Atomic Layer Deposited Al-doped ZnO Nanomembranes for Supercapacitor Application
NAEEM Farah, NAEEM Sumayyah, ZHANG Jing , MEI Yongfeng , HUANG Gaoshan
(1. State Key Laboratory for Modification of Chemical Fibers and Polymer Material Science and Engineering, Donghua University, Shanghai 201620, China; 2. Department of Materials Science, Fudan University,Shanghai 200433, China; 3. College of Science, Donghua University,Shanghai 201620, China)
【Abstract】 Electrochemical supercapacitors are continuously taking part to fulfill energy requirements. In this study, we described the potential application of Al-doped ZnO (AZO) nanomembranes in supercapacitor. The nanomembranes were fabricated by atomic layer deposition and their structures were stacks of alternate ultrathin ZnO and Al2O3 layers with different cycle numbers. The ultrathin AZO nanomembranes were well electrochemically responded and the results demonstrated the (10∶1)10 sample had the most suitable behavior of charging and discharging. The best specific capacitance of the AZO nanomembranes supercapacitor was 61 F·g-1 at 1 A·g-1 in 6 M KOH electrolyte. As for practical application, we demonstrate the supercapacitor can flash a red LED light for more than 60 min. The AZO nanomembranes have potential in supercapacitor and could be engaged in future wearable energy storage device.
【Key words】 Aluminum doped zinc oxide; Nanomembranes; Atomic layer deposition; Electrochemical characterizations; Supercapacitor
1 Introduction
With increasing awareness of environment pro-tection, the clean renewable energy resources have drawn vast attention among researchers[1]. The high electrical energy density and high-power density with sustainability and efficiency of energy storage devices are demanded[2-3]. Among all kinds of energy storage devices, supercapacitors have become the most promising ones due to their high-power density, fast charge/discharge rate, and good cycling performance, which are thousands of times higher than a battery or conventional capacitors[4-5]. Generally, supercapacitive behavior is competent in high power and large charge/discharge rates as compared to batteries, but the energy density is sacrificed[6-7]. The improvement of specific capacitance will promote the energy/power densities with device efficiency, which further enhances the energy storage performance[8-9]. Nowadays, 2D materials have become ideal electrode materials for energy generation and storage devices owing to their low cost, low toxicity, multiple oxidation states, and good flexibility[10]. In particular, a large surface-to-volume ratio of 2D nanomembranes is one of the substantial parameters in the contribution of electrostatic supercapacitors applications[11-12].
As candidates for transparent conductive oxides, ZnO-based materials have attracted considerable attention[13-16]. They have already demonstrated prominent roles in supercapacitors. For instance, JACOBetal.[17]studied the Cu/ZnO doped graphene nanocomposites, and the electrochemical analysis exhibited the high specific capacity of 630 mAh·g-1with 95% capacitance retains after 100 cycles. ARAVINDAetal.[18]used magnetron sputtering to fabricate ZnO film with a thickness of 186 nm on carbon nanotube, which generated the high capacitance of 59 F·g-1. Unfortunately, intrinsic defects of undoped ZnO leads to unstable thermal conductivity and poor charging-discharging behavior for commercial supercapacitor[19]. In order to improve physical and electrochemical properties of ZnO, group III (B3+, Al3+, Ga3+, and In3+) ions can be used to generate the extra electrons[20-23]. Among them, Al3+is a promising doping material due its small ion radius and the doping of Al3+ions can greatly improve electrical conductivity and thermal stability[24]. A number of fabrication techniques are introduced to deposit Al-doped ZnO (AZO) thin films such as sol-gel, chemical spray, thermal evaporation, pulsed laser deposition, and magnetron sputtering[25]. Among these techniques, despite of the low growth rate, atomic layer deposition (ALD) has the advantage of angstrom scale conformal coverage under self-limited surface reaction with high quality[26]. Especially, ALD is a suitable technique to fabricate the high density ultra-thin nanomembranes at relatively low temperatures on various substrates such as silicon, transparent glass, and polymers substrates[25-27]. In present work, AZO nanomem-branes (NMs) with layered structures were prepared and used as electrode of supercapacitor. In our experiment, the AZO NMs consisting of Al2O3and ZnO layers with different thicknesses were fabricated by tuning corresponding ALD cycles, demonstrating the excellent feasibility of ALD in producing composite structures. The obtained supercapacitor exhibited high specific capacitance up to 61 F·g-1at a current density of 1 A·g-1in 6 M KOH electrolyte. The electrochemical response of ultrathin AZO NMs in terms of 60 min red LED flashing suggests their great potential in future wearable and flexible energy storage devices.
