Ni, Co-Based Selenide Anchored g-C3N4 for Boosting Photocatalytic Hydrogen Evolution
Zhiliang Jin, Yanbing Li , Xuqiang Hao
School of Chemistry and Chemical Engineering, Key Laboratory for Chemical Engineering and Technology, State Ethnic Affairs
Commission, Ningxia Key Laboratory of Solar Chemical Conversion Technology, North Minzu University, Yinchuan 750021, China.
Abstract:Developing novel and efficient catalysts is a significant way to break the bottleneck of low separation and transfer efficiency of charge carriers in pristine photocatalysts. Here, two fresh photocatalysts, g-C3N4@Ni3Se4 and g-C3N4@CoSe2 hybrids, are first synthesized by anchoring Ni3Se4 and CoSe2 nanoparticles on the surface of well-dispersed g-C3N4 nanosheets. The resulting materials show excellent performance for photocatalytic in situ hydrogen generation. Pristine g-C3N4 has poor photocatalytic hydrogen evolution activity(about 1.9 µmol·h−1)because of the rapid recombination of electron-hole pairs.However, the hydrogen generation activity is well improved after growing Ni3Se4 and CoSe2 on the surface of g-C3N4, owing to the unique effect of these selenides in accelerating the separation and migration of charge carriers. The hydrogen production activities of G-C3N4@Ni3Se4 and g-C3N4@CoSe2 are about 16.4 µmol·h−1 and 25.6 µmol·h−1, which are 8-fold and 13-fold that of pristine g-C3N4, respectively.In detail, coupling Ni3Se4 and CoSe2 with g-C3N4 greatly improves the light absorbance density and extends the light response region. The photoluminescence intensity of the photoexcited Eosin Y dye in the presence of g-C3N4@Ni3Se4 and g-C3N4@CoSe2 is weaker than that in the presence of pure g-C3N4. On the other hand, the upper limit of the electrontransfer rate constants in the presence of g-C3N4@Ni3Se4 and g-C3N4@CoSe2 is greater than that in the presence of pure g-C3N4. Among the g-C3N4@Ni3Se4@FTO, g-C3N4@CoSe2@FTO, and g-C3N4@FTO electrodes, the g-C3N4@FTO electrode has the lowest photocurrent density and the highest electrochemical impedance, implying that the introduction of CoSe2 and Ni3Se4 onto the surface of g-C3N4 enhances the separation and transfer efficiency of photogenerated charge carriers. In other words, the formation of two star metals selenide based on g-C3N4 can efficiently inhibit the recombination of photogenerated charge carriers and accelerate photocatalytic water splitting to generate H2. Meanwhile, the right shift of the absorption band edge effectively reduces the transition threshold of the photoexcited electrons from the valence band to the conduction band. In addition, the more negative zeta potential for the g-C3N4@Ni3Se4 and g-C3N4@CoSe2 catalysts as compared with that for pure g-C3N4 leads to a notable enhancement in the adsorption of protons by the sample surface. Moreover, the results of density functional theory calculations indicate that the hydrogen adsorption energy of the N sites in g-C3N4 is −0.22 eV; further, the hydrogen atoms are preferentially adsorbed at the bridge site of two selenium atoms to form a Se―H―Se bond, and the adsorption energy is 1.53 eV. In-depth characterization has been carried out by transmission electron microscopy, scanning electron microscopy, X-ray photoelectron spectroscopy, X-ray diffraction,ultraviolet-visible diffuse reflectance spectroscopy, transient photocurrent measurements, and Fourier transform infrared spectroscopy; the results of these experiments are in good agreement with one another.
Key Words:Ni3Se4; CoSe2; g-C3N4; Hydrogen evolution
1 Introduction
Converting solar energy to clean energy to reduce dependence on fossil fuel and environmental pollution has attracted much attention1,2. Photo-catalytic and photoelectrocatalytic systems are recognized as one of the best choices for splitting water to generate hydrogen and oxygen to alleviate pressing needs3.However, how to improve the efficiency of separation and migration for photoproduced charge carriers still remains an open question4. Up to now, a vast majority of studies have shown that most single photocatalysts could not achieve highly photocatalytic activity because of their inherent band-gap structure and rapid recombination of electron-hole pairs5. It is an effective way to break this bottleneck by synthesizing a composite catalyst with a matching band gap and an ideal contact interface for separation and transfer of charge carriers. Clean,environmentally friendly, practical and low-cost hydrogen energy generated by solar-driven water-splitting not only has an important impact on mitigating energy crises and environmental problems, but also on ecological and economic development6,7.In order to realize this very promising goal, a lot of joint efforts have been made to develop effective photocatalysis semiconductor materials for improving hydrogen evolution reaction.
In the past few decades, there were many new materials with high catalytic performance have been successfully designed and built in the fields of photocatalysis and electrocatalysis.However, the development of sustainable and stable semiconductors for efficient photo-catalytic water-splitting today is still attracting great attention8, because this goal of solar-chemical energy and solar-electric energy is far from what people expected. In addition, if a photocatalyst can capture more photons to generate more electron-hole pairs, minimize the recombination of the electron carriers, enable the electrons to quickly separate and transfer and obtain a matching redox potential, it may drive water-splitting to produce more hydrogen9.The catalytic activity of numerous photocatalysts that have been reported for hydrogen evolution still suffered from many limitations10,11. Therefore, designing and developing effective procatalysts and efficient cocatalysts still faces many challenges12.
N-type graphitic carbon nitride as a metal-free polymer photocatalyst with a graphitic structure and a suitable band gap(Eg= 2.7 eV)for a visible light response has recently attracted extensive research interest, and it is a semiconductor that combines green energy and environmentally friendly ideas(metal-free, excellent chemical and physical stability, simple preparation, non-toxic and low cost and so on)13–15. Haoet al.reported the heterostructure constructed by combining g-C3N4with ZnS having a lot of zinc vacancy defects on its surface to provide more active sites and efficiently promote separation of charge carriers16. Physical properties, chemical performances and photo-catalytic activities of g-C3N4were modified by loading Cu2O nanoparticles (NPs)on its surface under visible light irradiation17. Ultra-thin g-C3N4nanosheets could also be used as a co-catalyst to modify porous BiVO4 to significantly enhance the photo-current density and hydrogen production rate by 7–12 times for BiVO418. N-CeOxNPs (nitrogen-doped CeOxnanoparticles)modified graphitic carbon nitride semiconductors showed that enhanced photo-catalytic H2-generation activity for the N-CeOx/g-C3N4hetero-structure is due to the boosted separation and transfer of charges between graphitic carbon nitride and N-CeOxas resulted from the close interface contact and Type II band alignment19. Wanget al.20reported that a rodlike g-C3N4/ZnS material could effectively degrade methyl orange under simulated sunlight irradiation. Pt/2D/2D C3N4/MoS2heterojunction exhibited more efficient electrocatalytic activity compared with traditional electrocatalytic reactions for the oxidation of methanol (6.5-fold), ethanol (2.2-fold), and formic acid (2.5-fold)under visible light irradiation due to the excellent photoelectric synergy and the effective electron separation in this two-dimensional hetero-junction21. Ni-Mo-S@C3N4complex showed around 14-fold more efficient photo-catalytic activity of H2-generation compared with pure g-C3N4 due to more active sites exposed, excellent synergistic effect between Ni-Mo-S and g-C3N4and improved efficiency of separation and transfer for charge carriers22. These modifications to graphitic carbon nitride in the fields of photoelectrocatalysis demonstrate the advantages of g-C3N4as a promising semiconductor.
However, alone graphitic carbon nitride also has disadvantages that are not conducive to photocatalytic reactions,such as poor light absorption, small specific surface area and high electron-hole recombination rate under visible light irradiation,etc. So developing new and effective co-catalysts can remedy this deficiency and also can significantly promote the evolutionary efficiency of hydrogen because recombination of electron-hole pairs is greatly suppressed, the band gap structure is effectively regulated and the optical properties of the composite material can be prominently improved23.
