Plane microscopic observation on instability of double emulsion clusters
Zhang Zhanhao,Qiu Yuheng,He Dongliang,He Liqun
Department of Thermal Science and Energy Engineering,University of Science and Technology of China,Hefei 230027,China
Abstract:Double emulsions have huge potential applications in many fields,but their wide use is limited by the instability.We have established a plane experimental observation method based on the microscope observation on the instability of double emulsion clusters to understand the key factors that affect the instability of droplets.A glass capillary device was used to produce the uniform monodisperse double emulsion droplets,and the dynamic information of the double emulsions was recorded through a microscope.We found that the instability of the double emulsions was caused together by diffusion and coalescence.And through the light curing experiment,the coalescence phenomenon of the internal droplets and the outer phase became visible.The experimental results show that the thicker the oil film thickness of the W/O/W droplets,the more difficult the coalescence of the inner droplets and the outer phase,and the more stable the double emulsions.The effect of glycerol on the stability of double emulsions was studied by changing the concentration of glycerol in the outer phase.We found that the stability time of the double emulsions increased with the decrease of the glycerol concentration,but when the glycerol concentration was reduced to 10 wt%,the stability of the double emulsions became worse.When the glycerol concentration was not less than 40 wt%,the instability of the double emulsions was more caused by the conversion of W/O/W droplets to O/W droplets.When the glycerol concentration was not more than 30 wt%,the instability of the double emulsions was more caused by the conversion of W/O/W droplets to W/W droplets.In addition,by using different surfactants,it was found that the double emulsions formulated with polyvinyl alcohol (PVA)had higher yield and a longer stable time.
Keywords:double emulsions;coalescence;glycerol;surfactant;stability
1 Introduction
Droplet-based microfluidics has become one of the most active research fields in microfluidics.It provides new methods for microencapsulating active ingredients such as microbes[1],living cells[2,3],drugs[4]and functional materials[5,6]in isolated spaces,and separating them from the external environment.In particular,the water-in-oil-in-water (W/O/W)double emulsion droplets and their resulting microcapsules are attractive candidates for microreaction[7-9],synthesis of functional particles[10,11]and controlled release[4,12-16]applications.The conventional method of producing W/O/W emulsions is to produce primary water emulsions in the oil phase,and then further emulsify the primary emulsions in the outer water phase[17,18].This method lacks control over the structure and size of the resulting double emulsions,which limits their application in the controlled encapsulation and release of active components.Now,microfluidic technology can precisely control their formation,so as to prepare double,triple,quadruple and even higher hierarchical emulsions with precisely controlled monodispersity and complexity[19].Among these methods,the glass capillary device with 3D geometry is one of the most widely used and reliable techniques for making complex emulsions[2,3].This method has many advantages,such as easy manufacturing,precise modification of local surfaces and precise control of the internal structure of complex emulsions.The glass capillary device can precisely control the size of the inner and outer droplets as well as the number of inner droplets encapsulated inside the outer droplets[21].
Double emulsions have great potential in a wide variety of applications.However,the stability of double emulsions,which is essential for practical applications,remains a big challenge due to their inherent thermodynamic instability[22].For instance,for W/O/W double emulsions,which stability depends on a variety of process parameters,including the type and concentration of surfactants[23],the osmotic pressure gradient of the inner and outer water phases[24],the addition of biopolymers such as proteins and polysaccharides[25],and the viscosity or gelation of the W1 phase[26].Previous studies have reported fat crystals have been shown to impart good stability on W/O emulsions by forming sintered “shells”around the water droplets[27].Some researchers have found that by increasing the adsorption of emulsifier molecules at the interface[28]and increasing the viscoelasticity of the interface[29],the stability of the W/O emulsion system can be improved to a certain extent.In addition,the aqueous phase (inner phase or outer phase)is converted into polymeric gel,which can improve the stability of the double emulsions[30].In the W/O/W double emulsions,due to the imbalance of Laplace pressure related to the curvature of the droplet surface and the osmotic pressure gradient of the inner and outer aqueous phases,which will cause the swelling and shrinkage behavior[31].Although there are many reports on the instability of multiple emulsions in the fields of food,cosmetics,medicine,etc.,to the best of our knowledge,the instability in the field of microfluidic droplets has not yet been widely studied.Moreover,there is no universal solution to the instability of multiple emulsions,and further research is still needed.
