Theoretical Study of High-Efficiency Organic Dyes with Different Electron-Withdrawing Groups Based on R6 toward Dye-Sensitized Solar Cells
Zhen Wei, Minjie Li , Wencong Lu
Department of Chemistry, College of Science, Shanghai University, Shanghai 200444, China.
Abstract:Dye-sensitized solar cells (DSSCs)are the most promising alternatives to traditional fossil energy because of their advantages of low production cost, facile structure,relatively low environmental impact, relatively high photoelectronic absorption efficiency, and overall high efficiency. In addition, several studies on sensitizers as vital components have been conducted over the last three decades. Compared to metal dyes, metal-free organic dyes have been considered as promising candidates because of their simple fabrication, multiple structures, high molar absorption coefficients, easily tunable properties, and environmental friendliness. In this study, we systematically investigated the optoelectronic properties of six metal-free organic donor-acceptor dyes (RD1–6)derived from the known dye R6 by using the density functional theory (DFT)and time-dependent DFT methods. Cell performance parameters were discussed, including the geometrical and electronic structures, absorption spectrum, adsorption energy, light harvesting efficiency (LHE)curve, predictive short circuit current density, predictive open circuit voltage, and theoretical power conversion efficiency (PCE). Results revealed that all the designed dyes exhibited high theoretical PCE. In particular,dyes RD1, 2, and 4–6 showed greater conjugations, and dyes RD1–3 had smaller energy gaps than those of the reference dye. In addition, dyes RD1–3, 5, and 6 exhibited better light harvesting capacities that covered the entire visible region and extended to the near-infrared region with obviously red-shift maximum absorption wavelengths (λmax), wider LHE curves,and higheras compared to the reference dye. It was critical that dyes RD1 and 2 not only have greater conjugations and narrow band gaps but also good light harvesting capacities with more than 56-nm red-shift maximum absorption wavelengths and broadened LHE curves than those of the reference dye. Notably, mainly because of an average increment of 12.0% of a remarkable increment of the theoretical power conversion efficiency was observed from 12.6% for dye R6 to 14.1% for dyes RD1 and 2. Thus, dyes RD1 and 2 exhibited superior cell performances and could be promising sensitizer candidates for highly efficient DSSCs. These results could be used to guide effective synthetic efforts in the discovery of efficient metal-free organic dye sensitizers in DSSCs.
Key Words:Metal-free organic dyes; DFT/TD-DFT; Light-harvesting ability; Optoelectronic property;Power conversion efficiency
1 Introduction
Dye-sensitized solar cells (DSSCs)have drawn considerable attention owing to their relatively high photoelectronic absorption efficiency, low production cost and facile structural,since the pioneering work was published by O’Regan and Grätzel in 19911. Over past three decades, The power conversion efficiency (PCE)of DSSCs has continuously increased from 7% to 13% under simulated air mass 1.5 global conditions2,3. As one of the crucial components of DSSCs, the dye-sensitizer plays a major role in optoelectronic conversion,which including Ruthenium(II)complexes, Zn-porphyrins, and metal-free organic dyes4–8. Thus far, the metal complexes have shown remarkably higher efficiencies, which provide PCEs up to 13%. However, in view of environmental issues of the Ruthenium (II)dyes, the organic sensitizers have been considered as promising candidates due to the simple fabrication, the multiple structures, the high molar absorption coefficients, the easily tunable property and the environmental friendliness9–12. Although metal-free dyes might have their own weaknesses, such as absorption spectrum in visible region,outstanding progress has been achieved, with the currently record PCE up to 12.6%13. It is believed that there still remains much room for improving the optoelectronic performance of metal-free dyes by the structural modification14–17.
To improve their power conversion efficiency, dyes should have the following properties9,18–20: (1)the dye should absorb light over the wide energy range of visible and near-infrared region to achieve excellent light harvesting efficiency; (2)The dye can adsorb firmly on the surface of the semiconductor; (3)The energy level of the highest occupied molecular orbital(HOMO)must be established below the electrolyte redox potential to guarantee the regeneration of dye, and the energy level of the lowest unoccupied molecular orbital (LUMO)must be situated above the semiconductor conduction band (CB)to ensure efficient electron injection.
