Properties and microstructures of a matrix graphite for fuel elements of pebble-bed reactors after high temperature purification at different temperatures
ZHOU Xiang-wen,ZHANG Kai-hong,YANG Yang,WANG Lei,ZHANG Jie,LU Zhen-ming,LIU Bing,TANG Ya-ping
(Key Laboratory of Advanced Reactor Engineering and Safety, Ministry of Education, Institute of Nuclear and New Energy Technology of Tsinghua University, Beijing 100084 China)
Abstract:The matrix graphite (MG) of pebble fuel elements for a High Temperature Gas-cooled Reactor (HTGR),composed of 71wt% natural graphite,18 wt% artificial graphite and 11 wt% phenolic resin-derived carbon,was purified by high temperature treatment (HTT),and its properties and microstructure were analyzed to investigate the effect of different HTT temperatures and optimize the purification temperature.Results showed that with increasing HTT temperature,its density and thermal conductivity gradually increased,but pore size and d002 gradually decreased.The rate of erosion caused by friction as the fuel pebbles move in the reactor also decreased.The ash content decreased significantly from to 18.2 to 12.3×10−6 after HTT at 1 600 ℃,but changed little when the HTT temperature was further increased to 1 900 ℃,especially for catalytic metals such as Fe,Ni and Ca that are related to its corrosion rate.The microstructure improvement and ash content reduction at high temperatures jointly contributed to the increase in the anti-corrosion performance of MG.Based on properties such as crushing strength,erosion resistance,and corrosion rate,a HTT of 1 600 ℃ is adequate although the MG gradually became more ordered with a further increase of HTT temperature from 1 600 to 1 900 ℃.This determination of an appropriate HTT temperature for the production of MG for the fuel elements of an HTGR should improve the production efficiency and reduce the mass production cost of this material for a commercial HTGR.
Key words:A3-3 matrix graphite;Thermal conductivity;Corrosion rate;High temperature purification;Ash content
1 Introduction
The demonstration plant of high temperature gascooled reactor (HTGR),high temperature reactor pebble-bed module(HTR-PM)are under construction in the coastal of east China[1].Around 800 000 pebble fuel elements are required and each pebble fuel element contains 7 g of uranium to initiate the HTR-PM.Since then,300 000 fuel elements are required to satisfy the normal operation of HTR-PM in full power every year[2].More than 90 wt% are A3-3 matrix graphite (MG) in a pebble fuel element of HTR-PM.A3-3 MG powder includes 64 wt% natural flake graphite,16 wt% artificial graphite,and 20 wt% phenolic resin binder to fabricate the green fuel pebbles by a cold quasi-isostatic pressing method[2,3].The green pebbles are carbonized first,and then purified at the high temperature of 1 900 ℃ under vacuum to remove the impurities in the MG,which is beneficial for improving their corrosion resistance and reducing the r adioactivity generated by the activated nuclides after irradiation.Commonly,such a heat treatment process is referred to as the“high-temperature purification(HTP)”[3,4].After carbonization and HTP,the A3-3 MG is approximately composed of 71 wt% natural flake graphite,18 wt% artificial graphite,and 11 wt%phenolic resin-derived carbon.With the progress and development of purification technology for graphite powder,the ash contents for fabricating the pebble fuel elements of HTR-PM decrease to about 1.0×10−5[5].Meanwhile,the ash content of phenolic resin is less than 5.0×10−5[4].Compared with those of raw materials used in the past,the purity levels of raw materials used to manufacture the pebble fuel elements for HTR-PM have been greatly improved.Hence,it is considered whether the HTP process is still necessary in the preparation of pebble fuel elements for HTR-PM.In our previous study[4],the comprehensive properties of carbonized MG with and without HTP treatment were comparatively analyzed and their microstructure features were characterized as well.Results showed that the crush strength,corrosion rate,and erosion rate of the carbonized MG without HTP treatment did not satisfy the specifications.After HTP,not only the purity but the comprehensive properties of MG were improved in various degrees.All the performances of MG treated with HTP met the technical requirements of A3-3 MG of pebble fuel elements for HTR-PM.The improvement of comprehensive properties of MG in HTP could be attributed to the synergistic effects of microstructure optimization and impurity reduction.
