Development of the Ag nanoparticle-decorated Co3O4 electrode for high-performance hybrid Zn batteries
Shang Wenxu,Yu Wentao,Ma Yanyi,Tan Peng
Department of Thermal Science and Energy Engineering,University of Science and Technology of China,Hefei 230026,China
Abstract:The hybrid Zn battery is a promising electrochemical system integrating the redox reactions of transition metal oxides and oxygen in a single cell,through which high energy efficiency and energy density can be achieved simultaneously.However,the positive electrode usually suffers from unsatisfactory capacity utilization of the active material and poor oxygen reduction and evolution reaction activity.Here,a novel nano-structured Co3O4 electrode with the decoration of Ag nanoparticles is developed.Benefiting from the synergistic function between Ag nanoparticles and Co3O4 nanowires,the electric conductivity is improved and the morphology is optimized effectively.With this electrode,a hybrid Zn battery delivers five-step discharge voltage plateaus from 1.85 to 1.75,1.6,1.55,and 1.3 V,a high active material utilization ratio of 18%,and a low voltage gap of 0.69 V at 1 mA·cm-2.Moreover,it can operate stably for 500 cycles with a voltage gap increase of only 0.03 V at 10 mA·cm-2.This work brings up a novel electrode for an ultra-high performance hybrid Zn battery with both high active material utilization and outstanding oxygen electrocatalytic activity.
Keywords:hybrid Zn battery;cobalt oxide;Ag nanoparticles;active material utilization;voltage gap
1 Introduction
Zn-air batteries have attracted research attention due to their low cost,high theoretical energy density,and intrinsic safety[1-4].However,restricted by the inferior oxygen reduction reaction (ORR)and oxygen evolution reaction (OER)catalytic activities,the high voltage gap causes unsatisfactory energy efficiency[5-7].Although tremendous efforts concerning the ORR and OER catalysts have been conducted to address this issue,the theoretical potential (1.65 V)limits its further development[8-12].
Recently,a new type of hybrid Zn battery integrating Zn-M (M represents the transition metal or its oxide/hydroxide)and Zn-air battery is proposed,in which the high energy density and energy efficiency can be presented simultaneously[13-16].During the discharge process,the reduction reaction of M occurs first and offers a discharge voltage of higher than 1.7 V,followed by the ORR to fully utilize the capacity of Zn;while during the charge process,the above reactions are reversed.Significantly,the positive electrode material can not only serve as the ORR and OER catalysts but also can serve as the active material of the redox reaction.Among them,Co oxides show superior performance due to their high theoretical capacity and excellent OER/ORR catalyst activity[17-20].For example,Tan et al.developed a hybrid Zn battery with Co3O4nanosheets electrodeposited on carbon cloth which delivered two discharge voltage plateaus:a high one of 1.85 V from the Zn-Co reaction and a low one of 1.0 V from the Zn-air reaction,effectively lifting the operating voltage[21].Based on the oxygen vacancy-rich Co3O4,Liu et al.constructed a hybrid Zn battery with a high capacity of 790 mAh·g-1and energy density of 928 Wh·kg-1,and it can also work stably after 300 cycles[22].
For the hybrid Zn battery,the evaluation for the battery performance includes both the utilization of active material corresponding to the Zn-Co reaction region and the ORR/OER activity to the Zn-air reaction region.In a Zn-Co battery,the utilization ratio of active material can be improved through the microstructure modified or metal substitution.For example,Shang et al.reported a Co3O4electrode with the heterogeneous porous nanowire,which shows a utilization ratio of 51.6%[23].After substituted with Ni,the value can be further lifted to above 60%[24].Even with these tremendous efforts,the intrinsic poor electric conductivity still limits its further improvement.When applied in the hybrid Zn battery,unfortunately,the utilization ratio is usually lower than 10% due to the addition of non-conductive binders,which hinders the contact between the active material and the electrolyte,therefore causing the ultralow utilization value[15].On the other hand,although cobalt oxide is a bifunctional ORR/OER candidate catalyst,the ORR activity is insufficient,which also limits the performance of the Zn-air reaction region[25].For instance,the discharge voltage is usually lower than 1.2 V even at a low current density of 1 mA·cm-2[21].Hence,the electrochemical performance for both two reaction regions should be further improved for the overall battery performance.