Recently, various electron-withdrawing groups have been designed to develop advanced sensitizers. Benzothiadiazole(BTD)has been considered as a promising electron-withdrawing group (EWG). Wanget al.synthesized some outstanding dyes with the introduction of benzothiadiazole (BTD), such as C275,C278, C281, C288, R2 and R6, and their dyes perform excellent PCE21–26. It is worth mentioning that Wanget al.synthesized organic D-A dye R6 with the intense absorption peak at 631 nm,and the optimized power conversion efficiency of R6 achieves 12.6% with the high short-circuit current density (19.36 mA·cm−2)and the high open-circuit voltage (850 mV)26. Thus,the introduction of potential EWGs can be favorable for achieve high power conversion efficiency.
In this paper, to improve the cell efficiency, we designed six dyes RD1–6 (Fig. 1)with different EWGs based on R6 and further simulated the optoelectronic properties using the density functional theory (DFT)and time dependent density functional theory (TD-DFT)methods, such as the geometrical structures and photoelectronic properties. In addition, many parameters of the cell performance including the light harvesting efficiency(LHE)curve, the predictive short circuit current densitythe predictive open circuit voltageand the theoretical PCE were discussed. We expect our work could offer efficient guideline and further in the discovery of high-efficiency DSSCs for experimentalists.

Fig. 1 Molecular structures of all the studied dyes.
2 Methods
The ground-state geometries of pure dyes RD1-6 and R6 were optimized at hybrid DFT levels by the B3LYP functional27with 6-311G(d,p)basis set28. Based on the fully optimized geometry,the frequency calculation was performed at the same theoretical level to confirm that the optimized geometry is at minimum point (no imaginary frequencies)on the potential energy surface.The conductor-like polarizable continuum model (CPCM)29was carried out in experimental solvent environment tetrahydrofuran(THF)26. The calculated value of the excitation energy as well as the absorption ones were dominated mainly by the exchangecorrelation (XC)functional with the ratio of Hartree-Fock (HF)to be used30. With the aim to select a trustworthy functional to evaluate the absorption spectra, we considered nine commonly used TD-DFT methods, including B3LYP (20%)31, CAMB3LYP (19%)32, PBE0 (33.33%)33, HSE06 (25%)34, MPW1K(42.8%), MPW1PW91 (25%)35, BHandHLYP (50%)36, M06(27%)and M062X (54%)37functionals. The maximum absorption wavelength (λmax), the oscillator strengthf, and the main excitation configuration are presented in Fig. S1 and Table S1 (in Supporting Information). Based on the well agreement with the experimental value, the BHandHLYP is a good choice as the functional for the UV/Vis absorption spectral calculation38. Thus, the absorption spectra of all of the designed dyes were simulated by the TD-CPCM-BHandHLYP/6-311G(d,p)method. The absorption spectra of the studied dyes were performed using the Originlab program package. The frontier molecular orbital (FMO)energies were determined at CPCM-B3LYP/6-311G(d,p)level in THF. DFT and TD-DFT calculations on the studied dyeswere performed using the Gaussian 16 program package39. The electron density differences maps (EDDMs)of all the studied dyes were investigated by Multiwfn 3.4 code40.
The adsorption of isolated dyes on the (TiO2)38clusters were investigated with DFT calculations using DMol3 program in Material Studio Version 7.041,42. The excitation energy of(TiO2)38cluster was in good agreement with the experimental value of TiO2semiconductor by De Angelis and co-workers41.Therefore, the model was used to simulate the semiconductor surface. The dye-(TiO2)38complexe configuration was fully optimized using the generalized gradient-corrected approximation (GGA)method43. The Perdew-Burke-Ernzerhof(PBE)functional44was used to account of the exchangecorrelation effects with the double numerical polarization (DNP)basis set.