In this work,we assembled a droplet generator with glass capillary and micro-connectors,and placed the prepared double emulsion droplet clusters under a microscope for observation.By recording the dynamic information of the double emulsions,we analyzed the instability of the droplets.We used two MicroTees connected in series to produce monodisperse double emulsions,and observed the disappearance and rupture process of droplets in the double emulsions.The influence of the oil film thickness on the stability of double emulsions was studied by changing the flow ratio of the three phases.Our outer phase was aqueous glycerol solutions.By changing the glycerol concentration of the outer phase,we observed the life-time of the double emulsion droplets and analyzed the mechanism of emulsion stability.In addition,we explored the effects of several different surfactants on the stability of double emulsions.Studying the stability of double emulsions can provide a reliable basis and optimization index for the rationality of the equipment used for various double emulsion droplets.
2 Experimental section
2.1 Materials
To generate monodisperse double emulsions,we chose ultrapure water as the inner phase,mineral oil (#M-3516,Sigma)added with 0.05 wt% Triton X-100 (T9284,Sigma)and 2 wt% ABIL EM90 as the middle phase.In the light curing experiment,the inner phase was ultrapure water with 2 wt% dyeing agent and 2 wt% Span 80,the middle phase was ethoxylated trimethylolpropane triacrylate (ETPTA,Sigma)with 2 wt% Span 80.For the outer phase,we used ultrapure water with glycerol of different concentrations containing 1 wt% Pluronic F-127.Glycerol has high viscosity and solubility,is non-toxic,friendly to the human body,and can meet our outer requirements for higher viscosity after being dissolved in water.Polyvinyl alcohol (PVA),PEG (20)-sorbitan monolaurate (Tween 20)and sodium dodecyl sulfate (SDS)were also selected as surfactants.The relevant physical parameters of the liquids used in this work are listed in Table 1.The physical properties,regarding density,viscosity and surface tension,of all three phases are listed in Table 2.Unless otherwise specified in this experiment,all chemical reagents used in the experiment were of analytical grade,and the ultrapure water was prepared by a pure water system (Milli-Q Advantage A10).

Table 1.Summary of physical parameters of the liquids.

Table 2.Physical properties of three phases and operating conditions at 24 ℃.
2.2 Device configuration
We used a standard micro-connector that can be assembled to form a droplet generator.The two-step droplet preparation device used in this article is schematically shown in Figure 1.The droplet generator includes stainless steel MicroTees (IDEX Health &Science,USA),fittings (IDEX Health &Science,USA),and quartz capillary (OD 360 μm,ID 150 μm).The outside of the quartz capillary is coated with polyimide,which can protect the capillary well and make it not easy to be broken.Insert the capillary into the fitting and tighten it to make a droplet generator based on the T-shaped shear flow method.This kind of droplet generator can withstand a pressure of up to 4000 psi (about 276 bar),so it can stably generate droplets with a higher frequency under a larger flow resistance.At the same time,the entire device is soaked in alcohol and cleaned with an ultrasonic oscillator for a period of time,and then dried with nitrogen to be reused.If a certain part is damaged,only the corresponding part needs to be replaced.Compared with the commonly used microfluidic chip that can only be used once,it greatly reduces the cost of experimental instrument consumables.
In order to facilitate the observation and measurement of the generated droplets,a glass slide was placed at the exit of the droplet generation to collect the droplets.After that,the glass slide was observed under a stereomicroscope (Zeiss V16,Germany),and the size of the generated droplets was measured using the CellSens Dimension software (provided by the Life Science Experimental Center of the University of Science and Technology of China,with an accuracy of 0.01 μm)in the computer.The uniformity of the droplet size is characterized by the coefficient of variance (CV),which is the ratio of the standard deviation of the droplet diameter to the average droplet diameter.Usually,the CV is less than 3%,it can be considered that the droplet size is uniform.

Figure1.Schematic illustration of the glass capillary device used to make double emulsions.
3 Results and discussion
3.1 Formation of monodisperse double emulsions
In the double emulsion droplets generator,the first stage W/O single emulsion was generated in the first MicroTee by adjusting the two phases flow rate.Then the first stage W/O droplets entered the second MicroTee along with the middle phase fluid,and the flow rate of aqueous glycerol solutions was adjusted for the second re-wrapping.Due to the extrusion of the outer fluid,the middle fluid containing the first stage droplets broke,thus forming W/O/W double emulsions,as shown in Figure 1.In the experiment,the size and frequency of droplets can be well controlled by adjusting the flow rates of the dispersed phase and the continuous phase.We used aqueous glycerol solutions containing 50 wt% glycerol as the outer phase,and kept the flow rates of the inner phase,middle phase,and outer phase at 3 μL/min,10 μL/min,and 15 μL/min,respectively.The resulting double emulsion droplets are shown in Figure 2(a).The diameter distribution of the generated droplets as shown in Figure 2(b).The average diameter of the W/O droplets was measured to be 105 μm,and the CV was 2.9%;the average diameter of the O/W droplets was 188 μm,and the CV was 2.0%.This shows that the size of both the inner and outer droplets is uniform,and it also shows that the experimental device has good working stability as a droplet generator.