2 Experimental
2.1 Fabrication of AZO NMs
To fabricate the AZO NMs on polymer substr-ate, diethylzinc (DEZ), trimethyl aluminum, and deionized water were used as precursors. The precursors used were purchased from J&K Scientific Ltd., China. The AZO NMs were deposited at 150 ℃ on polyurethane sponge templates in presence of 20 sccm flow rate nitrogen (N2) carrier gas with 20 ms purging. Alternate cycling process of diethylzinc-H2O with 50 ms pulse time and 30 s purge time was used to deposit ZnO layer. The doping of Al layer was introduced by Trimethylaluminium-H2O cycles with same pulse and purge times. Three AZO NM samples were prepared in our experiment. The first sample is 5 loops of ZnO (10 cycles)/Al2O3(1 cycle), which is named as AZO (10∶1)5, the second sample is 10 loops of ZnO (10 cycles)/Al2O3(1 cycle), which is named as AZO (10∶1)10, and the third sample is 20 loops of ZnO (10 cycles)/Al2O3(1 cycle), which is named as AZO (10∶1)20. The polyurethane sponge templates with AZO deposited were then removed after calcination in O2atmosphere (600 mL·min-1) at 700 ℃ for 3 h. The remaining AZO NMs were purified with ethanol and de-ionized water to remove the residual impurities.
2.2 Fabrication of AZO NMs electrodes
90 wt% of active materials (i.e., AZO NMs with different structures) were firstly mixed with 10 wt% of binder (polytetrafluoroethylene, PTFE). A milling process was used to prepare homogenous paste which was then deposited onto clean nickel foam substrate and degassed at 60 ℃ for 2 h in vacuum. The prepared electrodes were soaked into corresponding electrolyte for 12 h after being pressed under 10 MPa pressure.
2.3 Microstructural characterizations
To examine the morphologies, crystal structures, and compositions of AZO NMs, scanning electron microscopy (SEM, Zeiss Sigma), X-ray diffraction spectroscopy (XRD, Bruker D8A Advanced XRD with Cu Kα radiation, λ=1.5405 Å), X-ray photoelectron spectroscopy (XPS, PHI 5000C EACA, with C 1s peak at 284.6 eV) were used.
2.4 Electrochemical Characterizations
The electrochemical characterizations of AZO NMs electrodes were carried out on a Chenhua CHI 660E electrochemical workstation. The three-electrode system equipped with Ag/AgCl reference electrode and Pt foil counter electrode was used to determine the electrochemical properties of the samples. The AZO NMs were investigated in KOH electrolyte by means of cyclic voltammetry (CV), chronopotentiometry (CP), and electrochemical impedance spectroscopy (EIS, frequency range of 1-100 kHz with an amplitude of 5 mV) characteri-zations at room temperature. The calculation methods of specific capacitance (Cs) and energy/power densities were presented in Supporting Information.
