Fisetin,which is structurally similar to quercetin, also has been investigated for its anti-diabetic potential. A few studies have reported the potential activity of fisetin in displaying hypoglycaemic activities both in vitro[82]and in vivo[83,84]. An in vitro study by Kim et al. revealed treatment of fisetin could inhibit the expression of proinflammatory cytokines TNF-α and IL-6 in high glucose-treated-THP-1 monocytes[10]. Another study also reported that fisetin affected liver metabolism and glucose input in vitro. Fisetin inhibited pyruvate carboxylation and decreased NAD/NADratio leading to gluconeogenesis inhibition[82].Furthermore, fisetin may prevent hyperglycaemia by blocking glycogenolytic ability of hormones, and thus potentially works as an anti-diabetic agent.
An in vivo study by Prasath et al. reported anti-diabetic potential of fisetin on streptozotocin (STZ)-induced diabetes in male albino rats[83]. STZ increased hydroxyl radical production leading to oxidative damage of DNA, protein, and lipids and caused the necrosis of pancreatic β-cells. Diabetic rats displayed significant increases in food and water intake compared to the control group. Animals that were treated with fisetin at a dose of 10 mg/kg BW/day for 30 d markedly reduced blood glucose and haemoglobin A1c (HbA1c),as well as increased plasma insulin concentration. Moreover, fisetin prevented hyperglycaemia by downregulating glycogen breakdown and increasing NF-κB p65 unit and IL-1β[84]. Fisetin also decreased the mRNA and protein levels of pivotal gluconeogenesis enzymes,phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) in hepatic tissue of STZ-diabetic rats. PEPCK catalyses the conversion of oxaloacetate to phosphoenolpyruvate,whereas G6Pase hydrolyses glucose-6-phosphate to glucose[85,86].Overall, these researches suggested that fisetin may improve glucose homeostasis by inhibiting gluconeogenesis and may be potentially useful as an anti-diabetic agent.
3.7. Antihypertensive and cardioprotective activity
Hypertension, or high blood pressure, is one of the most significant risk factors for cardiovascular mortality across the world.Hypertension increases the possibility of health complications,including myocardial infarction, renal hypertension, and sudden cardiac arrest[87]. It has been reported that there were 17.5 million death cases due to cardiovascular diseases and its complications[88].Evidence from a number of in vitro and in vivo studies indicates that the consumption of fisetin from edible plants was associated with prevention of hypertension-related cardiovascular diseases through vasorelaxant activity[89-92]. Chen et al. have studied the protective activity of fisetin on H9c2 cells and spontaneous hypertension rats[89]. It was found that 50 µM (EC) of fisetin attenuated angiotensin Ⅱ-induced cellular hypertrophy on H9c2 cells.Angiotensin Ⅱ is a hormone that regulates sympathetic nervous stimulation, renal action, and causes vasoconstriction. It is widely known that angiotensin Ⅱ is responsible to raise blood pressure that leads to increased risk of cardiovascular-related diseases including myocardial infarction, hypertension, and ventricular hypertrophy[90].Treatment with fisetin attenuated elevated systolic, diastolic, and mean artery pressure in spontaneous hypertension rats[89,91]. Je et al. also reported that oral administration of fisetin induced vascular relaxation on agonist-induced vascular contraction mainly related to pERK1/2 and ERK signaling[92].
Myocardial infarction is a condition where the heart lacks oxygen because of the blockage of coronary artery. This condition leads to the irreversible death or necrosis of the heart muscle. A recent study by Shanmugam et al. reported that fisetin conferred cardioprotective properties against ischemia/reperfusion (I/R) injury (IRI)[93]. Fisetin at a concentration of 20 mg/kg attenuated I/R-induced myocardial tissue injury in an animal model with ischemia. Additionally,biochemical assay consistently showed similar results with molecular docking study which displayed that fisetin inhibited oxidative stress and augmented mitochondrial biogenesis through regulating glycogen synthase kinase 3β (GSK-3β). Another study also evaluated the potential activity of fisetin in attenuating myocardial damage in diabetic rats. Acute myocardial infarction is also well known to be associated with diabetes mellitus-related complications.Althunibat et al. observed the potential activity of fisetin against the circulating levels of the cardiac function markers, such as creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH), and troponin-I(cTnI) in STZ-induced diabetic rats[94]. Treatment with fisetin for 6 weeks effectively reduced the elevated CK-MB, LDH, and cTnI.Haematoxylin and eosin staining further confirmed that the treatment with fisetin effectively alleviated the myocardial injury in the heart of diabetic rats, showing its therapeutic potential for cardiovascular diseases[94].