Figure 2.(a)Highly monodisperse W/O/W double emulsions with one single water droplet in oil droplet. (b)The diameter distribution of the double emulsion droplets. The inner phase flow rate Qi=3 μL/min,the middle phase flow rate Qm=10 μL/min and the outer phase flow rate Qo=15 μL/min.
3.2 Diffusion and coalescence
We found that after the W/O/W double emulsion droplets were formed,the W/O droplets gradually became smaller with time.When it came into contact with the spherical interface,it suddenly disappeared,and the W/O/W double emulsions transformed into simple O/W emulsions.We observed the change process of this part of the double emulsions which internal water droplets disappeared at 13 minutes,as shown in Figure 3.This is because the internal water droplets and the outer continuous phase are separated by the oil film at the beginning,and coalescence cannot occur immediately,as shown in Figure 3(b).Under the combined action of the capillary pressure of the two curved interfaces between W2 and W1 and the osmotic pressure between the inner and outer water phases,the water molecules in the inner phase diffuse to the outer continuous phase through the oil film,and the W/O droplet diameter gradually decreases.Therefore,we see that the diameter of the W/O droplets becomes smaller,as shown in Figure 3(c).At the same time,the W/O droplets are getting closer and closer to the spherical interface.When the fluid between the droplet and the interface is exhausted until the critical film thickness is reached,a hole (or multiple holes)is created in the film due to Van der Waals force[32],as shown in Figure 3(d).And the film will be broken,the W/O droplets will coalesce with the outer continuous phase to completely fuse,as shown in Figure 3(e).Figure 3 (a)shows the change of the diameter of the W/O droplet with time.We see that before 12 minutes,the W/O droplet diameter gradually decreases.After 12 minutes,the W/O droplets suddenly disappear and the diameters become zero.
In order to explore the influence of oil film thickness on coalescence behavior and coalescence time,we kept the flow rates of the inner and middle phases at 3 μL/min and 10 μL/min,respectively.The diameters of the internal droplets generated at this time were about 105 μm.By changing the outer phase flow rate,the oil droplet size can be changed.Figure 4 shows the change process of the diameter of oil droplets with different initial sizes over time.Each curve in Figure 4 represents the average behavior of several oil droplets.It can be seen that the size change of oil droplets is divided into three processes.In the early stage,the diameter of the oil droplets became slightly smaller due to the diffusion of water.Over time,as the fluid (oil and surfactant)between the water-oil and oil-water interfaces emptied,the internal water droplets eventually contacted the oil-water interface and rapidly coalesced with the external continuous phase.As a result,the oil droplet diameter was significantly reduced.Since the W/O/W double emulsion has been converted to O/W single emulsion in the later period,the oil droplet diameter will remain unchanged.This is similar to the changing trend of oil droplets in previous studies[33].The difference is that in previous studies,the oil droplets contained a large number of small water droplets,so internal coalescence and external coalescence occurred at the same time,and the oil droplet size change was divided into four stages.In our experiment,the oil droplet contained only a single big water droplet.We found that when the initial size of the internal droplets was the same,as the diameter of the oil droplets increased,the occurrence of coalescence would be delayed.The delay of the coalescence event was caused by the slowing down of fluid discharge between the two interfaces as the thickness of the interface film increased.