This paper presented the results of property measurement and microstructure characterization of MG pebbles purified at high temperatures.Temperatures (1 600–1 900 ℃) were selected for subsequent HTP of carbonized MG pebbles due to the further decomposition of a tiny amount of organic functional groups remaining on the surface of carbonized A3-3 MG up to more than 1 300 ℃[4].As a comparison,the overall properties and microstructure of the carbonized MG were investigated as well.
2 Experimental
2.1 Specimen preparation
Because more than 90 wt% of pebble fuel elements for HTR-PM is A3-3 MG,the comprehensive properties of fuel elements are commonly characterized with MG or MG pebbles instead of the fuel elements.Green MG pebbles were manufactured in INET[3]with a fixed fabricating process and qualified raw materials.The ash contents and equivalent boron contents (EBCs) of high-purity raw materials are listed in Table 1,which are important to ensure the purity of the fabricated MG pebbles.

Table 1 The ash contents and EBCs of raw materials for A3-3 MG (μg g−1).
The fabricated green pebbles were carbonized at 800 ℃ first,and then some of carbonized MG pebbles were subjected to HTP under vacuum at 1 600–1 900 ℃.The carbonized MG pebbles and HTP treated ones at 1 600–1 900 ℃ were named as MG-800,MG-1600,MG-1700,MG-1800 and MG-1900,respectively.Finally the pebbles were lathed to certain dimensions that met the technical requirements.
As mentioned in our previous work[4],the properties of MG such as the crush strength,erosion rate and corrosion rate of MG-800 did not meet the technical requirements.Therefore,these properties of MG with different heat treatment temperatures will be mainly measured and compared.Meanwhile,their ash contents and thermal conductivities will be comparatively characterized.All the samples were machined without a lubricant by ceramic cutting tools.MG pebbles for crush strength were tested parallel and perpendicular to the pressing direction,which were defined as axial (AX) and transverse (TR),respectively.The thermal conductivities of specimens were measured in the direction of AX and TR.Properties such as the erosion rate,corrosion rate,ash contents and corresponding impurity contents were examined with MG pebbles.Table 2 lists the exact shape,dimension,orientation and amount of the specimens for characterizing the comprehensive properties and microstructures of MG with different heat treatment temperatures.

Table 2 Specimen information for property and microstructure characterization of MG.
2.2 Property measurement
A Shimadzu AG-X100 universal testing machine with a maximum load of 100 kN was used to measure the crush strength of MG pebbles at a loading rate of 1 mm min−1.5 MG pebbles in each batch were measured in each direction and the average values were taken.According to the equation (1),the thermal conductivity (k) was obtained by measuring the thermal diffusivity (α),density (ρ) and specific heat (C) of MG.A Netszch LFA457 thermal flash sys-tem was used to measure the thermal diffusivity.

The corrosion rate of MG is determined by oxidizing the MG pebbles at a flow rate of 80 L h−1in a horizontal tube at 1 000 ℃ for 10 h.The oxidant gas consisted of 99 vol% helium and 1 vol% water vapor.Three MG pebbles were oxidized and the average value was calculated to get the corrosion rate of MG.The erosion rate was measured on a self-made cylinder chamber experimental device by putting 20 MG pebbles into the test chamber and then rotating the chamber horizontally at a certain speed for 100 h.The average erosion rate of MG could be obtained by calculating the weight changes during the erosion process.