3 Results and discussion
3.1 Geometrical and electronic structure
Commonly, the degree of conjugation is a vital factor that affect the absorption spectra of the sensitizers, so the dihedral angles (Φ, Fig. S2)between the EWG group and the benzoic acid(BA)were calculated. In comparison with R6 (35.46°), we find that dye RD1 (7.81°), RD2 (23.26°), RD4 (15.59°), RD5(21.85°)and RD6 (0.01°)exhibit the lesser dihedral angles,showing that dyes RD1, 2, 4–6 are endowed with a better coplanarity owing to their smaller steric hindrance. However,dye RD3 (37.71°)displays a larger dihedral angle, which could be unfavorable to charge transfer from the EWG group to BA group from the geometrical point of view. Thence, dyes RD1, 2,4–6 could have efficient intramolecular charge transfer.
The distribution of frontier molecular orbitals (FMOs)significantly affect the electronic excitation and transition characters. The FMOs of R6 and dyes RD1–6 are illustrated in Fig. S3. It is obvious that the HOMOs of all the investigated dyes are largely localized on donor and partly spread over EWG group, while the LUMOs are mainly distributed on acceptor, less electron density around the donor group. As shown for the FMOs, the charge separated states might favor efficient electron injection from the sensitizer to the semiconductor, whereas the overlaps might be advantageous to the intramolecular electron transfer. To furthe probe the intramolecular electron transfer of the designed dyes, the EDDMs (Table 1)were investigated. For dyes RD1–6 and R6, the decreasing electron density (purple)are mostly localized on the donor, and the increasing electron density (blue)are largely distributed on the acceptor. The above results imply that the designed dyes display favorable abilities in intramolecular charge transfer.
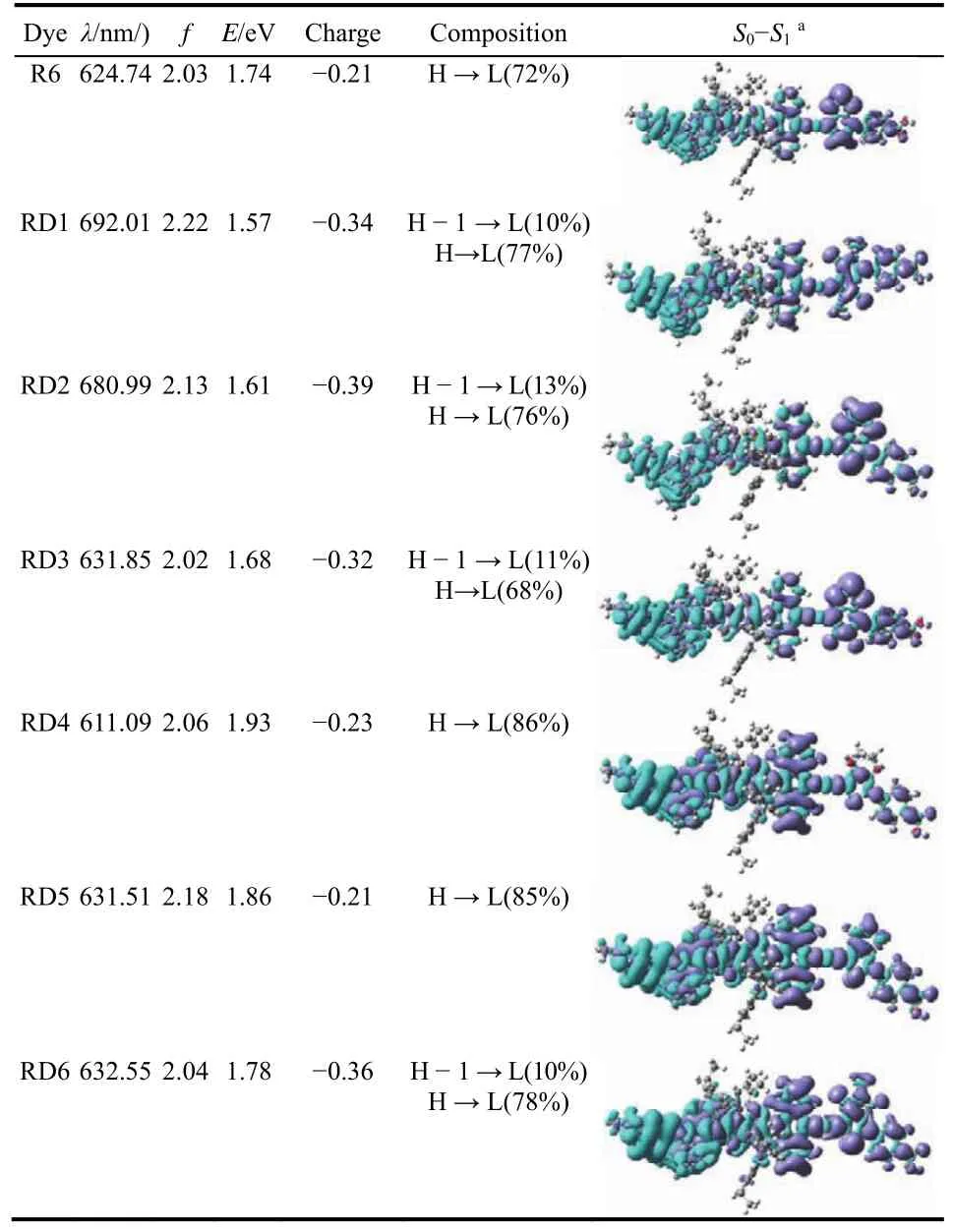
Table 1 Calculated absorption spectra (λ), oscillator strengths (f),excitation energies (E), Mulliken charge main,compositions and EDDs maps for the dyes in THF using TD-CPCM-BHandHLYP/6-311G(d,p)method.