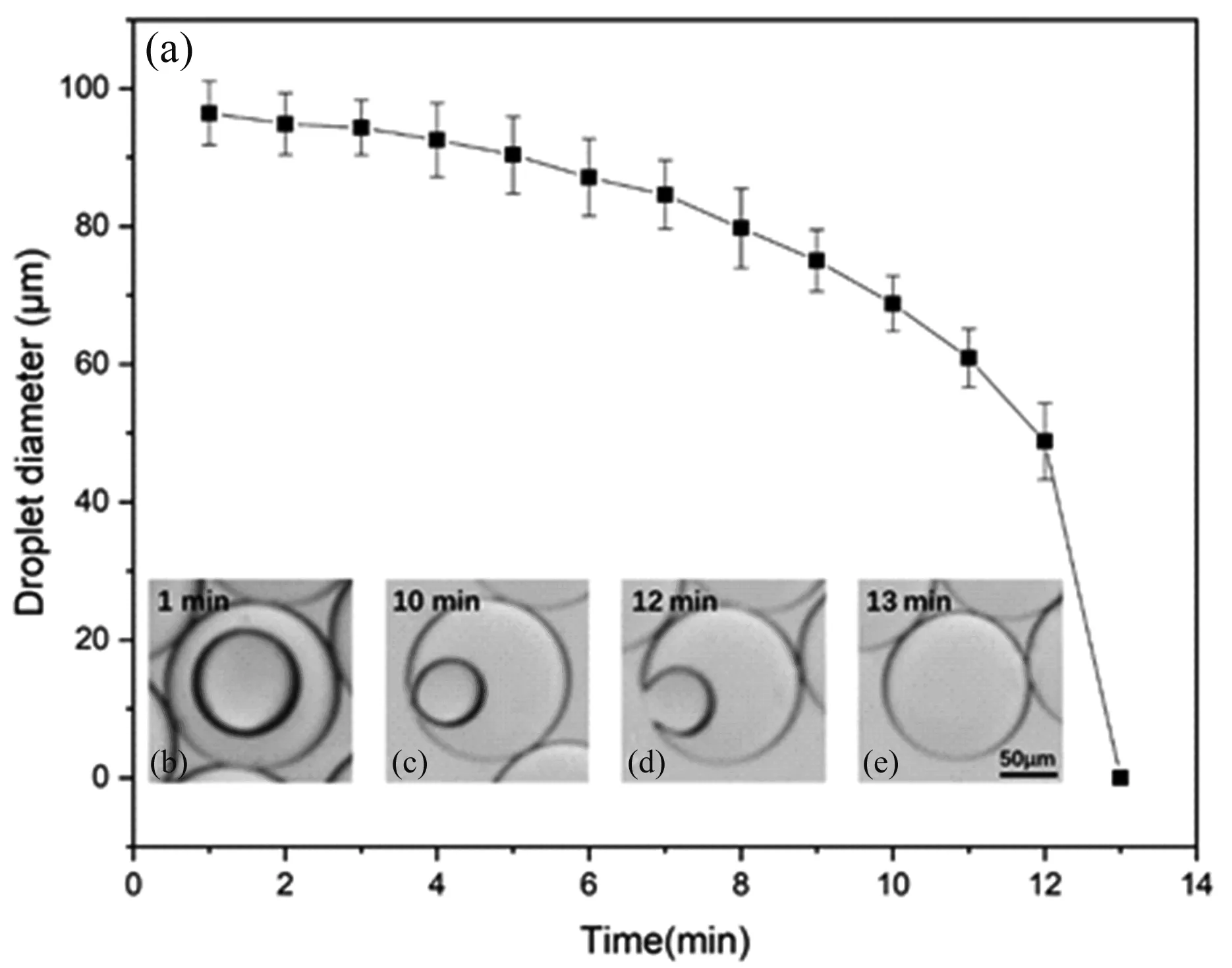
Figure 3.Time evolution of double emulsions. (a)Diameter of water droplets vs time. (b-e)The W/O/W double emulsion droplet transforms into the O/W single emulsion droplet. Data represents mean ± standard deviation with n=10.
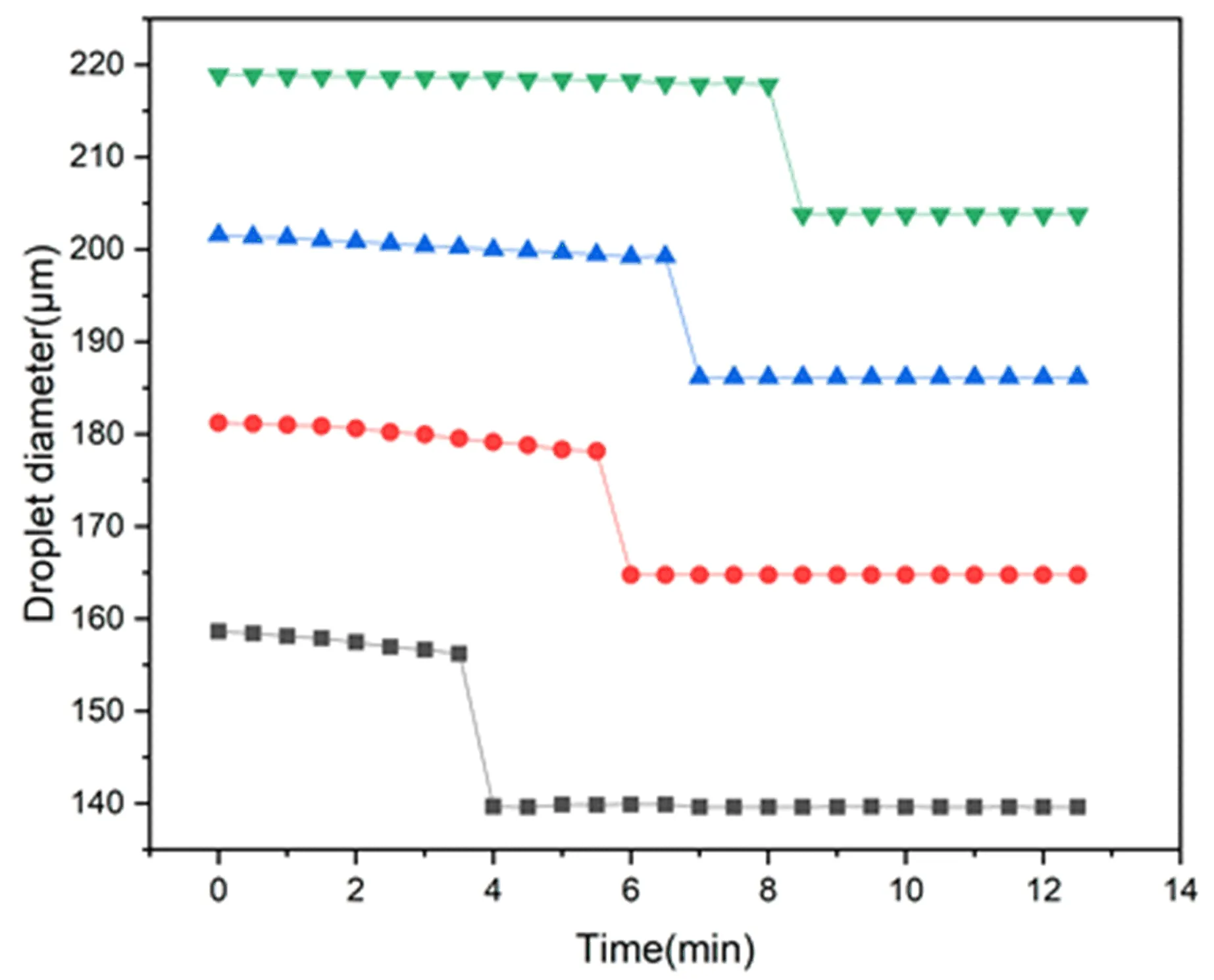