Ag is an excellent ORR catalyst with outstanding electric conductivity[26,27],and previous work has indicated that modification with Ag can dramatically improve the electric conductivity of pristine electrodes[28].Huang et al.reported a composite of Ag nanoparticles and ZnO,in which the overlapping of electron accumulation region produces a percolation path.Consequently,the electron concentration is effectively increased,leading to a 1000-time increase in the electric conductivity[28].Mao et al.fabricated a hybrid electrode made of Ni(OH)2grown on Ag nanowire,and the effective charge transfer between Ag and Ni(OH)2lifts the utilization of Ni(OH)2and avoids the useless zone,providing additional pseudocapacitance[29].After assembled into a supercapacitor,the electrode demonstrates an optimal capacitance of 3103.5 F·g-1and maintains 92.2% after 20000 cycles.Moreover,Ag particle has also been applied as the active material in a hybrid Zn battery,which exhibits high energy efficiency of 68% and operates stably after 100 cycles with almost no decay[26].Nevertheless,RuO2must be added into the electrode to improve the OER activity,which lead to the extra cost and complexity during the synthesis process.
Herein,a novel Co3O4electrode modified with Ag nanoparticles was fabricated for the hybrid Zn battery.Due to the addition of Ag,the utilization ratio of the Co3O4electrode was improved,and the ORR activity is also improved effectively.With this structure design,the long high-discharge-voltage region and excellent OER/ORR catalytic activity can be achieved in a single cell,thus the overall battery performance can be ameliorated.To systematically study the Ag nanoparticle-decorated Co3O4(Ag/Co3O4)electrode,the structure and morphology were characterized,and the multiple redox reactions were investigated.As for the battery behavior,the charge/discharge process under different current densities and the cycling stability were tested.This work paves a road of the electrode design for the high-performance hybrid Zn batteries.
2 Experimental
2.1 Fabrication of the Ag/Co3O4 electrode
The Co3O4electrode was fabricated through a hydrothermal reaction as reported[23].To obtain the Ag/Co3O4electrode,0.1 g of Ag powder with the size of 50 nm and 0.05 g of polytetrafluoroethylene (PTFE)with the size of 50 nm was mixed into 40 mL of water as precursor solution.Then the Co3O4electrode was soaked in it for 1 min and dried at 60 ℃ with several times.After that,the prepared sample was heat-treated under 300 ℃ for 1 h in an N2atmosphere with a heating rate of 10 ℃·min-1.The loading of the sample was measured to be 2.22 mg·cm-2.For comparison,the Co3O4electrode was fabricated with a similar method without adding Ag powder,and the loading of the sample was measured to be 2.0 mg·cm-2.
2.2 Material characterization
The material characterization was conducted at the Experimental Center of Engineering and Material Sciences in USTC.Detailedly,the X-ray powder diffraction (XRD,Smart lab)was conducted to investigate the spinel structure,the scanning electron microscope (SEM,XL-30 ESEM)was conducted to observe the morphologies and nanostructures.
2.3 Electrochemical measurement
The battery was assembled with the fabricated sample as the positive electrode,a zinc plate as the negative electrode,and 6 M KOH with 0.2 M Zn(Ac)2solution as the electrolyte.To investigate the electrochemical performance,the cycle voltammetry (CV)was conducted in an electrochemical workstation (Solartron Energy Lab)with the potential from 1.1 to 2.4 V (vs.Zn)using the scan rate from 1 to 20 mV·s-1.The battery discharge and charge behaviors were tested at 1,2,5,and 10 mA·cm-2,and the cycling performance was tested at 10 mA·cm-2with 3 min discharge followed by 3 min charge.The utilization ratio of active material is calculated from the quotient of the practical specific discharge capacity (mAh·g-1)and the theoretical capacity.In detail,the discharge areal capacity (mAh·cm-2)is calculated through multiplying the applied current density and the corresponding discharge time of the Zn-M section,while the practical specific discharge capacity is calculated by the quotient of the practical areal discharge capacity and the mass loading (mg·cm-2).