Before the measurement of ash and impurity contents,acetone and methanol were used to ultrasonically clean MG pebbles to remove the impurities that might be introduced during machining.The cleaned MG pebbles were dried at 120 ℃ for 24 h and their original weights were measured.Then the pebbles were burned in platinum crucibles in a muffle furnace until the weights of the crucibles were constant.Two pebbles were used for parallel measurement in each batch.The ash content of MG pebbles could be calculated by the equation (2):
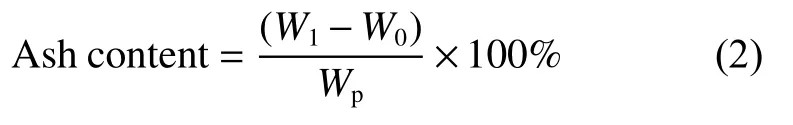
WhereW0is the initial weight of a platinum crucible andW1is the constant weight of the platinum crucible after burning the pebble,andWpis the initial weight of the pebble before burning.The residual ash powder in the platinum crucible was completely dissolved with hydrochloric acid,hydrofluoric acid,and nitric acid by microwave digestion technology.Inductively coupled plasma mass spectrometry (ICP-MS,Nexion 300,Perkin Elmer,USA) or inductively coupled plasma optical emission spectrometry (ICP-OES,Optima 8000DV,Perkin Elmer,USA) was used to further detect the solution to analyze the corresponding impurity element contents of MG.
2.3 Microstructure characterization
The pore size distribution and porosity of MG specimens were measured by mercury intrusion porosimetry in strict accordance with ASTM D4404-10[6].The pore volume of mercury intrusion was measured to determine the pore volume under different pressures.The absolute pressure was converted into apparent intruding pore diameter by the equation (3):

Wheredis the apparent diameter of the intrusion pore,γis the surface tension of mercury(485 dyne cm−1),θis the contact angle between mercury and MG (130°),andPis the absolute pressure causing the intrusion.
A Rigaku D/max 2500 X-ray diffractometer with Cu radiation (Kα=0.154 19 nm) was used to carry out the X-ray diffraction (XRD) analysis with a step of 0.02° at a scanning speed of 3° min−1in a 2θscanning mode from 10° to 90°.The equipment was calibrated and the test background was deducted before the test.Silicon powder was employed as the internal standard.Since the natural flake graphite powder and artificial graphite powder in MG had been completely graphitized,the heat treatment process would not change the microstructure of graphite powder,but only affect the microstructure of phenol resin derived carbon (PRC)in MG.In order to avoid the interference of graphite powder during XRD analysis,PRC specimens obtained under different temperatures were analyzed separately instead of MG.For the convenience of description and distinction,the PRC specimens were correspondingly named as PRC-800,PRC-1600,etc.
3 Results and discussion
The dimensional and weight changes of MG-800 pebbles through subsequent HTP under different temperatures are listed in Table 3.Take weight as an example,the change through HTP at 1 600 ℃ can be calculated as the equation (4):
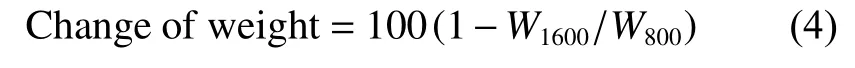
WhereW800andW1600are the weights of MG-800 and MG-1600,respectively.The volume for MG pebble could be obtained by the equation (5):
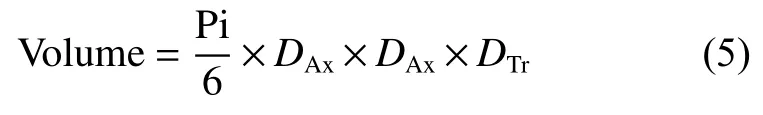
WhereDAxandDTrare the axial and transverse diameters of a MG pebble.In each batch,parameters of 40 pebbles before and after HTP were recorded and calculated.Then,the average was taken as the parameter changes as shown in Table 3.After carbonization,a three dimensional network of PRC was formed in MG-800.When MG-800 pebbles were further purified at different temperatures,a slight weight loss of~0.30% was observed due to the further decomposition of residual organic functional groups and removal of a trace amount of metal impurities in MG-800 pebbles.With the increase of purification temperature,the weight loss of pebbles slightly increased and maintained constant at temperatures higher than 1 800 ℃,mainly due to the removal of more trace metal impurities at a higher temperature.Meanwhile,the shrinkages of axial and transverse dimensions and volume increased gradually with increasing the heat treatment temperatures,which was probably due to the microstructural changes of PRC acting as the binder in MG pebbles.