Figure 4.Diameter of oil droplets of different sizes vs time. The inner phase flow rate Qi=3 μL/min,the middle phase flow rate Qm=10 μL/min.
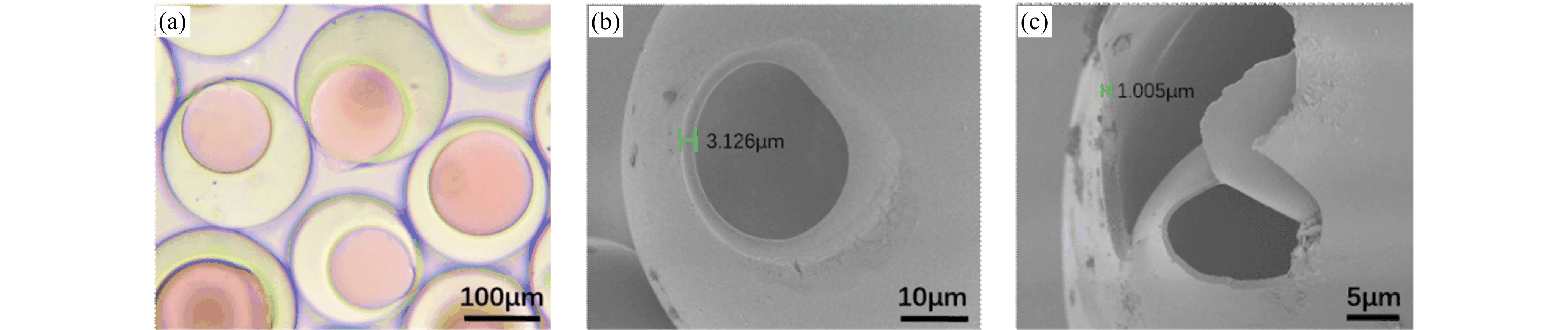
Figure 5.(a)Optical micrographs of microcapsules. (b,c)Field-emission scanning electron microscope (FE-SEM)images of microcapsules.