3 Results and Discussion
The schematic of fabrication of AZO NMs supercapacitor is represented in Fig. 1. The reactive precursors were injected into the ALD chamber at 150 ℃ to deposit the ZnO layers and Al2O3layers over surface reactions[28]. Alternate deposition of precursors on sponge template leads to layer-by-layer coating of oxides by following reactions (1)-(4) (* indicates the free radical)[29]:
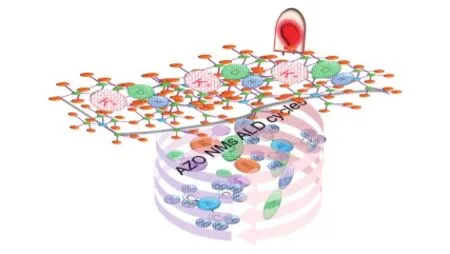
Fig. 1 Schematic representation of AZO NMs supercapacitor for potential application
ZnOH*+Zn(C2H4C2H6)→ZnOZn(CH2CH3)*+CH3CH3
(1)
Zn(CH2CH3)*+H2O→ZnOH*+CH3CH3
(2)
AlOH*+Al(CH3)3→AlOAl(CH3)2*+CH4
(3)
AlCH3*+H2O→AlOH*+CH4
(4)
The AZO NMs with different numbers of loops were obtained after thermal removal of sponge template at 700 ℃ for 3 h in O2atmosphere[30]. The morphologies of AZO (10∶1)5, AZO (10∶1)10, and AZO (10∶1)20NMs are demonstrated in the Figs. 2(a-c). The SEM images display that AZO NMs have uniform thickness. Fig. 2(a) show the morphologies of AZO (10∶1)5NMs. The red arrows in Fig. 2a indicates the typical thickness of NM is 10.5 nm. The variation of dimensions and increased thickness with the number of ALD cycles can be observed in AZO (10∶1)10NMs shown in Fig. 2(b). A typical AZO (10∶1)10NM in Fig. 2(b) has a lateral size of 306 μm, and a thickness of 18 nm. The uniformity of AZO (10∶1)20NMs can also be observed in Fig.2(c). The measured thickness is 33 nm. All these SEM images in Fig. 2 prove the feasibility of current approach in producing free-standing uniform AZO NMs.
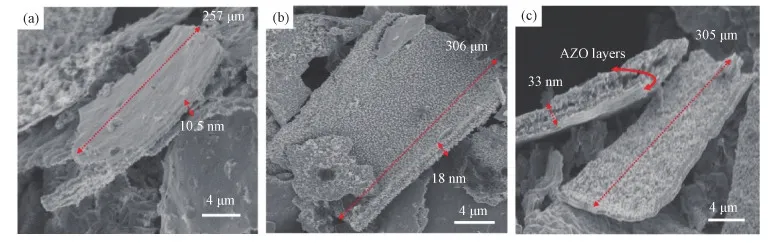
Fig. 2 SEM images of ALD deposited AZO NMs (a) AZO (10∶1)5 NMs, (b) AZO (10∶1)10 NMs, and (c) AZO (10∶1)20 NMs (see electronic version for color map)
In order to further reveal the crystal structure of AZO NMs, XRD characterization was carried out and the results are shown in Fig. 3. XRD patterns revealed Al substituting into ZnO matrix. The presence of (102) peak in all three samples with reduced intensity demonstrated the poor crystal quality of current AZO NMs[31]. The existence of dopant Al in the AZO NM should affect the diffraction peak of the host due to ionic size difference[32]. To ensure the effective doping of Al in the sample, here we selected AZO (10∶1)10NMs for XPS analysis. Fig. S1 demonstrates the O 1s peak at 531.6 eV, Zn 2p1/2, and Zn 2p3/2peaks at 1045.8 and 1022.8 eV, and Al 2p peak at 74.1 eV, indicating successful incorporation of Al ions[33].
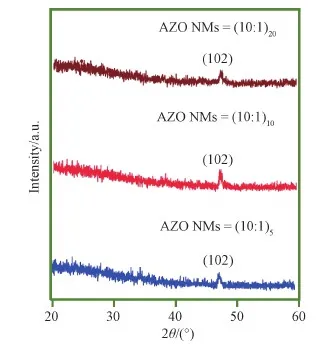
Fig. 3 XRD pattern of AZO (10∶1)5, AZO (10∶1)10, and AZO (10∶1)20 NMs. The peaks correspond to hexagonal wurtzite AZO
The electrochemical performances of electrodes made from different AZO NMs were evaluated in 6 M KOH aqueous solution. The CV curves shown in Figs. 4(a, c, e) and S2 exhibit oxidation and reduction peaks, which well represent the pseudocapacitor behavior of the AZO NMs. The anodic and cathodic peaks in the CV curves indicate the functional groups' activation on surfaces with well electrode/electrolyte surface interaction[34]. Fig. S2(a) provides the comparison of AZO NMs with different structures. The obvious redox activities are observed in all three electrodes. The large curves area is noticeable due to the good surface electrode-electrolyte interaction[35]. In addition, AZO (10∶1)10also shows the inner active site interaction[36]. Figs. 4 (b, d, f) provide the charging and discharging performance of supercapcacitor within the potential range of 0-0.5 V. The nonlinear CP curves represent the stability of all three electrodes due to faradaic behavior.