Recently, Wanget al.24reported that successfully synthesized metal selenides NiSe2used as a co-catalyst decorated CdS nanorods greatly increases hydrogen evolution activity. After the Ni-Bi-Se complex modified ultra-thin graphitic carbon nitride nanosheets with nitrogen defects, the photo-catalytic activity of Ni-Bi-Se@g-C3N4 sample was obviously enhanced under the condition of EY (Eosin Y)as a photosensitizer with visible light25.A new type Z-scheme heterojunction (In2O3-ZnIn2Se4)containing metal selenide was successfully designed to improve hydrogen production and carbon dioxide reduction performance26. A nanocage-like NiS2/NiSe2 heterostructure with rich-phase boundaries were successfully prepared through a simultaneous sulfuration/selenylation process using Ni-based acetate hydroxide prisms as precursor, tetrahedral NiS2/NiSe2 heterocages can expose more active sites with dual-phase synergy for efficiently triggering the oxygen evolution reaction27.The heterostructure combining NiSe2and Ni2P coupled nanowrinkles on Ni foam was synthesized by successive selenization and partial phosphorization treatments, which could promote the water adsorption process and speed up the whole catalytic kinetics for overall water splitting28. The dual-cation(Fe, Co)-incorporated NiSe2nanosheets (Fe, Co-NiSe2)was synthesized, the dual-cation incorporation can distort the lattice and induce stronger electronic interaction, finally the obtained Fe0.09Co0.13-NiSe2porous nanosheet electrode showed an optimized catalytic activity for overall water splitting29. The asprepared NiSe/NF, an efficient and stable 3D bifunctional electrode, exhibited high activity for full water splitting30.
As an antenna molecule, dye sensitizer plays an important role to collect energy, similar to the role of chlorophyll and carotene in natural photosynthesis. Therefore, the dye molecule is very important for hydrogen production from sensitized water based on the following advantages: (1)The dye molecule has a wide spectrum absorption range and a large molar absorption coefficient; (2)The energy of the lowest unoccupied orbit(LUMO)is higher than the energy of the edge of the conduction band of the semiconductor, which is conducive to the electron transfer of the light-excited dye; or the energy levels of the semiconductor and the dye have good orbit overlap, which is conducive to the energy transfer; (3)The stability of light and heat is good, and the regeneration ability of excited state or oxidized state is good under the action of electron sacrificial agent; (4)It can bond with the surface of semiconductor and other matrix materials firmly and effectively.
In this report, we successfully synthesized two composite photocatalysis materials g-C3N4@Ni3Se4and g-C3N4@CoSe2to promote the photo-catalytic hydrogen evolution reaction. The resulting g-C3N4@Ni3Se4and g-C3N4@CoSe2samples can effectively suppress the recombination of electron-hole pairs for enhancing photo-catalytic H2-evolution and extending the absorption range of visible light, thereby the efficiency of charge separation and transfer is improved. This photo-catalytic hydrogen evolution activity of g-C3N4@Ni3Se4and g-C3N4@CoSe2, which is more efficient than pure g-C3N4in the EY sensitization system, is mainly attributed to the excellent physicochemical properties of g-C3N4 and the close interface contact as well as interaction between graphitic carbon nitride and metal selenide.
2 Experimental section
All reagents are analytical grade and could be used without further purification. Urea (CH4N2O, Aladdin, CAS:57-13-6,Lot# C1806145, AR 99%), selenium powder (Se, Tianjin Damao Chemical Reagent Factory, CAS NO: 7782-49-2, AR), nickel nitrate (Ni(NO3)2∙6H2O, Tianjin Kaitong Chemical Reagent Co.,Ltd., AR > 98%), cobalt nitrate (Co(NO3)2∙6H2O, Aladdin,C112729, Lot # D1916072, CAS: 10026-22-9, AR 99%),triethanolamine (N(CH2CH2OH)3, Tianjin Damao Chemical Reagent Factory, AR > 99%), Eosin Y (C20H6Br4Na2O5, Aladdin,CAS: 17372-87-1, Lot# F182260).
2.1 The preparation of g-C3N4
Appropriate amount of urea was calcined in a muffle furnace at 550 °C for 4 h and cooled naturally to room temperature.Subsequently, the as-prepared yellowish solid (g-C3N4)was grinded to powder and collected. All operations were carried out at room temperature.
2.2 The preparation of g-C3N4@Ni3Se4 and g-C3N4@CoSe2
The specific preparation process of g-C3N4@Ni3Se4 was as follows: 30 mg of selenium powder and 110 mg of sodium borohydride were added to a 100 mL beaker containing 50 mL of purified water, followed by stirring with slow heating until the solution became completely clear and transparent. At this time,300 mg of the prepared g-C3N4was added to the above transparent solution and completely dispersed in the solution by using an ultrasound system, followed by stirring for further 30 min to ensure that the mixed solution became more uniform.Then, 75 mg of nickel acetate was added into the mixed system,followed by stirring for 30 min again to make the reaction proceed sufficiently. Finally, the mixed system was kept at 80 °C until the moisture completely dried, and then the sample was ground and washed several times, followed by drying again. The complexes g-C3N4@Ni3Se4containing different amounts of nickel was named CN@NS-1, CN@NS-3, CN@NS-5 and CN@NS-7, respectively. And the specific addition amount of each sample is shown in Table 1.

Table 1 The adding amount (mg)of different samples forpreparing g-C3N4@Ni3Se4 samples.
The specific preparation process of g-C3N4@CoSe2 is exactly the same as that of g-C3N4@Ni3Se4, and the specific quality of the different samples added is shown in Table 2.

Table 2 The adding amount (mg)of different samples for preparing g-C3N4@CoSe2 samples.
2.3 Characterization of as-prepared samples
The topographies of as-synthesized photocatalysts were observed on JSM-6701F JEOL scanning electron microscope (SEM), whose acceleration voltage was set at 5000 V. And TEM images of graphitic carbon nitride, g-C3N4@CoSe2 and g-C3N4@Ni3Se4samples were carried out on JEM1200EX JEOL transmission electron microscopy (TEM), whose acceleration voltage was set at 10000 V. XRD patterns of all resulting catalysts were obtained by using a CuKαX-ray diffractometer,whose model was Rigaku RINT-2000 and the tube current and tube voltage was set at 40 mA and 40 kV respectively, and at a scanning rate of 5 degrees per minutes in the 2θrange of 5°–80°.Fourier-transform infrared (FT-IR)spectra of all resulting catalysts were recorded on a Thermo Nicolet Avatar 380 FT-IR spectrometer. The surface chemical compositions of the photocatalst were detected on the ESCALAB 250Xi X-ray photo-electron spectrometer (XPS). BET specific surface areas of graphitic carbon nitride, CN@NS-3 and CN@CS-3 were determined by the nitrogen adsorption-desorption isotherms on a ASAP 2020M analyzer at 77 K. UV-visible diffuse reflectance spectra (UV-Vis DRS)of solid powders were recorded on an UV-2550 spectrophotometer using BaSO4as a reference.Photoluminescence (PL, containing steady-state fluorescence and transient fluorescence)spectra of the obtained catalysts were recorded on a FLUOROMAX-4 fuorescence spectrometer at room temperature. The photoelectrochemical tests (containing chronoamperometry, linear scan voltammetry, potentiostatic electrochemical impedance spectroscopy (EIS)and Mott-Schottky)were performed on a VersaStat4-400 electrochemical workstation in a standard three-electrode system with platinum plate and calomel electrode as counter electrode and reference electrode, respectively. Meanwhile, 0.2 mol∙L−1Na2SO4aqueous solution and 300 W xenon lamp were used as the electrolytic solution and a solar simulator illumination source (XMM65-63,C16). And I-T and LSV curves were recorded at a stationary bias of 0.3 VvsSCE. EIS curves of samples were recorded at a stationary bias of −0.2 V with AC amplitude of 10 mV and a frequency range of 10000–1 Hz. The Mott-Schottky curves were recorded from −0.6 to 0.2 V with AC amplitude of 10 mV at a frequency of 1000 Hz. The zeta potential was recorded by Litesizer 500.