In order to further clarify the process of coalescence,we chose the light-curable material ETPTA to prepare double emulsions.The ultrapure water solution,the ETPTA solution,and the aqueous glycerol solution were prepared as the inner,middle,and outer phase fluids,respectively.After the double emulsions were formed,the liquid ETPTA was irradiated with ultraviolet light,and the ETPTA monomer molecules underwent photopolymerization under the action of the photoinitiator 184.At this time,the liquid ETPTA became solid,and a thin film was formed around the liquid water-in-oil droplets.Figure 5(a)shows the optical micrograph of the double emulsions after curing.Figure 5(b,c)are scanning electron microscope (SEM)images of the microcapsule.As shown in Figure 5,when the inner droplet contacts the spherical interface,a small hole is formed in the film,which leads to coalescence.And the thickness of the thinnest part of the film is the nanometer level,which is very easy to break.
3.3 The effect of glycerol on the stability of double emulsions
In order to verify the effect of glycerol on the stability of double emulsions,we prepared 6 groups of aqueous glycerol solutions containing different concentrations of glycerol for experiments.Kept the flow rates of the inner phase,middle phase and outer phase of each group at 3 μL/min,10 μL/min and 15 μL/min,respectively.Observed the number of double emulsions in the field of view with a microscope,and recorded it every 30 seconds.Finally,the statistical results are drawn as curves of the percentage of double emulsion droplets over time.
Figure 6 shows the change process of double emulsion droplets with glycerol concentration between 10wt% and 60wt% over time.It can be seen from the figure that when the glycerol concentration in the outer phase is 60wt%,the double emulsion droplets disappear very quickly,and they have all disappeared in about 4 minutes.As the glycerol concentration decreases,the disappearance speed of the double emulsion droplets will be significantly slower.When the concentration of glycerol is 20wt%,nearly three-quarters of the double emulsion droplets can continue to exist stably after 12 minutes.However,when the concentration of glycerol is 10wt%,the disappearance speed of the double emulsion droplets became faster again,and almost all disappeared at 12 minutes.Figure 7 is the relationship curve between the percentage of the number of double emulsion droplets and time under different glycerol concentrations,which can statistically reflect the stability of the droplets.
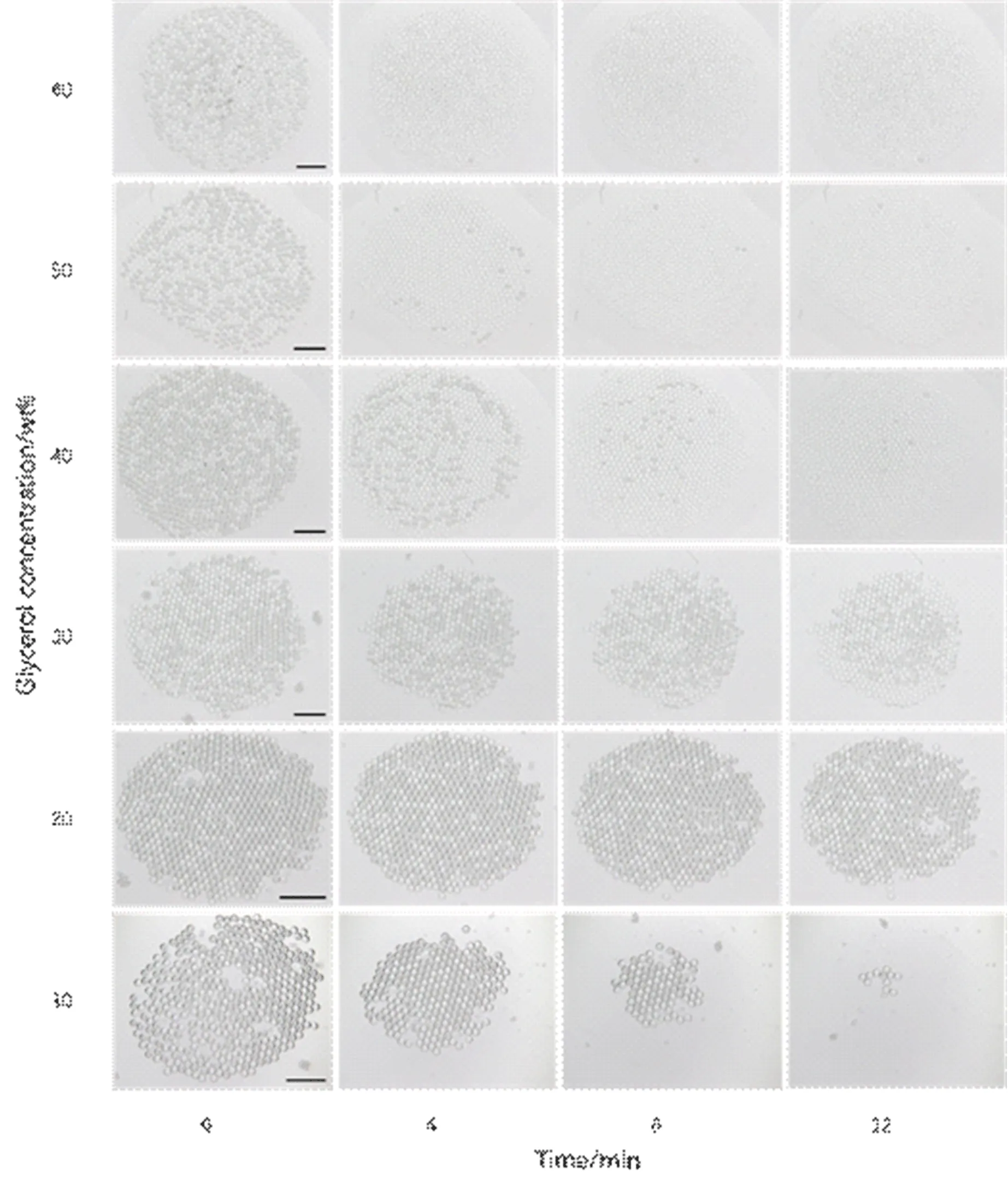
Figure 6.The change process of double emulsion droplets with glycerol concentration between 10 wt% and 60 wt% over time. The scale bars are 1000 μm.