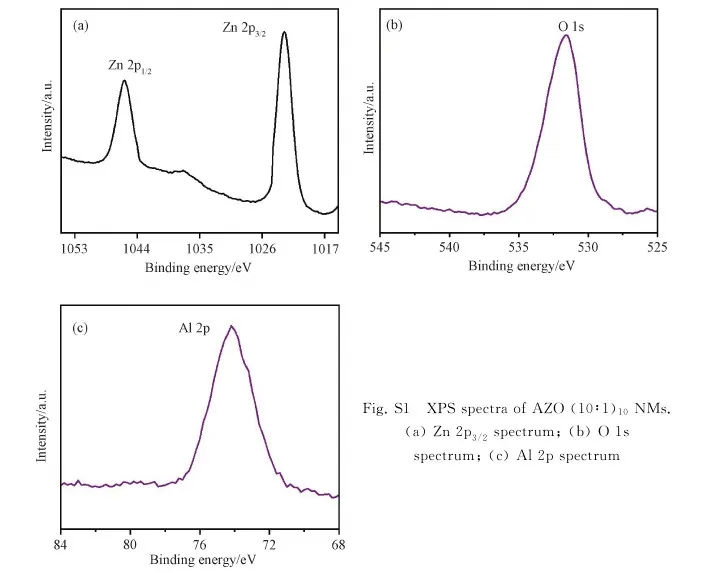
Fig. S1 XPS spectra of AZO (10∶1)10 NMs. (a) Zn 2p3/2 spectrum; (b) O 1s spectrum; (c) Al 2p spectrum
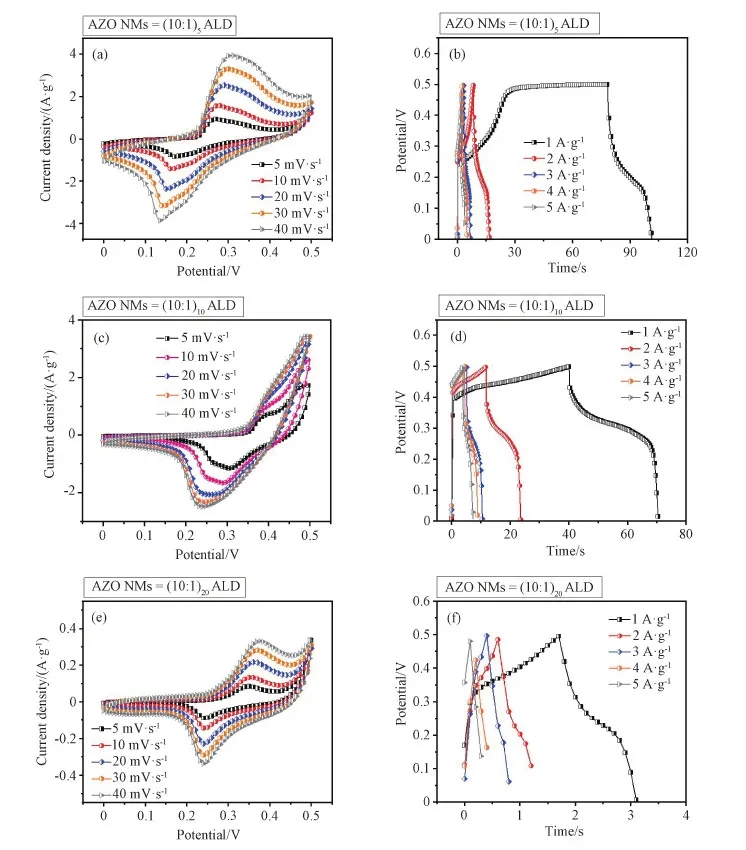
Fig. 4 Electrochemical characterizations of electrodes made from AZO (10∶1)5 NMs, AZO (10∶1)10 NMs, and AZO (10∶1)20 NMs.(a, c, e) CV profiles at scan rates of 5-40 mV·s-1. (b, d, f) Charge/discharge profiles at current densities of 1-5 A·g-1
In current results, all gravimetric curves exhibite obvious electrolyte/surface interaction at determined current densities. The appeared pseudocapacitive behavior might be due to electrolyte ions adsorption onto the surface of AZO NMs electrode[37]. In Fig.S2(b), AZO (10∶1)5NMs electrode demonstrates prolonged charging behavior at 1 A·g-1whereas AZO (10∶1)20NMs electrode clearly exhibits the fast pseoudcapacitor gravimetric performance[38]. The study of these behaviors demonstrates the surface electrode-electrolyte interaction with minimized ion diffusion length and charge transfer length, while thick AZO (10∶1)20NMs allows limited access of ions transportation. Fig. 4(f) exhibits the current mobility proportion-ality towards the electrode[39]. At low current densities, electrode-electrolyte demonstrate strong polarity interaction while at higher current density the interaction is reduced with weaker polarity[40]. In addition, the discharging behaviors of the AZO NMs electrodes (Fig. S2) are considered to be due to the influence from oxide/electrolyte interfaces, polarization bonds at surface, and contribution from inner active materials[41-42].
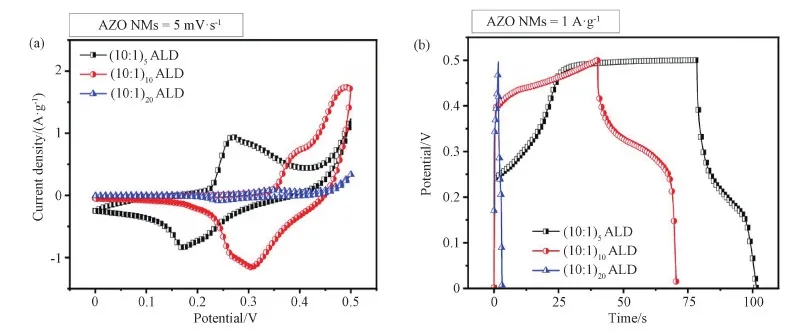