2.4 Photo-catalytic activity of H2 evolution
Photo-catalytic H2-generation activity experiments were operated in a PCX50A Discover nine-channel photo-catalytic reaction system with stirring system and 5 W LED white light as the simulated solar light at room temperature. In a typical process, 10 mg of catalyst was suspended in a bottom-irradiation quartz glass vessel having a 63 mL volume containing 30 mL of 15%V/Vtriethanolamine solution (pH = 10). Subsequently, the air in this quartz glass vessel was completely removed through the prepared nitrogen. The produced hydrogen gas was detected periodically (hydrogen was detected every hour)by using a Tianmei GC7900 gas chromatograph (TCD, 13Xcolumn)with N2as carrier gas and the test date of hydrogen evolution was recorded in time. The total time of light duration was designated as 5 h.
2.5 DFT calculation details
Density functional theory calculations were achieved by taking VASP with the DFT exchange-correlation of GGA-PBE(Generalized Gradient Approximation, Perdew Burke Ernzerhof). The structure cells of g-C3N4and Ni3Se4were constructed for this calculation. The types of calculation and smearing respectively was Single Point and Gaussian with a cutoff energy of 500 eV and a threshold of SCF convergence was 2 × 10−5. The adsorption energy of H on g-C3N4 and Ni3Se4 surface were calculated according to the following formula:Eads=Etotal− (Esurface+ 1/2EH2), whereEadsis the adsorption energy of either hydrogen atom,Etotalis the total energy for the adsorption state,Esurfaceis the energy of the pure g-C3N4and Ni3Se4surface,EH2is the energy of hydrogen molecule.
3 Results and discussion
3.1 Morphology and structure
Fig. 1a shows characteristic nanosheet structure of g-C3N4. It can be clearly observed from Fig. 1b that CoSe2nanoparticles neatly grew on the surface of g-C3N4nanosheets, and the particles size are very uniform. Furthermore, the well-dispersed nanosheets suggest that the general aggregation state of g-C3N4 is efficiently broken compared with that in Fig. 1a, which can provide an appreciable surface forinsituanchoring of CoSe2nanoparticles. Compared to serious aggregation state of g-C3N4,it can expose more active sites for photo-catalytic hydrogen generation that CoSe2nanoparticles as an efficient cocatalyst coupled with more dispersed g-C3N4 sheets. A same result also is found for g-C3N4@Ni3Se4as shown in Fig. 2b. The sheet structure of g-C3N4is still distinct in the TEM images as displayed in g-C3N4@CoSe2and g-C3N4@Ni3Se4(Fig. 1c and Fig. 2c). Evidently, pure g-C3N4in g-C3N4@CoSe2and g-C3N4@Ni3Se4 exhibits a dispersed and irregular sheet-like structure. Meanwhile, the successful synthesis of g-C3N4@CoSe2and g-C3N4@Ni3Se4hetero-junctions are shown in the TEM and HRTEM images in Fig. 1c,d and Fig. 2c,d. And it can be clearly observed that g-C3N4nanosheets in g-C3N4@CoSe2and g-C3N4@Ni3Se4samples are more dispersed compared with highly aggregated state of pure g-C3N4 nanosheets, which signifies that the rational design efficiently removed the agglomeration phenomenon of unprocessed g-C3N4nanoflakes. It is a common knowledge that a higher dispersity of g-C3N4nanosheets in hybrid samples can efficiently shorten transport path of charge carriers and reduce resistance of electrons transfer, which is conductive to hydrogen evolution reaction under visible light-driven. Furthermore, the amorphous phase structure of CoSe2and the typical g-C3N4@CoSe2heterostructure is clearly showed in Fig. 1d, which is equal to the result of XRD analysis. Fig. 1e shows the element species in g-C3N4@CoSe2and no other elements are found except C, N, Co and Se elements, indicating the fairly high purity of the assynthesized g-C3N4@CoSe2catalyst. Meanwhile, it can also be clearly perceived from the element mapping images (Fig. 1f)that all elements are very evenly distributed, which corresponds to the result of SEM and TEM images. However, unlike CoSe2,Ni3Se4exhibits a crystalline structure as shown in Fig. 2d. A close interface between Ni3Se4 and g-C3N4 is successfully formed, and the lattice spacing of 0.27 nm represents the (−112)face. Meanwhile, it still can be clearly seen that the existence of C, N, Se and Ni elements in g-C3N4@Ni3Se4(Fig. 2e)and the uniform distribution is observed in Fig. 2f, which implies the high purity and the successful preparation of the g-C3N4@Ni3Se4.
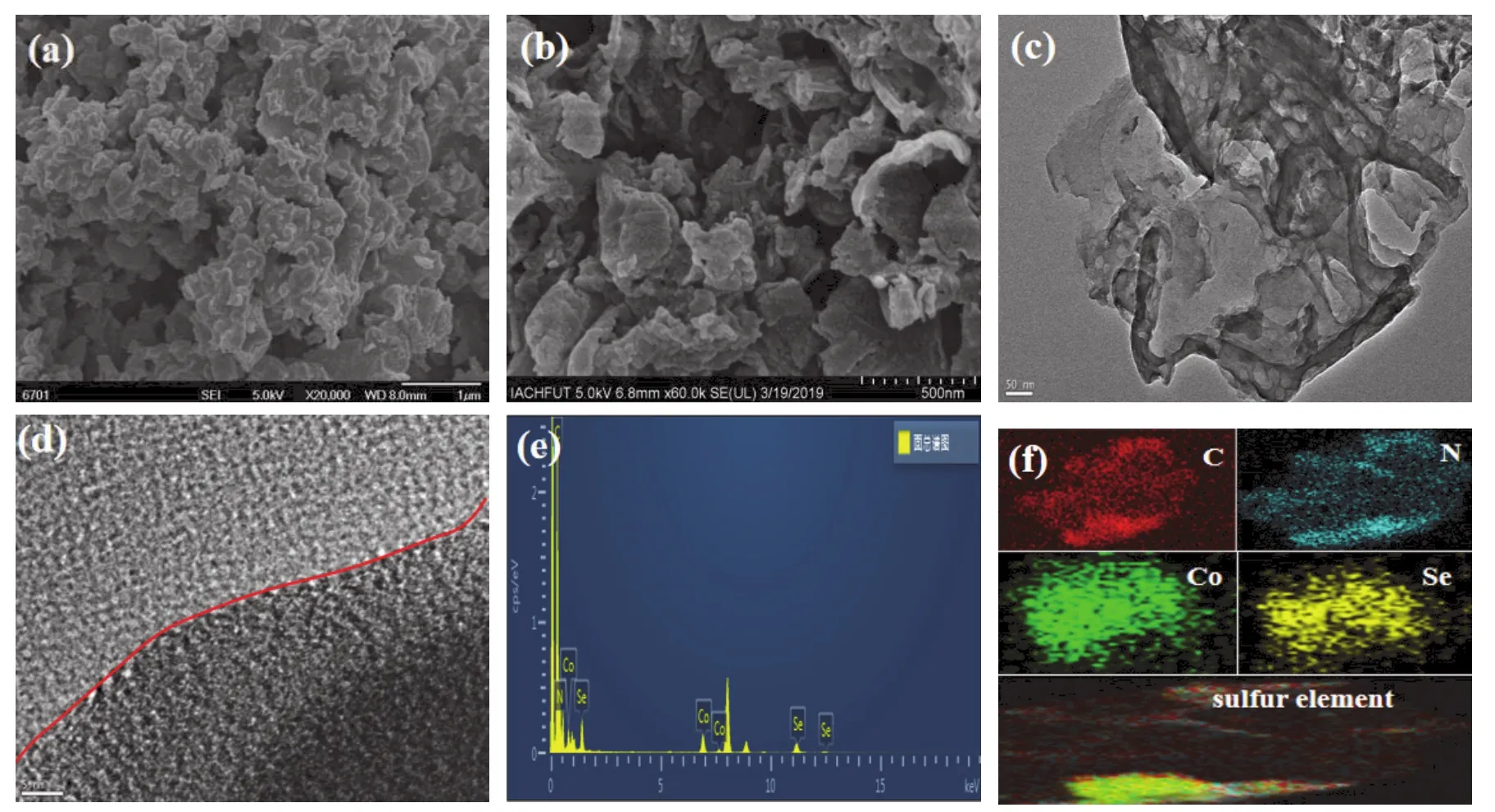
Fig. 1 SEM, TEM, EDX images and element mapping of g-C3N4@CoSe2.