3 Results and discussion
3.1 Characterization of Ag/Co3O4 electrode
Figure 1 shows the XRD results from 20° to 80° of the Ag/Co3O4electrode.Apart from the diffraction peaks of the nickel substrate,the other peaks can well assign to Co3O4(PDF #42-1467)and Ag (PDF #04-0783),indicating the successful fabrication.The morphology of the sample was observed by SEM.As shown in Figure 2,the nanowires distributed uniformly on the surface of the Ni foam.From the high-magnification SEM images,the Ag nanoparticle can attach to the nanowires,which can greatly improve the contact surface with Co3O4,therefore lifting the electric conductivity of the electrode.To further confirm the addition of Ag nanoparticles in the electrode,the element mapping is illustrated in Figure 3.The metal of Ni,Co,O,Ag can be detected,which represent the Co3O4and Ag on the Ni foam,manifesting the successful composition of Ag.
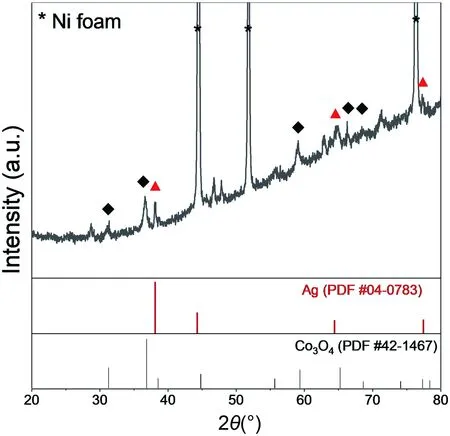
Figure 1.XRD pattern of the Ag/Co3O4 electrode.

Figure 2.SEM image of the Ag/Co3O4 electrode at different magnifications:(a-b)overall microstructure coated on Ni foam;(c-d)distribution of Ag nanoparticles on nanowires.
3.2 Electrochemical performance of the Ag/Co3O4 electrode
The electrochemical performance of the Ag/Co3O4electrode was first investigated in a two-electrode system.As shown in Figure 4,the CV curves were conducted from 1.1 to 2.4 V.Figure 4(a)displays four pairs of peaks,which represent Ag ↔ Ag+(Peaks 1 and 2),Co2+↔ Co3+(Peaks 3 and 4),Co3+↔ Co4+(Peaks 5 and 6),and Ag+↔ Ag2+(Peaks 7 and 8),respectively.In contrast,the Co3O4electrode only shows two pairs including the Co2+↔ Co3+and Co3+↔ Co4+,indicating the existence of multiple reactions after the addition of Ag.Besides,the specific capacitance also increases from 873 to 1108 F·g-1after the composite of Ag.Moreover,the signs from 1.1 to 1.4 and 2.2 to 2.4 represent the oxygen reduction reaction (ORR)and the oxygen evolution reaction (OER)section,respectively.Obviously,the Ag/Co3O4electrode show much higher current densities in both ORR and OER sections than the Co3O4electrode,demonstrating the improvement after the decoration of Ag nanoparticles.Figure 4(b)illustrates the CV curves of the Ag/Co3O4electrode at different scan rates from 1 to 20 mA·cm-2.As the scan rate increases,the position of each peak change towards two sides gradually,which was caused by the increasing polarization.While the shape of CV curves can remain well and the peak can retain clearly,manifesting excellent stability.

Figure 3.The elemental distribution of the Ag/Co3O4 electrode:(a)Pristine SEM image;(b)Layered image;(c-h)different elemental distribution images:(c)O,(d)Ag,(e)Co,(f)Ni,(g)C,and (h)F.