Fig.S2 Electrochemical characterizations of electrodes made from AZO (10∶1)5 NMs, AZO (10∶1)10 NMs, and AZO (10∶1)20 NMs. (a) CV curves; (b) charging-discharging behaviors
The specific capacitance (Cs) of three electrodes made from AZO NMs with different ALD loops are calculated based on the CP curves in Fig. 4 (see Supporting Information for details), and the results are shown in Figs. 5(a, c, e). The specific capacitances of AZO (10∶1)5NMs in 6 M KOH are 46-19 F·g-1for current densities of 1-5 A·g-1. Similarly, the specific capacitances of AZO (10∶1)10NMs are determined to be 61-34 F·g-1under same circumstance. However, the capacitances of AZO (10∶1)20NMs are only 3-2 F·g-1(see Table 1). The decreased specific capacitances at higher current densities are considered to be due to the weak electrolyte polarity on electrode surface, increased resistance of diffusion ions, and low electrical conductivity[40]. The best specific capacitance perfor-mance of AZO (10∶1)10NMs electrode may be related to appropriate ions diffusion, availability of active sites, and small interfacial resistance[43]. Figs. 5(b, d, f) represented the corresponding energy density as a function of power density for three electrodes (see Table 2). The observed specific capacitances and energy-power densities of three electrodes demonstrate the good redox electro-chemical activity during ions transportation into electrode-electrolyte[38-40]. It is worth noting that the electrode made from AZO (10∶1)10NMs exhibits the largest specific capacitance as well as the highest energy density, and it is considered to be due to the fastest ion's transportation and connection between electrode/electrolyte interface[44].