Fig. 2 SEM, TEM, EDX images and element mapping of g-C3N4@Ni3Se4.
3.2 XRD analysis
The phase-structure of g-C3N4, Ni3Se4, CN@NS, CoSe2and CN@CS catalysts are investigated by X-ray diffraction patterns as displayed in Fig. 3a–c. The pristine g-C3N4shows the two specific diffraction peaks at about 13.0° and 27.2°,corresponding to (100)plane that originates from the tri-striazine units arranging in the lattice flats parallel to thecaxis and (002)plane that comes from a periodic stack from the graphitic phase layers of pure g-C3N4(PDF#87-1526), respectively. The result is the same as the previous reports31,32. The as-synthesized Ni3Se4shows the four obvious diffraction peaks from 5° to 80°, which suggests that the Ni3Se4is crystallized well. And in Fig. 3a, the diffraction peaks at 35.1°, 44.8°, 50.6° and 62.0° belong to(−112), (−114), (310)and (−402)planes of Ni3Se4(PDF#18-890). In addition, the XRD patterns of g-C3N4@Ni3Se4 hybrids also are revealed. However, the feature peaks of Ni3Se4 in g-C3N4@Ni3Se4cannot be clearly observed mainly because the content of Ni3Se4in g-C3N4@Ni3Se4is too low, which implies the high efficiency of Ni3Se4as electron accelerator for improving H2-evolution based on g-C3N4. But it is evident that the diffraction peaks density of g-C3N4 semiconductor gradually decreased as the content of Ni3Se4in g-C3N4@Ni3Se4increased,which implies that the g-C3N4@Ni3Se4composites are successfully synthesized. Meanwhile, the XRD patterns of CoSe2and g-C3N4@CoSe2are also characterized as shown in Fig. 3b. However, no any diffraction peak density is found in the XRD pattern of CoSe2, demonstrating that amorphous crystal structure of CoSe2, which has been known in Fig. 1d. All g-C3N4@CoSe2samples exhibit very similar diffraction peaks, and the peaks intensity of g-C3N4decreased significantly as the content of CoSe2in g-C3N4@CoSe2grew, demonstrating that a successful hybridization between g-C3N4and CoSe2material. In addition, many irrelevant peaks in samples like CN@NS-5,CN@NS-7 and CN@CS-1 are assigned to Se element that was not fully reacted (Fig. 3c).

Fig. 3 XRD and FT-IR patterns of the resulting samples.
The FTIR spectra of the pure g-C3N4, g-C3N4@Ni3Se4and g-C3N4@CoSe2samples are obtained as displayed in Fig. 3d and Fig. 3e. The absorption peaks at 1237, 1323, 1414, 1571 and 1638 cm−1belong to the typical stretching vibration modes of the C―N in g-C3N4hetero-cycles33,34. In addition, the stretching modes of N―H and the surface-adsorbed OH also are observed from 3000 to 3500 cm−135. Meanwhile, the obvious peak at 807 cm−1is ascribed to characteristic breathing mode of triazine units36,37.
3.3 XPS analysis
The element species in the as-prepared photo-catalysts are detected by X-ray photo-electron spectroscopy (XPS)characterization. As shown in Fig. 4a, the main element signals,Se, C, N, Co and Ni, are observed and the intensities of Se, Co and Ni are smaller than that of C and N due to low adding amount in the preparing process, which is consistent with the results of the XRD analysis. The high resolution C 1sspectrum of layered g-C3N4 can be deconvolved into two peaks with the binding energies at 284.7 eV and 288.1 eV (Fig. 4b), which are assigned to the C―C groups andsp2-hybridized N=C―N species,respectively38,39. In high resolution N 1sspectra of laminated g-C3N4(Fig. 4c), the two main peaks having binding energies of 398.6 and 401.1 eV belong to triazine rings and amino species(C―N―H), respectively40,41. Fig. 4d portrays the Ni 2pspectrum of Ni3Se4within the binding energy range from 850 eV to 890 eV. The deconvolution peak with binding energy of 855.9 eV can be attributed to Ni 2p3/2and an additional peak at 862.0 eV is its satellite peak. Additionally, the characteristic peak having a binding energy at 873.4 eV corresponds to Ni 2p1/2and a broad peak with lower intensity at 880.3 eV belongs to its satellite peak. The high-resolution Co 2pXPS spectra also is obtained by the Lorentzian-Gaussian function and it is fitted into four different peaks as shown in Fig. 4e. The two peaks at 781.1 and 796.9 eV correspond to Co2+coordinated to Se ions, and Co 2p1/2of Co groups in CoSe2and other two signals located at 785.5 eV and 803.1 eV are assigned to the satellite peaks due to the anti-bond orbit between the Co atom and the Se atom42,43.Fig. 4f shows the XPS analysis result of Se 3dhaving three deconvolution signals at 54.3, 58.3 and 59.1 eV, corresponding to Co―Se bond, Ni―Se and Se 3d3/2of Se atom, respectively44,45.

Fig. 4 XPS spectra analysis of all elements.
3.4 BET study
The Brunauer-Emmett-Teller (BET)specific surface area and the pore diameter distribution of pure g-C3N4, CN@CS-3 and CN@NS-3 samples are calculated by the nitrogen adsorptiondesorption isotherm measure at 77 K. And the obtained results are displayed in Table 3 and Fig. 5a, b. All isotherms show a typical Type IV curves having a H3 hysteresis loop atP/P0of 0.7–1.0, implying a multilayer adsorption process for mesoporous materials. This phenomenon is the same as the result presented in Fig. 5b and that of SEM and TEM for g-C3N4,CN@CS-3 and CN@NS-3. The average pore diameters of pure g-C3N4, CN@CS-3 and CN@NS-3 are 15, 19 and 16 nm respectively and it can be obviously seen from the Fig. 5b that the pore diameter for pristine g-C3N4, CN@CS-3 and CN@NS-3 is concentrated between 0–50 nm, which clarifies their mesoporous structure. In addition, pure g-C3N4 has a specific surface area of 44 m2∙g−1, is bigger than CN@CS-3 with a specific surface area of 25 m2∙g−1and CN@NS-3 having a specific surface area of 23 m2∙g−1. The decrease of the specific surface area of CN@CS-3 is related to the interaction between g-C3N4 and CoSe2 and the same is true for CN@NS-3, and it may be mainly due to the decrease in the content of g-C3N4 in unit mass CN@CS-3 and CN@NS-3, that is, the difference in density between them as well as the size of their respective specific surface areas in the unit mass are the main causes of the change for the specific surface area of the final composite.
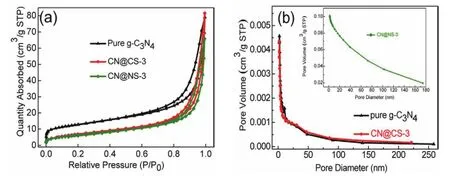
Fig. 5 N2 adsorption-desorption isotherms and pore diameter distribution.
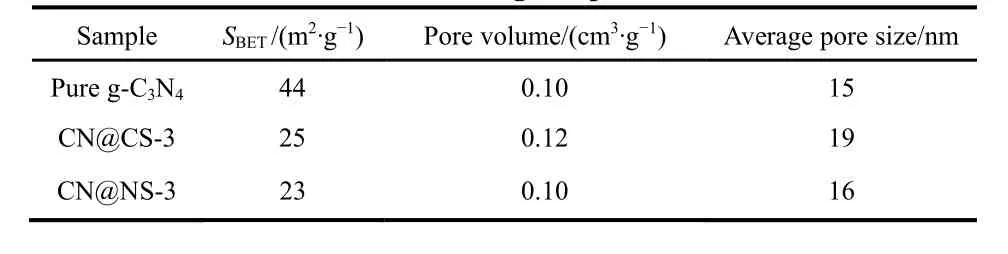
Table 3 The SBET, pore volume and pore diameter for the resulting samples.