The comprehensive properties of MG with different heat treatment temperatures are presented in Table 4.Some properties such as the transverse crush strength,corrosion rate,and erosion rate of MG-800 were not qualified,which coincided well with the results reported in our previous study[4].Meanwhile,the comprehensive properties of MG after HTP all satisfied the technical specifications.
As shown in Table 4,the density of MG increased gradually with increasing the heat treatment temperatures.This order is the same as for the volume changes and a high density is associated with a high volume shrinkage.The axial and transverse crush strengths of MG increased at different degrees after HTP at different temperatures,which totally met the technical requirements.Compared with those of MG-800,the density of MG-1600 increased by only 0.17%,while its axial and transverse crush strengths increased remarkably by 21.8% and 11.1%,respectively.The improvement of crush strength was probably due to the microstructure upgradation of MG through HTP.When the purification temperature further increased,unlike the slightly increasing trend of density of MG,the axial and transverse crush strengths almost maintained constant.Meanwhile,the average erosion rate of MG-800 was as high as 7.94 mg h−1·(Pebble),which was not qualified.After HTP at 1 600 ℃,the average erosion rate of MG-1600 decreased remarkably by 61.3% to 3.07 mg(h−1·Pebble),which satisfied the criterion of the specification.When the purification temperature further increased to 1 700,1 800 and 1 900 ℃,the average erosion rates of MG-1700,MG-1800 and MG-1900 were 2.03,1.81,1.48 mg (h−1·Pebble) respectively,which gradually decreased with the increase of HTP temperature.Based on the previous surface investigation results of the eroded MG pebbles,the relatively high erosion rate of MG-800 should be caused by the peeling off the fillers due to the poor interfacial forces between the graphite fillers and binder in the MG-800[4].The interfacial binding force between the fillers and binder might get much stronger by the microstruc-ture optimization with a HTP at 1 600 ℃,which led to the remarkable improvements of anti-erosion properties of MG.When the purification temperature further increased,the microstructure of MG was further optimized which could be considered as the main factor for the gradual improvement of anti-erosion properties of MG.
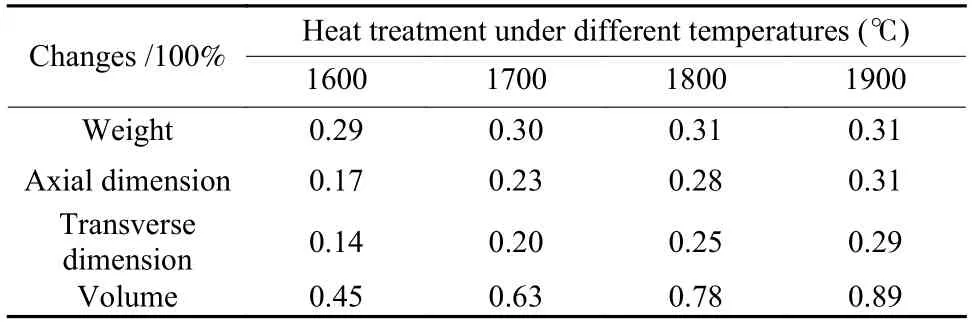
Table 3 Weight and dimensional changes of MG pebbles through HTP.