3.8. Senolytic effect and neuroprotective activity of fisetin
Senolytic is a compound that encourages damaged senescent cells to destroy themselves thereby extending health span[95]. Cellular senescence is associated with age-related diseases, including cardiovascular diseases, neurodegenerative diseases, T2DM,osteoarthritis, atherosclerosis, and various types of tumours[95-99].Fisetin has been shown to exert anti-osteoporotic activity in a number of experimental studies, however, only a few studies explained its capacity in modulating cellular senescence. Several study displayed a senotherapeutic effect of fisetin in extending the average lifespan of Saccharomyces cerevisiae and Drosophila melanogaster by 55% and 23%, respectively[100,101].
Yousefzadeh et al. showed that fisetin was one of potent senolytic flavonoid compounds both in vitro and in vivo[102]. Flavonoid polyphenols, including fisetin, were screened for their senolytic effect using murine embryonic fibroblasts and primary HUVECs[102].Fisetin at a dose of 5 µM was found to effectively reduce a senescence marker, SA-β-gal, without affecting cell numbers.Meanwhile, fisetin selectively induced apoptosis in senescent cells,but not in proliferating HUVECs as measured by caspase 3/7[102,103].It was also displayed that fisetin intervention in progeroid and old mice reduced senescence markers in multiple tissues. Ercc1progeroid syndrome mice treated with fisetin showed a significant decrease of senescence-associated secretory phenotype factors in multiple organs such as spleen, liver, and kidney[102]. These results showed fisetin's effect on senescence markers and age-related histopathology[102].
In addition, several studies also reported that fisetin possesses neuroprotective activity by activating key neurotrophic factor signaling pathways[104,105]. Neurological disorders are associated with multiple factors leading to disruption of the cellular system.Metabolic diseases, particularly diabetes and its complications, have been known to have an impact on the brain microvascular system,leading to impairment in central nervous system[106,107]. Sagara et al. disclosed that fisetin promoted the differentiation of PC12 cells through Ras-ERK pathway activation[104]. This pathway is a pivotal signaling pathway in nerve cells and its activation is related to neuroprotective activity[108]. Moreover, fisetin prevented the behavioural and pathophysiological changes related to Alzheimer's disease (AD) and dementia. Fisetin-fed AD mice behaved indistinguishably from wild-type mice, compared to AD mice[105].AD mice spent 16% of their time in target quadrant, showing developed memory loss, while fisetin-fed mice demonstrated the ability to recall the platform location[105]. All these studies indicate that fisetin may have positive effects on neurological disorders.
4. Conclusion
This current review briefly summarized the potential pharmacological properties of fisetin. Various scientific evidence has documented that dietary compound fisetin demonstrates a wide range of biological and therapeutic activities against chronic diseases such as cancer, metabolic diseases, and degenerative diseases. Although a large number of studies have reported that fisetin may serve as a potential agent for the prevention and treatment of a number of diseases through in vitro and in vivo experiments, further studies are required to evaluate its potential use in human clinical trials.
Conflict of interest statement
The authors declare no conflict of interest.
 Asian Pacific Journal of Tropical Biomedicine2021年1期
Asian Pacific Journal of Tropical Biomedicine2021年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Immunostimulatory role of rBmHSP60 from filarial parasite Brugia malayi
- Sang-Yod rice bran hydrolysates alleviate hypertension, endothelial dysfunction,vascular remodeling, and oxidative stress in nitric oxide deficient hypertensive rats
- Network pharmacology-based analysis of effective components and mechanism of Rhizoma coptidis in treating diabetes
- Identification of climatic and environmental factors associated with incidence of cutaneous leishmaniasis in Central Iran using satellite imagery