3.5 Optical performance study
The optical characteristics and electronic band structure of g-C3N4nanosheets, CN@CS-3 and CN@NS-3 samples are investigated by UV-Vis diffuse reflectance spectroscopy (DRS)as illustrated in Fig. 6. It can be seen from Fig. 6a that g-C3N4nanosheet has an about 430 nm light-absorption edge, which is consistent with the intrinsic absorption from valence band to conduction band. The photo-absorption edges of both CN@CS-3 and CN@NS-3 exhibit a notable right shift compared with pure g-C3N4and that of CN@CS-3 is biggest, demonstrating that the light-absorption region successfully broadened after loading Ni3Se4and CoSe2on the surface of g-C3N4nanosheets. The improved absorbance should be caused by hyperchromicity effect of Ni3Se4 and CoSe2 nanoparticles. Additionally, the absorbance of composite samples also is higher than g-C3N4,which helps to capture more photons to promote water-splitting reaction. The UV-Vis diffuse reflectance spectrum of obtained catalysts is determined by transformed Kubelka-Munk function42:F(R)=K/S = (1 −R∞)2/(2R∞)(Kis the absorption coefficient,Srepresents the scattering coefficient andR∞ represents relative diffuse reflectance). The changes of energy band structure for g-C3N4, CN@CS-3 and CN@NS-3 are calculated through the Tauc method and the equation is as follows46: (αhʋ)=A(hʋ)n/2(where a represents absorption coefficient,hrepresents Planck’s constant,ʋrepresents light frequency,Egrepresentes band-gap energy andArepresentes a constant, respectively). The band gap value (Eg)of g-C3N4obtained by the above method around is 2.8 eV andEgof CN@CS-3 and CN@NS-3 significantly decrease,which reduces the threshold for the transition of photo-excited electrons from the valence band to the conduction band. This makes that H+have more chances to get electrons for participating hydrogen generation reaction under same lighting conditions and lighting time.
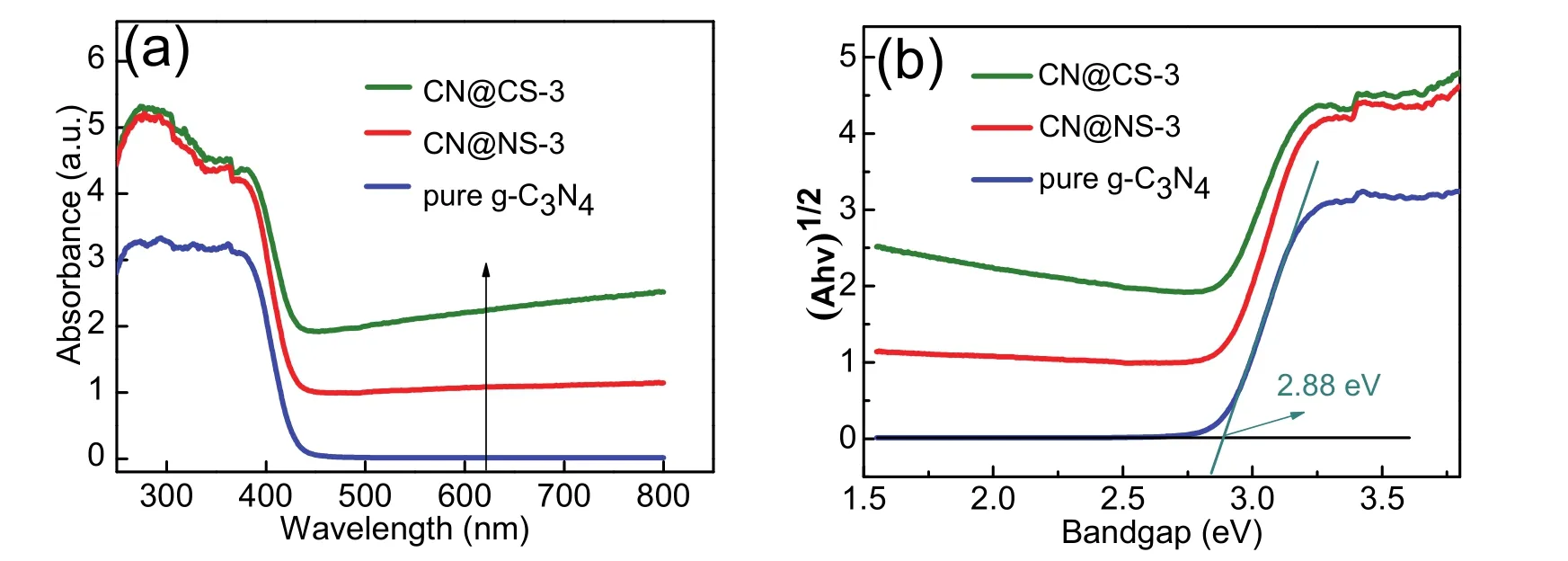
Fig. 6 UV-Vis diffuse reflectance spectroscopy and The plot of (ahv)1/2 versus energy for the band gap energy.
3.6 Photo-catalytic H2 evolution performance
Fig. 7 shows the photo-catalytic H2-evolution performance of all as-prepared samples in EY sensitization system. One can clearly see from Fig. 7a that pristine g-C3N4exhibits a very poor photo-catalytic hydrogen evolution activity with about 1.9µmol∙h−1mainly due to the serious recombination of electronhole pairs. However, the hydrogen generation activity is prominently improved after growing the Ni3Se4and CoSe2on the surface of g-C3N4, implying the unique effect of Ni3Se4and CoSe2for accelerating the separation and migration of charge carriers. CN@NS-3 exhibits a photo-catalytic activity that is 8 times and CN@CS-3 shows a hydrogen production activity that 13-fold that of g-C3N4. The enhanced photocatalytic hydrogen production suggests that the rational design and construction,anchoring Ni, Co-based metal selenide on the surface of welldispersed g-C3N4nanosheets, can inhibit the serious recombination of electron-hole pairs for g-C3N4. Interestingly,CN@CS-3 showcases more efficient hydrogen evolution performance than CN@NS-3, which may be related to the amorphous structure of CoSe2 because of the fact that a longrange disorder and short-range disorder of CoSe2can expose rich active sites. Furthermore, CN@CS-3 exhibits stronger light absorbance intensity and wider light response region compared with CN@NS-3, which demonstrates that the introduction of amorphous CoSe2on the surface of g-C3N4can utilize more light. It can be evidently observed from the hydrogen-production activity of CN@NS-xand CN@CS-x(Fig. 7b,c)that CN@CS-xsamples exhibit more efficient photo-catalytic activity compared with CN@NS-xsamples, which may imply that the excellent photo-catalytic performance of amorphous CoSe2semiconductor. The effect of pH for the hydrogen evolution activity of CN@CS-3 samples is evaluated as illustrated in Fig.7d. CN@CS-3 catalyst clearly exhibits the highest catalytic activity under the reaction system of pH = 10, showing that the optimum circumstance of hydrogen evolution for CN@CS-xsamples. And the stronger basicity of the reaction system, the more severe inhibition of the photocatalytic hydrogen evolution activity of CN@CS-3 sample due to stronger alkaline environment can cause the decrease of H+. In addition, the catalytic activity of CN@CS-3 catalyst is smothered because triethanolamine is easily protonated in a strong acidic environment. The photo-catalytic hydrogen evolution stability of CN@CS-3 also is evaluated as shown in Fig. 7e. The decline in the evolutionary stability of hydrogen may be due to the consumption of sacrificial reagents and the degradation of photosensitizers. In EY sensitized photocatalytic hydrogen evolution system, the excited state EY* can be quenched by TEOA, as a sacrificial reagent, to form strong reducing EY−•.Otherwise, a large amount of EY−•would be accumulated, which would lead to the rapid degradation of EY and affect the stability of hydrogen evolution system.