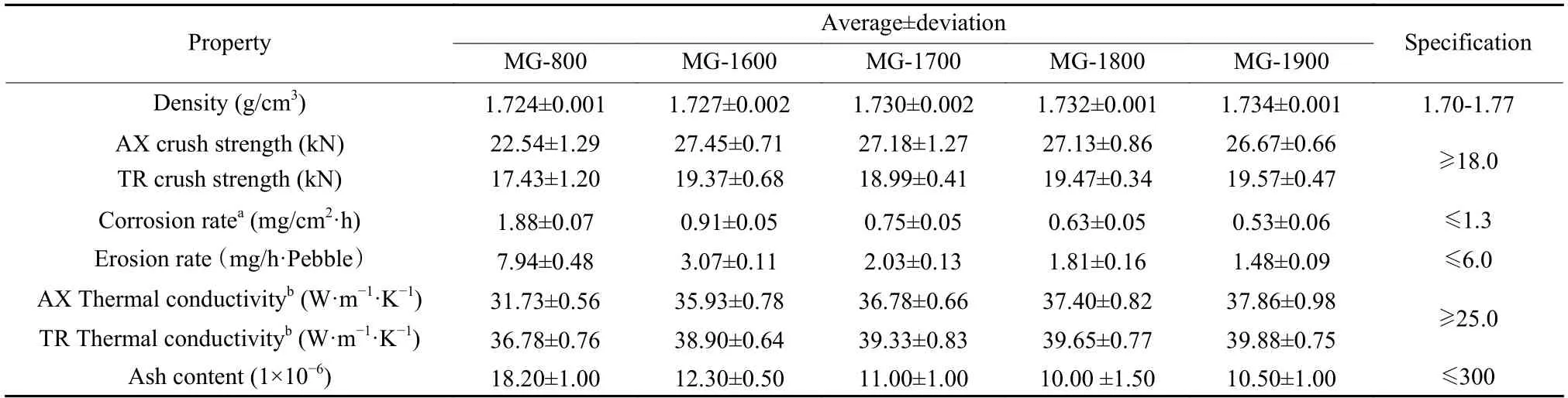
Table 4 Comprehensive properties of MG treated with different heat treatment temperatures.
As shown in Table 4,the thermal conductivities of all the MG specimens met the technical requirement.It could be seen that the transverse thermal conductivity of each sample was higher than that of the axial one,which was due to the preferred texture orientation in the molding process of MG[2,7].With the increase of heat treatment temperature,the difference of thermal conductivity of MG samples between the two orthogonal directions gradually reduced,which indicated that the microstructure and corresponding texture of MG were further optimized under high temperature.Compared with those of MG-800,the axial and transverse thermal conductivities of MG-1600 increased by around 13.2% and 5.8%,respectively.With increasing the heat treatment temperature,both the axial and transverse thermal conductivities of MG increased gradually,which further proved the microstructure optimization of MG through HTP[8].For instance,the axial thermal conductivities of MG-1700,MG-1800 and MG-1900 were respectively 2.4%,4.1%,and 5.4% higher than that of MG-1600.The increase of thermal conductivity and mechanical strength of the MG-800 through HTP were beneficial to improve the thermal shock resistance of MG,which was crucial for the integrity and safety of pebble fuel elements during their cycle in HTGRs under normal operation.
As shown in Table 4,the average corrosion rate of MG-800 was 1.88 mg cm−2·h−1,which did not satisfy the specification.After MG was further purified under different high temperatures,i.e.,MG-1600,MG-1700,MG-1800 and MG-1900,their average corrosion rates remarkably decreased and met the technical requirement.The corrosion rate of MG-1600 was 0.91 mg cm−2·h−1,which was less than half of that of MG-800.When the purification temperature further increased,the corrosion rate decreased gradually.The corrosion rate of MG-1900 was less than 30% of that of MG-800,which indicated the significant improvement of the anti-oxidation corrosion property.Based on literatures,the corrosion rate of graphite was determined by both of the graphitization degree and contents of impurity elements especially the transition metals such as Fe,Cr and Ni that would play the role as the catalysts in the oxidation of graphite[9,10].The ash contents of MG pebbles with different heat treatment temperatures are presented in Table 5.Since high-purity raw materials were used,the ash contents of all MG samples were less than 2.0×10−5and met the technical requirement of no more than 3.0×10−4.Compared with that of MG-800,the average ash content of MG-1600 decreased by about 30%from 1.82×10−5to 1.23×10−5.When the purification temperature further increased to 1 700,1 800 and 1 900 ℃,the ash contents of MG just decreased slightly and remained almost a constant value of 1.0×10−5.In order to find out the detailed changes of MG through HTP,the contents of more than 30 impure elements were analyzed by the methods of ICPOES or ICP-MS.Contents of some typical impurity elements of each MG sample are listed in Table 5.