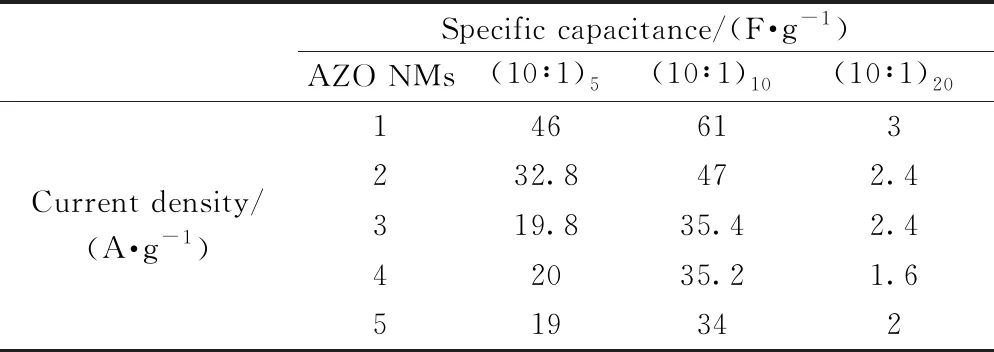
Table 1 Specific capacitances of three kinds of AZO NMs
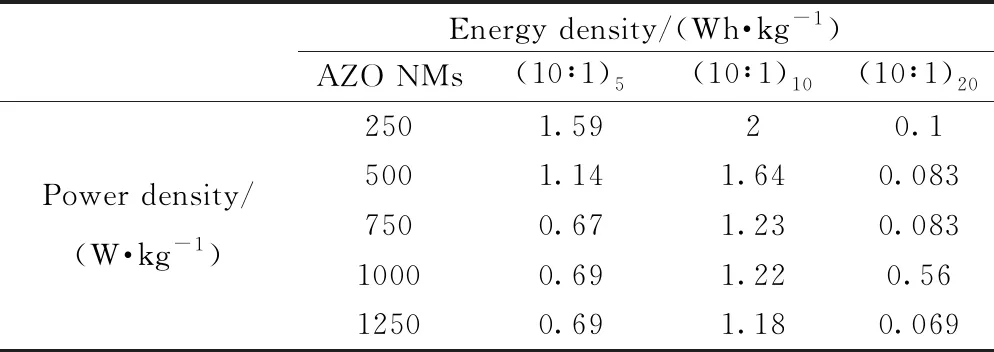
Table 2 Energy density and power density of three kinds of AZO NMs
The Nyquist plots of three electrodes are shown in Fig. S3. All the electrodes exhibit low charge transferred resistance at the electrode-electrolyte interface. The inclined resistance region at low frequency of AZO (10∶1)10NMs electrode indicates the lowest resistance compared with electrodes made from AZO (10∶1)5NMs and AZO (10∶1)20NMs. These experimental results demonstrate that the structure of AZO NMs can significantly influence electrochemical properties over charge transfor-mation, electrolyte resistance, Warburg impedance, and pseudocapacitance. The fast charge transfor-mation of AZO (10∶1)10NMs electrode might be due to the influence of suitable doping and optimized electrolyte/electrode interface[45]. The slow ion diffusion in AZO (10∶1)5NMs and AZO (10∶1)20NMs causes increased resistivity with reduced specific capacitance and this also leads to small surface activation for electrons transformation[46]. The satisfactory diffusion rate with sustainable active sites improve the redox transition in AZO (10∶1)10NMs electrode as compared to electrodes made from AZO (10∶1)5NMs and AZO (10∶1)20NMs[40]. All our results represent the contribution of electrode with redox activity[47].