Fig. 7 Evaluation H2 evolution activity
3.7 The photo-luminescence (PL)experiments
In photo-catalysis, the recombination of electron-hole pairs is generally considered to be a bottleneck limiting the efficiency of photon conversion47. Steady-state fluorescence spectroscopy is used to investigate the radiative recombination process of charges as displayed in Fig. 8a. All samples show similar spectra in a wavelength range of 500–600 nm and display a photoluminescence emission signal at 538 nm corresponding to the return transition process of the excited state electron for EY(Eosin Y). After EY absorbs the energy of photon, the electron would transition from ground state to excited state. Because the lifetime of excited state electron of EY is very short, if it can not be effectively transferred, it would emit fluorescence in the form of radiation transition and quench, thus greatly reducing the utilization efficiency of excited state electron in photochemical reaction. Evidently, the EY shows the highest photoluminescence intensity compared to g-C3N4, CN@NS-3 and CN@CS-3, which elucidates that the excited state EY is not oxidatively or reductively quenched in the absence of other samples. However, the photoluminescence intensity is prominently suppressed when the g-C3N4was introduced,demonstrating that the introduction of g-C3N4can inhibit the return transition process of excited EY, namely, promoting the migration of electrons of excited EY. Importantly, CN@NS-3 shows the lower fluorescence density compared to primitive g-C3N4 under the light excitation at 480 nm, but the weakest fluorescence signal is detected in CN@CS-3, manifesting that radiative carrier recombination can be restrained to a greater extent in CN@CS-3 photo-catalyst. This result also illustrates the faster transfer of charge between excited EY and CN@CS-3,which implies that amorphous CoSe2with the efficient role accelerated electron transfer for hydrogen generation reaction.Thus the recombination of charge carriers is greatly inhibited,boosting the photocatalytic hydrogen evolution reaction.

Fig. 8 Steady-state fluorescence and transient fluorescence.
Additionally, the charge carriers behavior between excited state EY and samples is further investigated by time-resolved photoluminescence spectra as shown in Fig. 8b and the fluorescence lifetime of electrons and electron-transfer rate constants are displayed in Table 4. The decay lifetime value for EY, g-C3N4, CN@NS-3 and CN@CS-3 are recorded at an excitation wavelength of 454 nm. The obtained decay curves are fitted by using the following formula:I(t)=B+A1e−t/τ1 +A2e−t/τ2(τ1andτ2are emission lifetimes, andA1andA2are the corresponding amplitudes), Meanwhile, the average emission lifetime is evaluated by the following formula:τ=A1τ12/A2τ22,Electron-transfer rate can be evaluated through the following formula:ket= 1/τF,donor*-acceptor− 1/τF,donor-acceptor*(ketis the upper limit of electron-transfer rate constants,τF,donor*-acceptorandτF,donor-acceptor*are the lifetimes of electron donor and acceptor,respectively). The fluorescence lifetimes of charge carriers, the corresponding amplitude and the weighted mean life-span for EY, g-C3N4, CN@NS-3 and CN@CS-3 are obtained as illustrated in Table 4. It can be expressly observed from the Table that bare g-C3N4shows single-exponential decay lifetime of 0.02 ns and CN@NS-3 and CN@CS-3 exhibit a double-exponential decay lifetime dominated by short life-span. The long life-time and short life-time of charges in CN@NS-3 are 3.07 and 0.05 ns having the corresponding amplitude of 1.87% and 98.13% respectively, and the long life-span and short life-span of electrons in CN@CS-3 sample are 3.7 and 0.31 ns having the amplitude of 1.41% and 98.59%, respectively. The longer lifetime for CN@CS-3 sample implies the longer period that electrons return from lowest energy level of excited state to ground state, which may make H+get a greater probability to harvest e−. Additionally, the transfer rate constants (ket)of charges of CN@NS-3 and CN@CS-3 samples are calculated and the value is 3.0 × 1010and 4.7 × 1010, respectively. Hence, the largest transfer rate for CN@CS-3 catalyst means the fastest transfer rate of photo-produced electrons and the highest photocatalytic activity for hydrogen evolution.
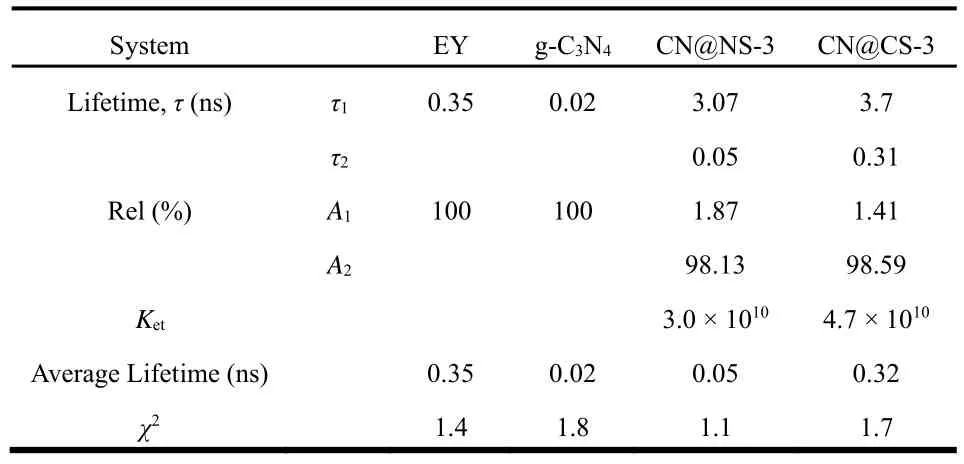
Table 4 Fluorescence lifetime of electrons and electron-transfer rate constants.
3.8 Photoelectrochemical measurements (I-T, LSV,EIS and Mott-Schottky)
The transient photocurrent-time curves of as-synthesized samples are tested by couple on/off runs as displayed in Fig. 9a.Remarkably, the g-C3N4@FTO electrode exhibits the lowest photo-current density compared with CN@NS-3@FTO and CN@CS-3@FTO electrodes, meaning the poor photocatalytic hydrogen evolution activity. The introduction of CoSe2and Ni3Se4on the surface of g-C3N4enhance the photo-current response. It is apparent that the CN@CS-3/FTO electrode shows the highest photo-current intensity, meaning that the accelerated transfer of charge carriers at the close interface between g-C3N4and CoSe2and more efficient photo-catalytic activity. The consistent results have been obtained at aforementioned fluorescence spectra.