From Table 5,it could be seen that the major impure elements in MG-800 were iron (4.473×10−6),calcium (1.936×10−6),copper (1.861×10−6) and nickel(1.313×10−6),which may act as catalysts and accelerate the oxidative corrosion of MG.After the MG-800 was treated at 1 600 ℃,the typical impurity elements in MG-1600 were iron (3.151×10−6),calcium(0.849×10−6),nickel (0.523×10−6).The decrease of the contents of impure elements in MG,especially the transition metal elements,was beneficial to reduce thecorrosion rate of MG.As mentioned above,compared with that of MG-800,the corrosion rate of MG-1600 correspondingly reduced by more than 50%.When the purification temperature further increased,compared with those of MG-1600,the contents of impure elements of MG samples decreased slightly and even kept constant,which was in good compliance with the ash content close to 1.0×10−5of each sample.However,the value of the corrosion rate of MG samples reduced gradually with increasing the purification temperature.Compared with that of MG-1600 of 0.91 mg cm−2·h−1,the corrosion rate of MG-1700,MG-1800 and MG-1900 were 0.75,0.63 and 0.53 mg cm−2·h−1,which decreased by around 17.6%,30.8% and 41.8%,respectively.As the ash contents almost maintained stable,the improvement of the oxidative corrosion resistance of MG samples purified at temperatures higher than 1 600 ℃ could be mainly attributed to the microstructure optimization.

Table 5 The ash contents and typical impurity elements of MG pebbles (1×10−6).
Because natural and artificial graphite powder had been completely graphitized,their microstructure will not change during the heat treatment of MG.In order to avoid the interference of graphite powder,the PRC samples with different heat treatment temperatures were used instead of the MG for XRD analysis to further investigate the effects of HTP on the microstructure of MG.XRD patterns of PRC samples were collected and presented in Fig.1.Since the PRC belonged to the glassy carbon having a turbostratic structure,it was characteristically observed that (10)and (11) diffraction profiles were asymmetric and broad,as compared with the symmetrical and sharp(100) and (110) diffraction profiles of natural graphite,as well as broadening of (00L) diffraction profiles[11].In the turbostratic structure,each hexagonal net layer formed a two-dimensional lattice independently,in other words,the c-axis could not be defined,and correspondingly these diffraction peaks had to be indexed to behk,not three-dimensionalhkl.As shown in Fig.1,compared with the diffraction pattern of PRC-800,the diffraction profiles such as(002)and 10 of PRC purified at temperatures from 1 600 to 1 900 ℃ were sharpened and narrowed,and the (11)diffraction profiles became more obvious,which indicated the improvement and optimization of carbon microstructures after HTP at higher temperatures.Based on the (002) diffraction profiles shown in Fig.1,the d-spacing of (002) (d002) of PRC samples purified at different temperatures could be calculated and the values are listed in Table 6.In the carbon materials with a turbostratic structure,spacing between adjacent layers was usually much larger than 0.335 4 nm because of weak van der Waals interaction due to irregularity in stacking,andd002values larger than 0.344 nm were often observed.As shown in Table 6,the values ofd002of all the specimens were larger than 0.344 nm,which showed the existence of the turbostratic structure in the glassy PRC samples.With the increment of the purification temperature,thed002values of heat-treated PRC samples decreased gradually because the more ordered structure occurred randomly in the crystallite of PRC purified at higher temperatures.Therefore,the gradually decreasedd002value indicated the optimization of microstructures of PRC[12],which was beneficial to improve the oxidative corrosion resistance of MG samples.

Fig.1 XRD patterns of PRC samples treated at different temperatures.
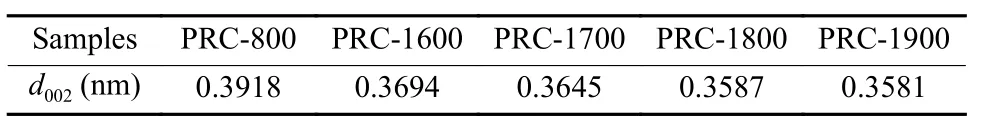
Table 6 The d002 values of PRC samples treated at different temperatures by XRD.