Moreover, the HOMO-LUMO gaps of the investigated dyes are displayed in Fig. 2. The HOMO energy levels of the investigated dyes match well with those of their electron donors 9,19-dihydrobenzo[1',10']phenanthro[3',4':4,5]thieno[3,2-b]benzo[1,10]phenanthro[3,4-d]thiophene (BPT2), while the different band gaps of the investigated dyes are predominantly owing to the different electron-withdrawing capacity of the investigated dyes. Obviously, the band gap of dyes RD1–6 could be tuned by the introduction of EWGs. In order to probe the further effect of the EWGs on the band gaps of the dyes, the Mulliken charges of the EWGs are calculated45(Table 1). The Mulliken charges of the EWGs are in the order of R6 = RD5 > RD4 > RD3 > RD1 >RD6 > RD2. The result implies that dyes RD1–3, 6 would have stronger intramolecular interaction and their LUMO energy levels would down-shift compared to other dyes. Moreover, dyes RD1–3 have the lower LUMO energy and narrower band gaps than R6, indicating that dyes RD1–3 would display red-shift in the absorption spectra and their electrons being more advantageous to excitation.
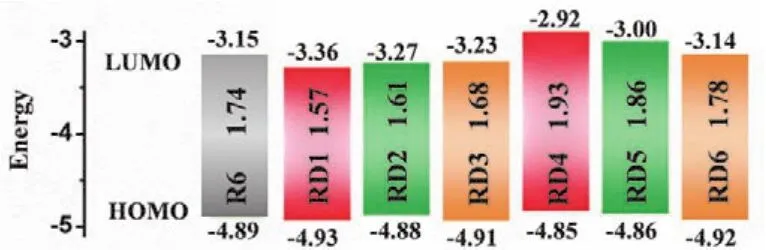
Fig. 2 Frontier molecular orbital energy levels obtained at CPCM-B3LYP /6-311G(d,p)level in THF.
3.2 Absorption spectra
To figure out the effect of EWG groups on the light harvesting capacities, the absorption spectra of the investigated dyes were calculated. As shown in Fig. 3 and Table 1, dyes RD1–3, 5, 6 exhibit red-shifts in the absorption spectra. As shown in Fig. 3 and Table 1, the maximum absorption wavelengths of investigated dyes are in the order of RD1 (692.01 nm)> RD2(680.99 nm)> RD6 (632.55 nm)> RD3 (631.85 nm)≈ RD5(631.51 nm)> R6 (624.74 nm)> RD4 (611.09 nm). Obviously,in comparison with R6 of BTD, the dyes RD1, 2 of EWGs exhibit significantly red-shift in their absorption spectra. And dyes RD1, 2, 5 have higher oscillator strengths than R6, which indicate dyes RD1, 2 have the superior light harvesting capacities.