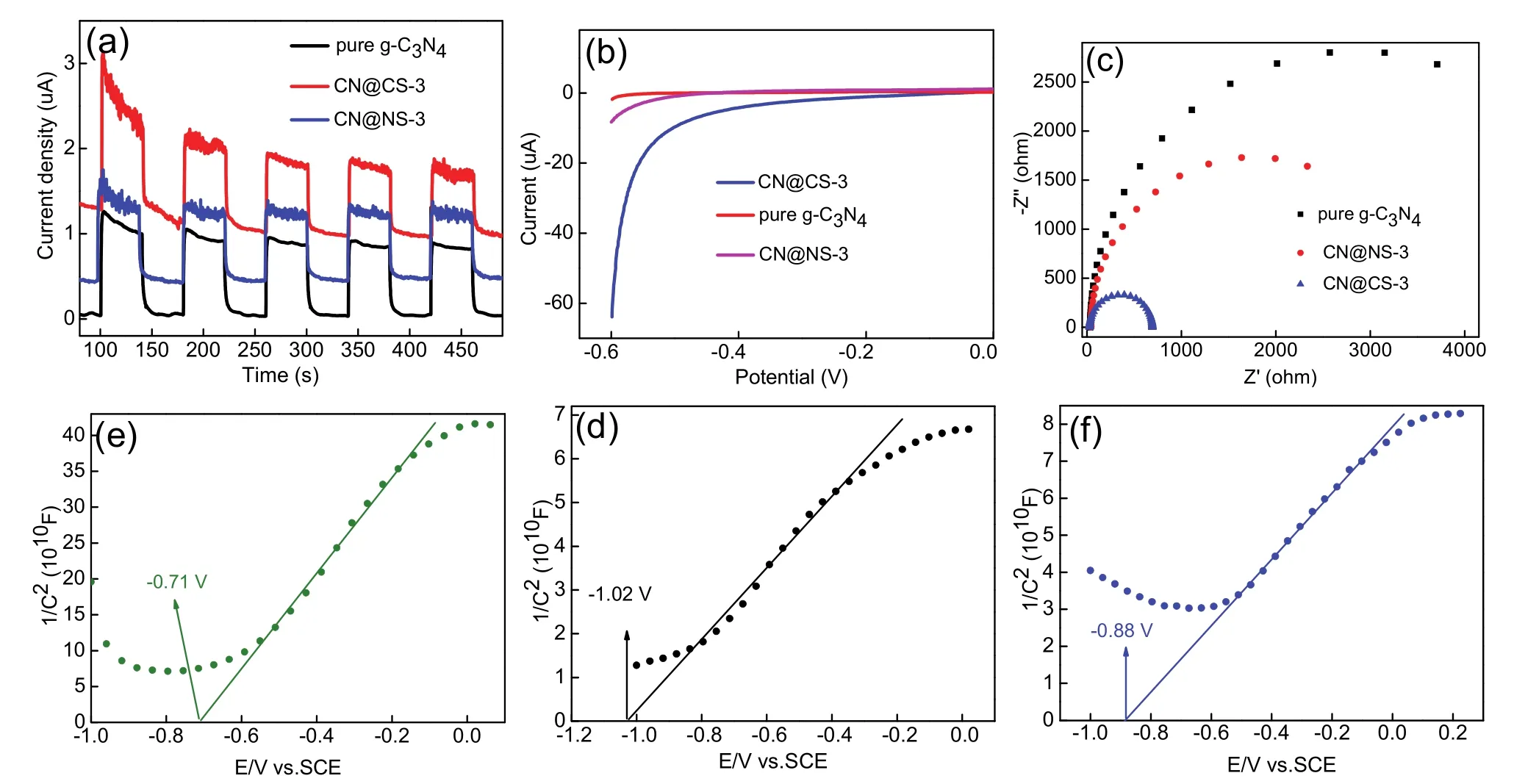
Fig. 9 Photoelectrochemical measurements (I-T, LSV, EIS and Mott-Schottky).
In addition, the electrochemical hydrogen production activities of CN@CS-3/FTO, CN@NS-3/FTO and g-C3N4/FTO electrodes are studiedviathe LSV measures as shown in Fig. 9b.The g-C3N4/FTO electrode exhibits an increased cathodic current under the equal conditions, demonstrating that the primitive g-C3N4material is an excellent carrier for a hydrogen evolution reaction. The both CN@CS-3/FTO and CN@NS-3/FTO electrodes show the lower cathodic current than g-C3N4/FTO electrode, which manifests that CoSe2and Ni3Se4as co-catalysts greatly improved the separation and transfer efficiency of photo-generated charge carriers based on g-C3N4.Obviously, it can be proved from the results of LSV measures that the hydrogen evolution performance of CN@CS-3 is more excellent.
Additionally, the Nyquist plots of EIS of CN@NS-3/FTO,CN@CS-3/FTO and g-C3N4/FTO electrodes are recorded as shown in Fig. 9c. It is apparent that the CN@CS-3/FTO electrode exhibits the smallest diameter of the Nyquist plots compared with CN@NS-3/FTO and g-C3N4/FTO electrodes,suggesting that the existence of CoSe2nanoparticles in CN@CS-3 sample accelerated the charge carriers transfer from the g-C3N4semiconductor thereby it makes that the recombination of electrons and holes are efficiently inhibited. Therefore,CN@CS-3 catalyst exhibits the higher photo-catalytic watersplitting activity.
The Mott-Schottky measure can be used to reflect the types of semiconductors and calculate Fermi level potential (EF)of g-C3N4, CoSe2and Ni3Se4. Fig. 9d,f display their Mott-Schottky(MS)curves under non-photo-excited condition, they all exhibit the positive slopes ofE–C−2plots, signifying that they are allntype performance semiconductors48,49. Meanwhile, the flat-band potential (Efb)of g-C3N4, CoSe2and Ni3Se4samples can be gained, namely, theEfbof g-C3N4, CoSe2and Ni3Se4materials are approximately −1.02, −0.71 and −0.88 VversusSCE,respectively. Hence the Fermi level potential (EF)of g-C3N4,CoSe2 and Ni3Se4 samples can also be obtained according to the meaning of flat-band potential:Efb=EF, Thus the Fermi level potential of g-C3N4, CoSe2and Ni3Se4are −1.02, −0.71 and−0.88 V, respectively. The conduction band potential (ECB)for n-type property semiconductor is proverbial, it is more negative about 0.1 or 0.2 V than itsEfb50, that is,ECB=Efb− 0.1 orECB=Efb− 0.2, therefore theECBof g-C3N4, CoSe2and Ni3Se4 are approximately −1.22, −0.91 and −1.08 VversusSCE,respectively. And the normal hydrogen electrode potentials(ENHE)for g-C3N4, CoSe2and Ni3Se4could be appraised by the following formula:ENHE=ECB+ 0.241 V. So theENHEfor g-C3N4, CoSe2and Ni3Se4are −0.979, −0.669 and −0.839 V,respectively. The obvious difference of energy levels between g-C3N4and CoSe2(Ni3Se4)ensure a vital driving force for electron transfer. And the redox potential (−1.05 V)excited state EY* is more negative compared with that of g-C3N4, CoSe2 and Ni3Se4,which confirms the interaction between excited state EY* and the resulting samples. Further, the energy level difference between excited state EY* and theENHEof CoSe2is more significant, which could more efficiently promote separation and migration of charge carriers.
The zeta potential of g-C3N4, Ni3Se4, CN@NS-3, CoSe2and CN@CS-3 in aqueous solution are measured to evaluate the adsorption property of them for protons as shown in Fig. 10. g-C3N4as a catalytic carrier has a relatively negative zeta potential of −23.5 mV in aqueous solution, which is beneficial for the adsorption of protons at water-splitting reaction. Both Ni3Se4and CoSe2also show negative zeta potentials of −19.4 and −8.5 mV at a same system. Expectantly, CN@NS-3 and CN@CS-3 exhibit more negative zeta potentials of −26.8 and −27.1 mV compared with alone g-C3N4, indicating that the coupling of Ni3Se4 and CoSe2 with g-C3N4 efficiently enhanced the adsorption ability of H+by photocatalysts. It is common knowledge that the improved adsorption of H+is conductive to hydrogen evolution reaction.
3.9 Theoretical calculation
To further evaluate the enhanced photocatalytic activity of the as-prepared catalysts based on g-C3N4, the interaction of hydrogen atom on the g-C3N4and Ni3Se4surface are explored by calculating the adsorption energy with density functional theory (DFT). Fig. 11a,b show the top and side views of optimized g-C3N4 and H adsorption at N site. As shown in Fig.11b, compared with the initial g-C3N4, the H would like to absorb the N site to form N―H bond, and the adsorption energy is −0.22 eV. Fig. 11c, d display the top and side views of optimized Ni3Se4and H adsorption at Se site. As shown in Fig. 11d, the hydrogen atom prefers to adsorb at the bridge site of two selenium atoms to generate Se―H―Se bond, and the adsorption energy is 1.53 eV. Thus the adsorption energy of Se site to H is closer to the energy of hydrogen atom (EH2)compared to that of N site on the g-C3N4surface to H, which is better for hydrogen evolution reaction. However, the adsorption of g-C3N4to hydrogen atom is more stable than that of Ni3Se4 to hydrogen atom, implying the improved adsorption of hydrogen, that is, the anchoring of Ni3Se4based on g-C3N4efficiently enhanced hydrogen evolution reaction under visible irradiation, which also may imply the excellent performance of amorphous CoSe2 coupling with g-C3N4due to star elements Ni and Co coupling with same metalfree elements has similar catalytic properties.