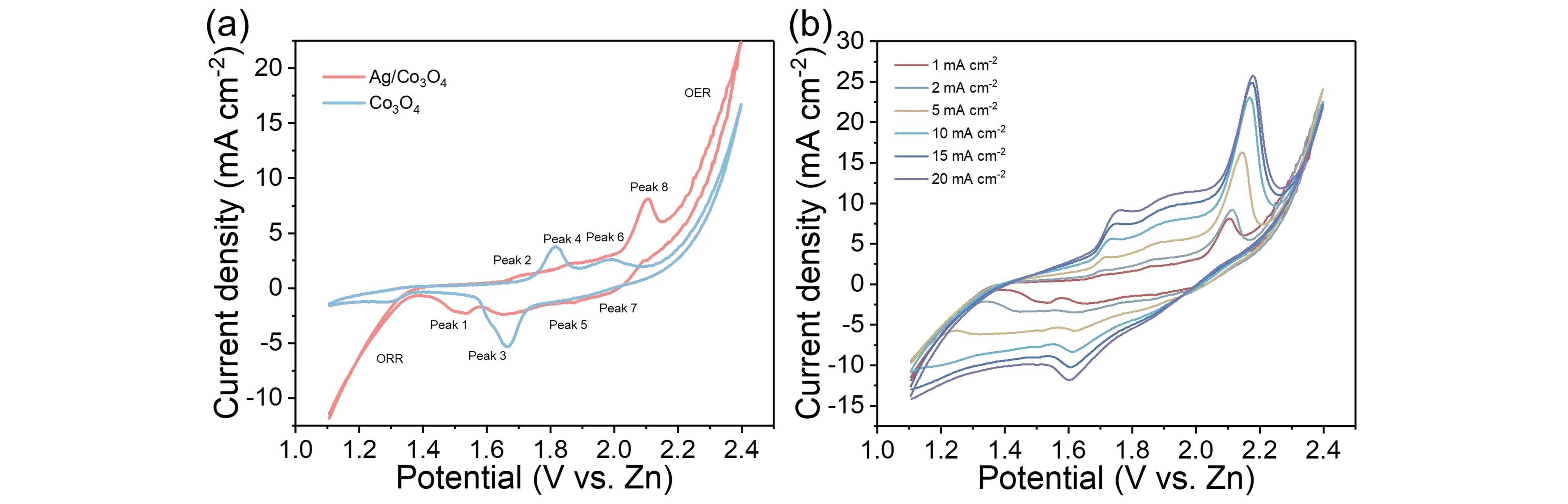
Figure 4.(a)Comparison of the CV curves between Co3O4 and Ag/Co3O4;(b)the CV curves of Ag/Co3O4 under different scan rates from 1 to 20 mA·cm-2.
To evaluate the discharge-capacity behaviors,a home-made battery was assembled.As shown in Figure 5(a),the battery displays five discharge voltage plateaus at 1 mA·cm-2:1.85 V corresponds to Ag2+→ Ag+,1.75 V for Co4+→ Co3+,1.6 V for Co3+→ Co2+,1.55 V for Ag+→ Ag,and 1.3 V for the ORR process,manifesting the multiple reactions.When operated under different current densities from 1 to 2,5,and 10 mA·cm-2,the battery shows different discharge and charge voltages for Zn-air reaction of 1.30 V/1.99 V,1.25 V/2.01 V,1.16 V/2.05 V,and 1.10 V/2.07 V,respectively,and the corresponding voltage gap is 0.69,0.76,0.89,and 0.97 V,respectively (Figure 5(b)).Table 1 summarizes the discharge voltages of recent hybrid Zn batteries at 10 mA·cm-2,from which this battery shows four voltage plateaus from 1.8 to 1.6,1.55,1.1 V,more than the reported one.Significantly,the voltage during the Zn-air reaction is higher than others,such as NiO/Ni(OH)2-CNT (1.08 V)[30],Co3O4nanosheet (1.0 V)[21],Co3O4/carbon cloth (0.98 V)[31],and Ag-RuO2/CNT (1.08 V)[26].The optimal catalytic behavior in the Zn-air reaction can be attributed to the following reasons:① the excellent ORR activity of Ag particles can effectively reduce the overpotential of the electrode,leading to a high discharge voltage;② the intrinsic good OER activity of Co3O4can be also presented,reducing the charge voltage.As reported in the literature,to construct a hybrid Zn-Ag/air battery,RuO2is usually added due to the poor OER activity of Ag particles,which could increase the cost and complex the synthesis process[26].Through modifying with Ag particles,the excellent ORR and OER activity can be achieved simultaneously with low cost,manifesting great market competitiveness.Besides,two parts of the battery performance are included for the hybrid battery:the utilization of active material in the Zn-M reaction and the discharge voltage in the Zn-air reaction.Therefore,the behavior of the Zn-M reaction should also be comprehensively considered to evaluate the battery performance.Figure 5(c)compares the utilization ratios of active materials in recent reported hybrid batteries.Due to the poor reaction surface between the active material and the electrolyte,the utilization ratio is generally insufficient,which is even lower than 10%.This electrode displays the optimal utilization ratio of 18%,which is much higher than the other hybrid Zn-Co/air batteries,such as Co3O4/carbon cloth (6.5%)[31],Co3O4nanosheets (6.7%)[21],coordination polymer derived oxygen vacancies rich Co3O4(7%)[32],NiCo2S4/3DNCC (11.2%)[33],oxygen vacancy-rich Co3O4particles (12%)[22].The great increase in the utilization ratio can be attributed to the following reasons:① the decoration of Ag particles improve the conductivity of Co3O4electrode,and therefore lifting the utilization ratio of active materials;② the uniform distribution of Ag particles can effectively modify the electrode morphology,which is prone to the electron and ion transport.