Pore information of MG samples were characterized by mercury porosimetry.In order to show more clearly on pore evolution throughout HTP,only MG-800 and MG-1600,of which pore characteristics were chosen and presented in Fig.2.The porosity and corresponding skeletal density of all MG samples measured by mercury porosimetry are listed in Table 7.As shown in Fig.2,compared with that in MG-800,the larger pore size in MG-1600 was ascribed to the residual small amount of C―H bonds decomposition and removal of a tiny amount of impurities in MG-800 at a higher temperature.As a result,the pore cumulative volume and log differential volume of MG-1600 was higher than those of MG-800.The difference in pore cumulative volume became more remarkable when the intruding pore sizes were smaller than 50 nm,which was probably due to the damage of graphite microstructure under the high intruding pressures.
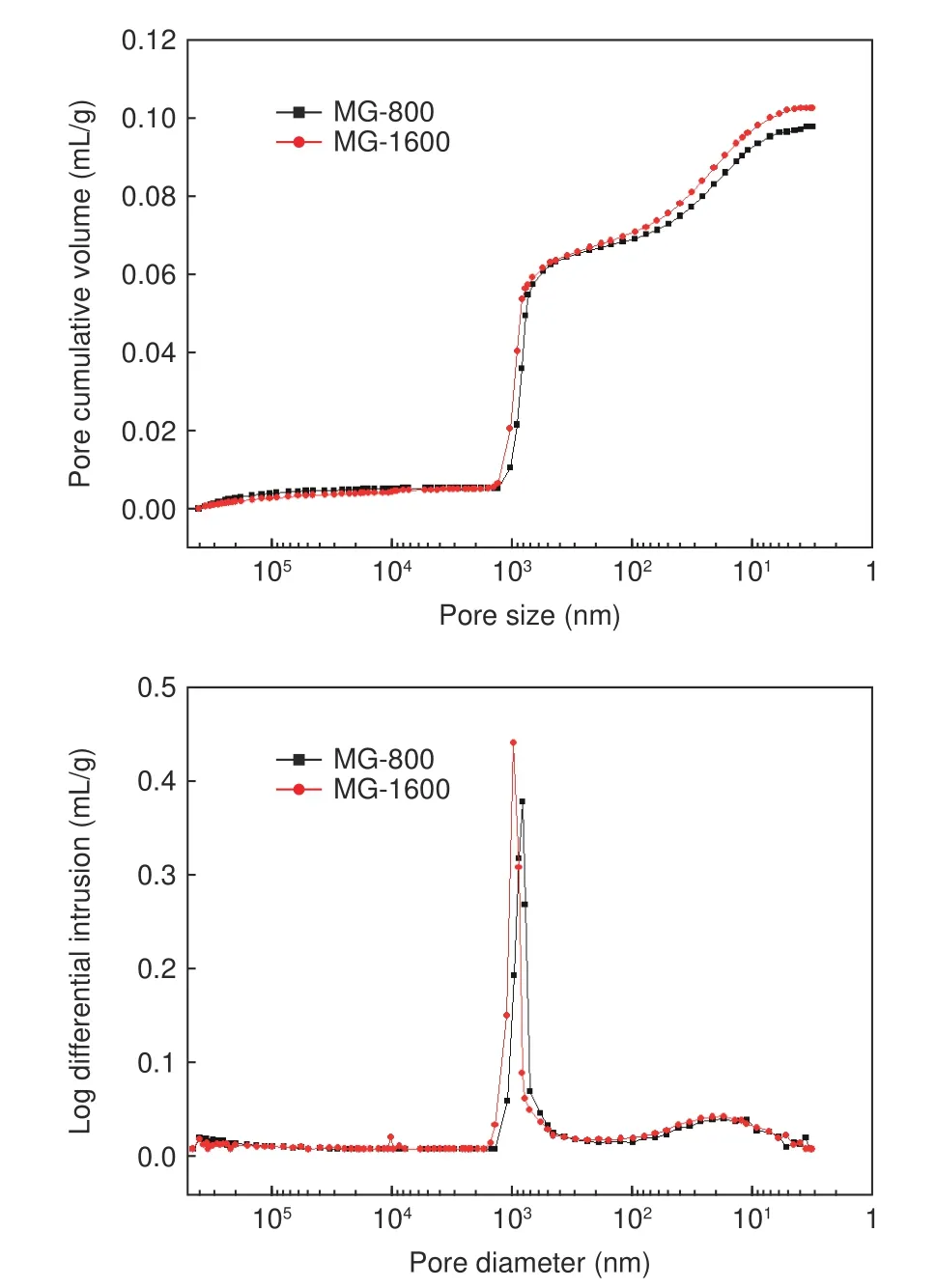
Fig.2 Pore information of MG-800 and MG-1600 characterized by mercury porosimetry.