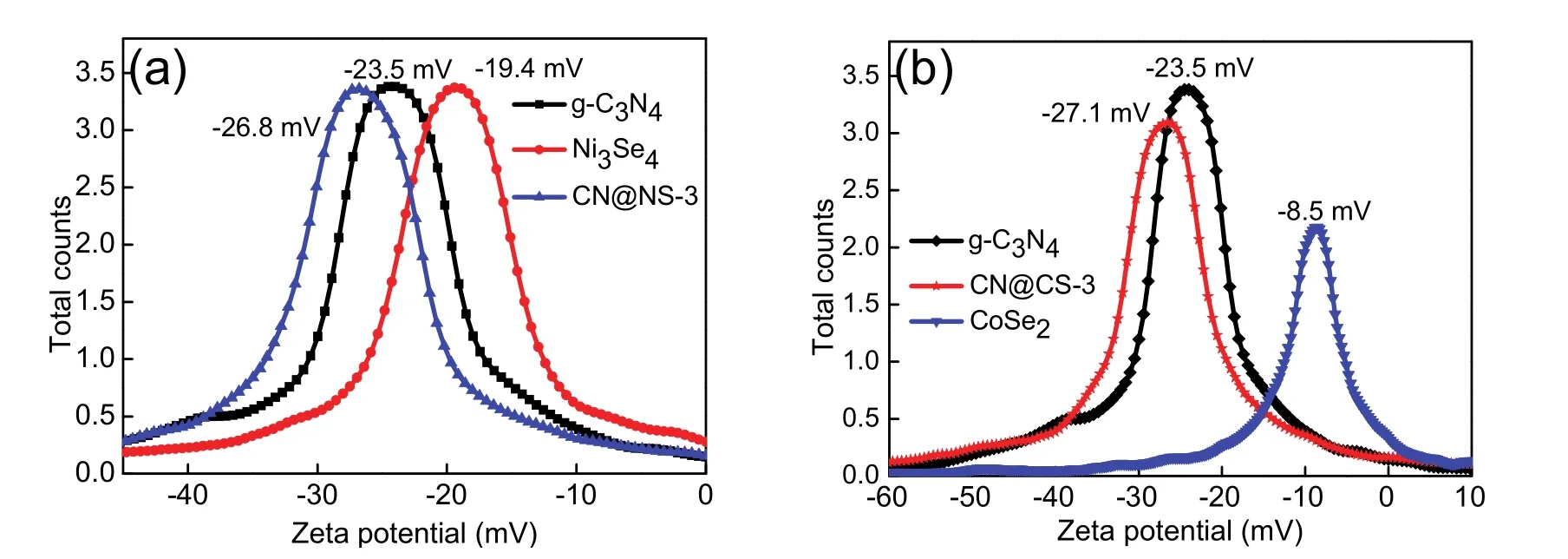
Fig. 10 Zeta potential measurements.

Fig. 11 Top and side views.
Additionally, the band structures and density of states of optimized g-C3N4and Ni3Se4also were obtained as shown in Fig. 12. It can be clearly perceived from the calculated results that g-C3N4 is a direct semiconductor and Ni3Se4 has the properties of like-metalloid, which suggests the prominent ability to transfer charges as well as implies same characteristics of CoSe2, which may be also an essential factor that Ni3Se4and CoSe2 can boost photocatalytic hydrogen generation activity based on g-C3N4with light driven. Meanwhile, the results of band structure are consistent with that of density of state for g-C3N4and Ni3Se4.
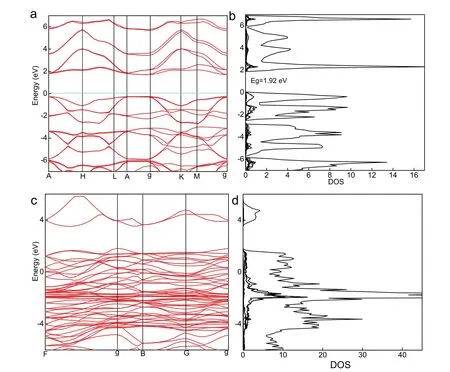
Fig. 12 The band structure and density of state.
3.10 Photo-catalytic mechanism of hydrogen evolution
Based on the above-mentioned studies, the possible mechanism of highly efficient photo-catalytic hydrogen production for EY-sensitized g-C3N4@CoSe2 catalyst under visible light irradiation is described as shown in Fig. 13. For EY-sensitized g-C3N4@CoSe2, it is EY molecules, not g-C3N4@CoSe2, that are excited by light. The excited EY molecules inject electrons into the conduction band of g-C3N4,which promotes the charge separation of the excited EY dye.This is also equivalent to extending the light absorption range of g-C3N4. EY adsorbed on g-C3N4@CoSe2is excited by visible light, and ground state EY formed one-excited state EY1*and next formed more stable triplet-excited state EY3*through intersystem transition, and finally forming EY−•having reduction abilityviathe interaction between EY3*and TEOA, then the electrons transfer to the conduction-band of g-C3N4 due to the difference of energy level between them (The redox potentials of ground state EY and excited state EY* respectively are 1.10 V and −1.05 Vversusthe standard hydrogen electrode.According to the formula:E(eV)= −4.5 −ENHE(V), the corresponding work functions are calculated to be −5.60 eV and−3.45 eVversusthe vacuum level, respectively). Meanwhile, the charges transfer from the valence-band to conduction-band of g-C3N4, so the electrons collected on the conduction-band of g-C3N4are then rapidly transferred to the CoSe2active sites,making H+continually get e-to produce hydrogen. And the holes remaining in the valence-band are consumed by the sacrificial reagent (TEOA). Meanwhile, the strong adsorption of samples surface to hydrogen accelerates water-splitting reaction to evolve H2.
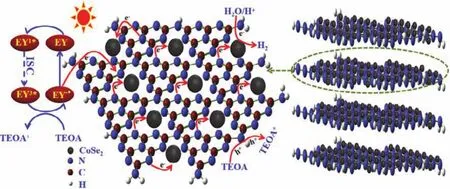
Fig. 13 H2 evolution mechanism of EY-sensitized g-C3N4@CoSe2 catalyst under visible light.
4 Conclusions
In brief, the two metal selenide semiconductors Ni3Se4and CoSe2were successfully synthesized and used as a highly efficient photo-catalyst for visible-light-driven water-splitting to generate hydrogen based on g-C3N4. And the morphology,crystal phase, specific surface area, optical properties and catalytic activity for as-prepared samples are studied in detail.More significantly, the photo-catalytic activity is greatly improved after Ni3Se4and CoSe2coupled with g-C3N4. And the as-synthesized g-C3N4@CoSe2exhibits the higher hydrogen evolution activity compared with the g-C3N4@Ni3Se4 due to amorphous CoSe2 may expose more active sites as well as more negative electronegativity, which helps to accelerate the rapid transfer of electrons and enhance the sorption of protons. And the formation of close inter-face between g-C3N4and metal selenide accelerated separation and transfer electrons.
Author contributions:Yanbing Li and Zhiliang Jin conceived and designed the experiments; Yanbing Li performed the experiments; Zhiliang Jin and Xuqiang Hao contributed reagents/materials and analysis tools; Yanbing Li and Zhiliang Jin wrote the paper.
Conflicts of interest:The authors declare that they have no competing interests.
- 物理化学学报的其它文章
- Hollow Nitrogen-Rich Carbon Nanoworms with High Activity for Metal-Free Selective Aerobic Oxidation of Benzyl Alcohol
- Photocrosslinking-Immobilized Polymer Vesicles for Lowering Temperature Triggered Drug Release
- CO Hydrogenation to Ethanol over Supported Rh-Based Catalyst:Effect of the Support
- CdTeSe合金幻数团簇的室温合成和形成机理研究
- 体相界面导通的复合快离子导体
- 异氰酸苯酯诱导的类胶原多肽自组装