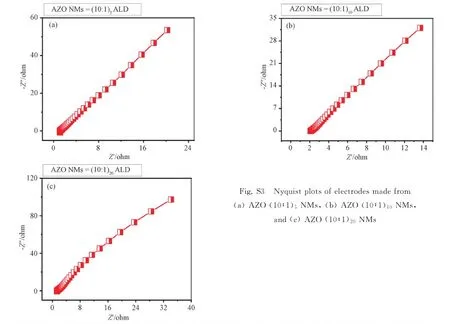
Fig. S3 Nyquist plots of electrodes made from (a) AZO (10∶1)5 NMs, (b) AZO (10∶1)10 NMs, and (c) AZO (10∶1)20 NMs
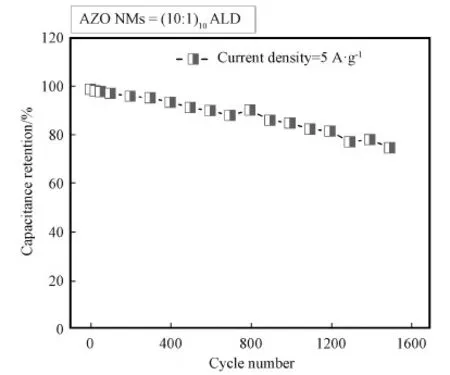
Fig. S4 Cycling performance of AZO NMs at a current density of 5 A·g-1
In order to evaluate the cycling stability of AZO (10∶1)10NMs electrode, cycling test was carried out at a current density of 5 A·g-1. As shown in Fig. S4, a great cycling performance can be seen and about 70% of initial capacitance is maintained after 1600 cycles. To further evaluate the practical application for AZO (10∶1)10NMs, we prepared a supercapacitor device equipped with electrode made from AZO (10∶1)10NMs. As shown in Video 1 in Supporting Information, the device can flash one red LED light over 60 min, implying the great potential of this kind of supercapacitors in practical applications.
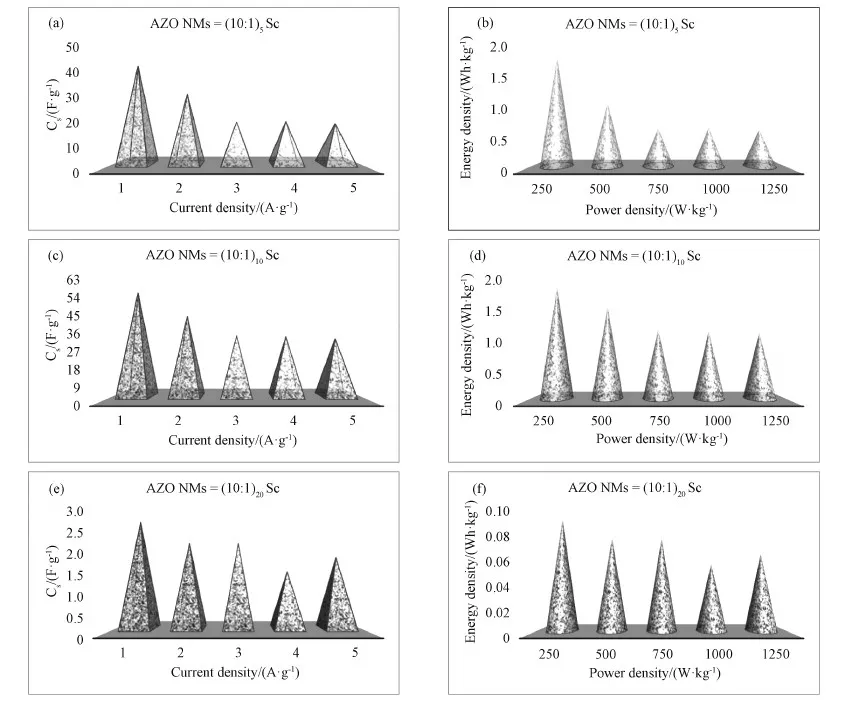
Fig. 5 (a, c, e) Specific capacitance (Cs) as a function of the current density. (b, d, f) Ragone plot of energy and power densities of AZO NMs
4 Conclusions
In summary, we have fabricated AZO NMs for supercapacitor applications. The NMs are prepared by ALD technique and the structure of the NMs can be easily tuned. The electrochemical characterizations were carried out in 6 M KOH and the electrode fabricated from AZO (10∶1)10NMs demonstrates the best performance and a about 70% capacitance retention after 1600 cycles was observed. For practical applications, we demonstrate that the obtained supercapacitor can power a red LED for at least 60 min. The ultrathin AZO NMs have important potential in future wearable, light, and flexible energy storage devices.
(s1)
where,Jis current density (A·g-1), Δtis discharge time (s), Δvis voltage window (V).
The energy density (E) was calculated according to the following equation:
(s2)
where,Cis specific capacitance (F·g-1), ΔVis voltage window (V).
The power density (P) was calculated according to the following equations:
(s3)
where,Eis energy density (Wh·kg-1), Δtis discharge time (s)