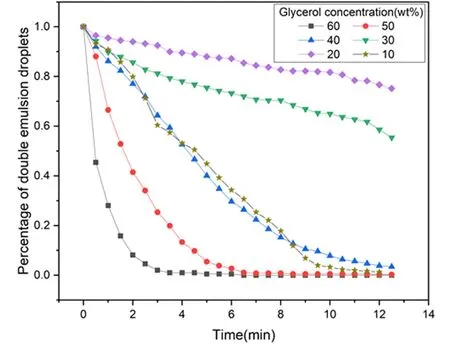
Figure 7.The relationship between the percentage of double emulsion droplets and time under different glycerol mass concentrations.

Figure 8.The diameter of O/W droplets of double emulsions produced with different concentrations of glycerol. Data represents mean ± standard deviation with n=50.
First,we measured the diameter of O/W droplets of double emulsions produced with different concentrations of glycerol,as shown in Figure 8.It can be seen that as the glycerol concentration increases,the diameter of the O/W droplets decreases.This difference in oil droplet size can be explained by the ability to reduce interfacial tension.Studies have shown that the surface tensions of aqueous glycerol solutions as functions of water concentration follow a continuous function[34].The greater the glycerol concentration,the lower the surface tension and interfacial tension.As we said before,the thicker the oil film,the longer it takes for the fluid to drain between the two interfaces,so the coalescence event will be delayed.This also explains that the higher the glycerol concentration,the faster the double emulsion droplets disappear.
Secondly,we found that when the glycerol concentration is not less than 40 wt%,the instability of the double emulsions is more caused by the conversion of W/O/W emulsions to O/W emulsions.We have discussed this earlier.When the glycerol concentration is not higher than 30 wt%,the instability of the double emulsions is more caused by the transformation of W/O/W emulsions into transient W/W emulsions.The internal water droplets of this W/W emulsion are wrapped with a thin film,which is a composite film composed of oil and surfactant.Transient W/W emulsions are very unstable,and Ostwald ripening occurs between them.And the film of the internal water droplets will continuously become thinner (perhaps by automatically emulsifying the oil in the film into a continuous water phase with a large amount of hydrophilic surfactant)until it finally ruptures,thereby releasing the water in the droplets to the continuous phase.We added fluorescent reagent to the inner phase for a clearer observation of the change process of the double emulsions,as shown in Figure 9.Figure 9 (a-d)shows the phenomena observed under bright field conditions.It can be seen that the W/O/W emulsions have transformed into W/W emulsion.And the internal water droplets are wrapped with a thin film,because the refractive index is different,the existence of the thin film can be clearly observed.Figure 9 (e-h)shows the phenomenon observed under dark field conditions,and it can be seen that Ostwald ripening occurs between the W/W droplets.The small droplets gradually shrink,the large droplets continue to grow,and finally the droplets burst.

Figure 9.The change process of double emulsions containing fluorescent dye. (a-d)Bright field. (e-h)Dark field.

Figure 10.Double emulsions made by microfluidics from formulations containing (a)2 wt% PVA,(b)1 wt% F-127,(c)2 wt% Tween 20 and (d)2 wt% SDS.