Fig. 3 The absorption spectra of R6 and the designed dyes.
3.3 Dye adsorption on (TiO2)38
To simulate the performance of the DSSCs more realistic, we investigated the electronic properties of the designed dyes adsorbed on the (TiO2)38 cluster (Fig. S4). The calculated bond distances between the Ti of TiO2and O of dyes are in the range of 0.213–0.222 nm (Table 2), and the minimum atomic distances between the dyes and TiO2are in the range of 0.197–0.212 nm.The HOMO and LUMO orbitals of dye-(TiO2)38complexes are presented in Fig. S5, with the HOMO of the designed dyes are entirely localized on the dyes, while the LUMO of designed dyes are mainly distributed on the anchoring groups and (TiO2)38clusters, indicating that the electron injection from dye to semiconductor can be sufficiently achieved.
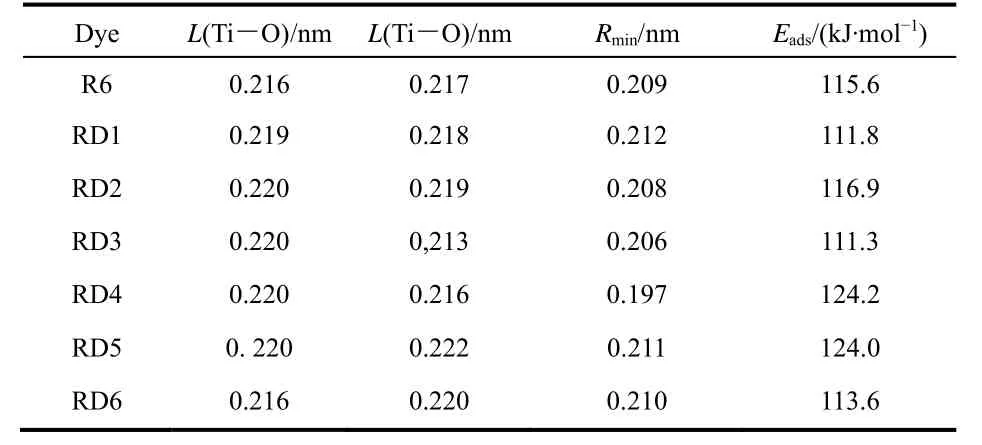
Table 2 Calculated bond length (L), and adsorption energy of dye-(TiO2)38 complexes
In order to investigate the adsorption capability of dyes, the adsorption energies (Eads)were investigated. Generally, the adsorption energies of the dye-(TiO2)38complexes (Eads=Edye+TiO2− (Edye+ETiO2)), whereEdyeis the total energy of isolated dye,ETiO2andEdye+TiO2are total energy of (TiO2)38cluster and dye-(TiO2)38complex, respectively46. The adsorption energies of the investigated dyes are presented in Table 2. The calculated adsorption energies of dyes RD1–6 are in the range 111.3–124.2 kJ·mol−1. The results imply that dyes RD1–5 are adsorbed on the TiO2surface tightly. It is manifested that dyes RD1–5 have a better performance in DSSCs.
3.4 Overall efficiency (η)
Generally, the overall efficiency (η)of DSSCs can be expressed as the product of the short-circuit current density (Jsc),the open-circuit voltage (Voc), the fill factor (FF), and the incident solar power (Pin)47.

whereas FF andPinare the experiment value, and the FF/Pin is assumed to experiment factor, according to experiment of R6.According to Eq.(1), theJscandVocare two key factors determining the PCE of DSSCs. In the succeeding sections, we focus to discussion them in detail.