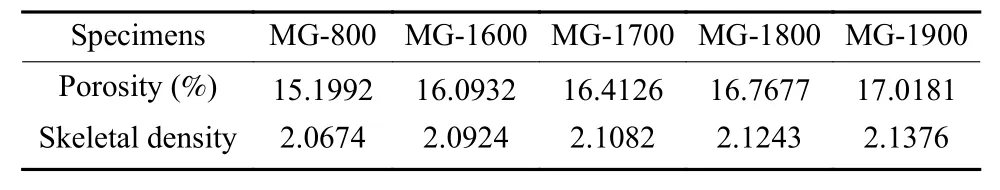
Table 7 Porosities of MG specimens measured by mercury porosimetry.
From Table 7,with the increase of purification temperature the porosity of MG specimens increased gradually,but the increasing rate of porosity tended to decrease,which was attributed to the larger pore size related to the removal of residual organic functional groups and impurities at a higher temperature.In the meantime,the improvement of graphitization degree and microstructure optimization of MG samples purified at a higher temperature might cause shrinkage,which led to a higher porosity and the increase of the skeletal density of MG.Compared with that of MG-800,the skeletal density of MG-1600 increased by about 1.2% from 2.067 4 to 2.092 4 g cm−3.With a further increase of purification temperature,the increment of skeletal density of MG specimens slowed down gradually,which could be ascribed to the gradual decline of the increase rate of graphitization degree as mentioned before.The gradual increase of skeletal density with increasing the purification temperature led to the gradual increase of density of MG samples as shown in Table 4,which was beneficial to the improvement of comprehensive properties,especially the mechanical properties of MG.
4 Conclusions
The comprehensive properties and microstructure of MG specimens treated at different temperatures were investigated and characterized.Unlike the crush strength,the erosion rate and corrosion rate of MG-800,which did not meet the technical requirements,the properties of all the MG samples after HTP fully satisfied the specifications,even if the purification temperature was reduced from the commonly used 1 900 ℃ to 1 600 ℃.As the purification temperature increased,the density,anti-erosion rate,and thermal conductivity of MG samples gradually increased,which was mainly due to the gradual microstructure optimization of MG based on the XRD analysis results.Compared with those of MG-800,the ash content and contents of typical impurity elements of MG-1600 significantly decreased.The combined effect of the reduction of impure elements and the optimization of microstructure made the oxidation resistance of the MG specimens remarkably improve.When the purification temperature continued to rise,the ash contents of MG samples remained basically unchanged while the corrosion rates continued to decrease,which was mainly due to the further optimization of the microstructure of MG specimens.In general,the purification of carbonized MG under high temperature was necessary and crucial,which was not only to purify the MG,but also to optimize the microstructure.Moreover,the microstructure optimization of MG under high temperature conditions plays a more important role in improving the comprehensive performance of MG.
Acknowledgements
Chinese National S&T Major Project(ZX06901);Key R &D plan of Shandong Province(major scientific and technological innovation project,2020CXGC010306).
- 新型炭材料的其它文章
- Microstructure of high thermal conductivity mesophase pitch-based carbon fibers
- 高导热聚酰亚胺石墨膜/环氧树脂复合材料的制备与性能表征
- TiC-modified CNTs as reinforcing fillers for isotropic graphite produced from mesocarbon microbeads
- One-pot modified“grafting-welding”preparation of graphene/polyimide carbon films for superior thermal management
- Thermal conductivity of graphite nanofibers electrospun from graphene oxide-doped polyimide
- A mini review:application of graphene paper in thermal interface materials