Finally,when the glycerol concentration is 10 wt%,the double emulsion droplets disappeared faster.This may be because the viscosity of the continuous water phase W2 is too low,which increases the fluidity of the oil droplets and accelerates the emulsification and coalescence of the double emulsions[35].At the same time,the presence of glycerol may change the characteristics of the solvent,thereby changing the solubility and adsorption characteristics of the surfactant[36].The reason for this phenomenon needs further research in the future.
3.4 The influence of surfactants on the stability of double emulsions
We also investigated the effectiveness of different surfactants (polymers)in stabilizing the double emulsion droplets produced by the glass microcapillary device.The surfactants (polymer)were dissolved in the outer water phase.In order to explore a wide range of chemical properties,two surfactants and two polymers with very different properties were evaluated.Partially hydrolyzed PVA and F-127 were selected as examples of surface active polymers.Tween 20 and SDS were selected as typical nonionic and anionic surfactants.
Figure 10 shows the double emulsions produced by using various surfactants (polymers).We found that the double emulsion droplets formulated with PVA were the most stable and the highest yield.The emulsions using F-127 can also be successfully stabilized,but its double emulsion droplets ratio is lower than that of PVA.The conventional surfactants Tween 20 and SDS will cause the emulsification of the emulsions,as shown in Figure 10(c,d).In the double emulsion droplets produced with Tween 20,the internal water droplets occasionally coalesced with the continuous water phase,leaving small satellite droplets in the larger oil droplets.In the emulsions containing SDS,the stable double emulsion droplets cannot be formed.We also observed the change of the emulsions after standing for a period of time,and found that the double emulsion droplets prepared with PVA basically did not change after 60 minutes of standing.It can be seen that the droplets generated by using the PVA aqueous solution as the outer continuous phase in the double emulsion droplets have good stability,and the internal water droplets can stably maintain the initial state without breaking.
4 Conclusions
In this work,we observed the instability phenomenon of the monodisperse double emulsion droplet clusters prepared by microfluidic technology from the plane based on the microscope,and explored the factors that affect the instability of the double emulsions.We used a glass capillary microfluidic device to produce W/O/W double emulsion droplets of the uniform size.We show that the instability of the double emulsions is caused together by diffusion and coalescence.The thicker the oil film thickness of the W/O/W droplets,the harder it is to coalesce and the longer the stable time of the droplet.The light curing experiment further shows that coalescence is the main reason for the instability of the double emulsions.By changing the glycerol concentration of the outer phase,we found that the greater the glycerol concentration,the smaller the O/W droplet diameter.The stability time of the double emulsions increases with the decrease of the glycerol concentration,but when the glycerol concentration decreases to 10 wt%,the stability of the double emulsions becomes worse.We also studied the effects of several surfactants on the stability of the double emulsion,and found that the double emulsions formulated with PVA had higher yield and longer stable time.These findings provide useful guidelines for the efficient stabilization of double emulsions produced with microfluidic approaches.The in-depth mechanism analysis of the droplet instability will be further studied in our future work.
Acknowledgments
The work is supported by the National Natural Science Foundation of China(31970754).
Conflictofinterest
The authors declare no conflict of interest.
Authorinformation

ZhangZhanhaois currently pursuing the Master degree with the Department of Thermal Science and Energy Engineering,University of Science and Technology of China.He received the BS degree in Energy and Power Engineering from Anhui University of Technology from 2018.His research interests include microfluidic and aptamer screening.

QiuYuhengis currently pursuing the Master degree with the Department of Thermal Science and Energy Engineering,University of Science and Technology of China.He received the BS degree in Energy and Power Engineering from University of Science and Technology of China in 2019.His research interests include single cell analysis.

HeDongliangis currently pursuing the Master degree with the Department of Thermal Science and Energy Engineering,University of Science and Technology of China.He received the BS degree in Building Environment and Energy Application from Anhui Jianzhu University in 2020.His research interests include microfluidic chip design.

HeLiqunreceived the BS,and PhD degrees from Harbin University of Civil Engineering and Architectur in 1988 and 1994,respectively.He is now a professor in University of Science and Technology of China.His research interests include building energy-saving technology,long-term preservation of biological materials,microfluidics and single cell analysis.
- 中国科学技术大学学报的其它文章
- The optimal logistics service strategy choices between the platform and e-retailers
- Vocational interests and informal learning in the workplace:The mediating role of goal orientation
- Corporate resistance to pandemic:Evidence from China
- The performance analysis on a novel purified PV-Trombe wall for electricity,space heating,formaldehyde degradation and bacteria in activation
- Development of the Ag nanoparticle-decorated Co3O4 electrode for high-performance hybrid Zn batteries
- Estimation based approximating control for wireless networked control systems