3.4.1 Short-circuit current density (Jsc)
In DSSCs, the short-circuit current density (Jsc)can be determined by48

whereeis the unit charge, IPCE(λ)is incident the photon-tocurrent conversion efficiency at a fixed wavelength. The IPCE(λ)term is made up of the light-harvesting efficiency (LHE(λ))49at a given wavelength, the electron injection efficiency (Φinj), and the electron collection efficiency (ηcoll).Is(λ)is the corresponding photon flux for air mass (AM)1.5 G (global)solar radiation spectrum. The LHE can be estimated by

whereε(λ)is the molar absorption coefficient at the relevant wavelength.bandcare thicknesses of TiO2 film and concentration, respectively, which derived from the experimental parameters of structurally similar derivatives R2 with 9.5 μm and 1.92 × 10−8mol·cm−2·μm−1, respectively25. The simulated LHE curves of dyes RD1–6 are displayed in Fig. 4.The LHE curves of dyes RD1–3, 5, 6 are red-shifted and broaderedcompared to R6, which reveal their higher light harvesting capacity. Importantly, the curves of dyes RD1, 2 represent significantly red-shifted and broadened. We can further infer that they match better between the absorption spectra of the dyes and the photon flux spectrum, this means that dyes RD1, 2 would display the highJsc.
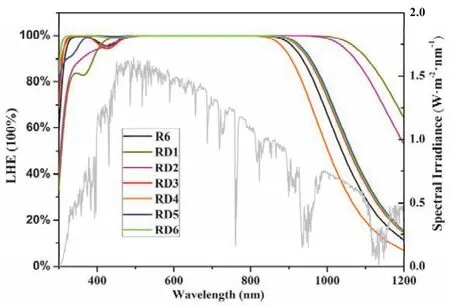
Fig. 4 The LHE curves of R6 and dyes RD1–6.
According to Eq.(2), the predictive short-circuit current densitycan be approximately estimated by the following equation

whereαis the correction parameter, which can be simulated by Eq.(5)as the product of theΦinj andηcoll.

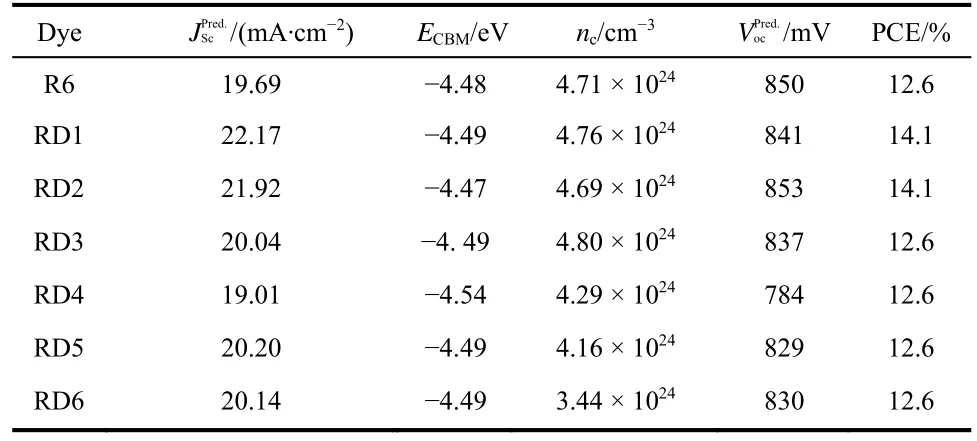
Table 3 Calculated optoelectronic properties of the dye-(TiO2)38 complexes.
3.4.2 Open-circuit voltage (Voc)
As another key parameter, the open circuit voltage (Voc)be estimated with the following expression50of dyes RD1–3, 5, 6 are higher than that of R6 (19.69 mA·cm−2).

wherekbTis the thermal energy, and the temperature is 300 K, a typical value forkbis 8.617 × 10−5eV·K−126.NCB is the density of accessible states in the conduction band (7 × 1020cm−3), andEredoxis the redox potential of the Co-pen redox couple in the electrolyte (−5.06 eV)51. TheECBMis the shift of conduction band minimum edge of the semiconductor, which leads to efficient electron injection from the excited dye to the conduction band (CB)of semiconductor.ncis the number of electrons in the TiO2, which can be calculated by employing the integral of the wave function distributions of the LUMO of the chromophore on the semiconductor substrate, which can be described by Eq.(7)15,52–54.
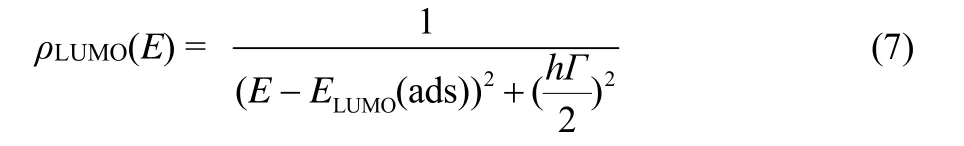
wherehΓis a lifetime broadening.ρLUMO(E)andELUMO(ads)are Lorentzian distribution and weighted average calculated energy, respectively. The calculatedncvalues of studied dyes(Table 3)decrease in the order RD3 > RD1 > R6 > RD2 > RD4 >RD5 > RD6. The result shows that dyes RD1–3 could inject more electrons into (TiO2)38cluster, which indicates that they will show better charge-transport properties that would be similar to R6.
The predictive open circuit voltage (, Table 3)is calculated according to Eq.(8)
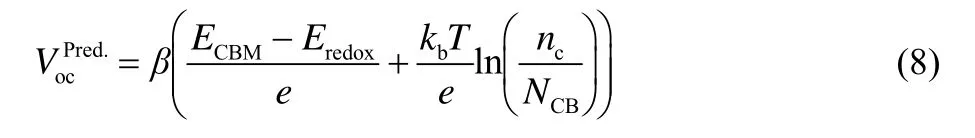
whereβis the correction factor, which can be described by

The calculated PCE values of dyes RD1–6 and R6 are listed in Table 3. The results show that the PCE of the designed dyes are in the range of 12.6%–14.1%. In particular, the DSSCs sensitized with RD1, 2 are expected to produce the PCE of 14.1%, indicating that dyes RD1, 2 could be the promising DSSC sensitizers.
4 Conclusions
We have systematically investigated designed dyes RD1–6 with different electron-withdrawing groups based on the experimentally dye R6 using the DFT and TDDFT methods. The parameters related to the cell performance including the absorption spectra, the light harvesting efficiency (LHE)curve,the predictive short circuit current densitythe predictive open circuit voltageand the theoretical PCE were investigated in detail. The results illustrate that all the designed dyes exhibit the high PCE. In particular, dyes RD1, 2, 4–6 show the greater conjugations and dyes RD1–3 have the smaller energy gaps compared with R6. In addition, dyes RD1–3, 5, 6 exhibit the better light harvesting capacities with covering the entire visible region and extending to near infrared region with obviously red-shift maximum absorption wavelengths (λmax),wider light harvesting efficiency (LHE)curves and higher predictive short-circuit current densitiescompared to R6.It is worth mentioning that dyes RD1, 2 not only exhibit the greater conjugations, obviously narrower band gaps and greater than 56 nm red-shift maximum absorption wavelengths contrast to R6, but also show the remarkably better light-harvesting capacities with broadened LHE curves. It is worth mentioning that mainly contributed by an average increment of 12.0% of the short circuit current density, the remarkable increment of the PCE from 12.6% for R6 to 14.1% for RD1, 2. The results conclude that dyes RD1, 2 could be the most promising sensitizer candidates for the highly efficient DSSCs. We hope our work could be efficiently guide the experimentalists to the discovery of advanced sensitizers.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- Hollow Nitrogen-Rich Carbon Nanoworms with High Activity for Metal-Free Selective Aerobic Oxidation of Benzyl Alcohol
- Photocrosslinking-Immobilized Polymer Vesicles for Lowering Temperature Triggered Drug Release
- CO Hydrogenation to Ethanol over Supported Rh-Based Catalyst:Effect of the Support
- CdTeSe合金幻数团簇的室温合成和形成机理研究
- 体相界面导通的复合快离子导体
- 异氰酸苯酯诱导的类胶原多肽自组装