Figure 5.(a)Charge-discharge voltage curves at 1 mA·cm-2 with 15 min charge and 15 min discharge (The inset marks the corresponding reaction regions);(b)charge and discharge curves at different current densities from 2 to 10 mA·cm-2 of the hybrid Zn battery;(b)comparison of the utilization of active materials in the Zn-M reaction region.
To further investigate the electrochemical performance of the battery,the cycling behavior was tested under a current density of 10 mA·cm-2with 3 min charge and 3 min discharge (Figure 6(a)).Initially,the battery shows a discharge voltage of 1.1 V and a charge voltage of 2.06 V with a potential gap of 0.96 V.During 500 cycles,the battery shows stable charge and discharge voltages.After the 500thcycle,the discharge and charge voltage is 1.08 and 2.07 V,respectively,and the voltage gap only increases to 0.99 V with an increase of only 30 mV,indicating excellent cycling stability.In comparison,the stability of Ag/Co3O4composite is better than most reported electrodes in hybrid Zn batteries,such as Co3O4nanosheets (200 cycles at 1 mA·cm-2)[21],Co3O4/carbon cloth (400 cycles at 10 mA·cm-2,voltage gap increases 0.032 V)[31],oxygen vacancy-rich Co3O4particles (300 cycles at 1 mA·cm-2)[22],MnCo2O4/nitrogen-doped reduced graphene oxide (100 cycles at 1 mA·cm-2)[34],NiO/Ni(OH)2nanoflakes (70 cycles at 1 mA·cm-2)[35],and NiO-Al-Co/Carbon cloth (192 cycles at 5 mA·cm-2)[30].As for the Zn-M reaction region,it delivers the specific capacity of 0.21 mAh·cm-2with a utilization ratio of 23.5%.After the 500thcycle,the capacity increases to 0.25 mAh·cm-2,and the ratio lifts to 28%,which may be ascribed to the electrode activation during the cycle.Furthermore,the size of Ag nanoparticles also has a great effect on battery performances.When smaller Ag nanoparticles are applied,they can distribute more uniformly and the aggregation can be alleviated,leading to the larger specific surface area and electrochemical reaction area[36].Therefore,both the active material utilization ratio and the catalytic activity can be improved.As for tuning the size of Ag nanoparticles,some effective strategies can be applied[37,38].For instance,in the conventional hydrothermal reaction,the size decreases with an increase of the stabilizer during the reduction reaction (prevent the aggregation),decreases with an increase of the methanolic NaOH until an optimal amount,and decreases with the decline of water content[37].Besides,some other reduction regents (e.g.,NaBH4)can also effectively decrease the size of Ag nanoparticles[38].

Table.1.Performance comparison of hybrid Zn batteries based on different electrode materials.
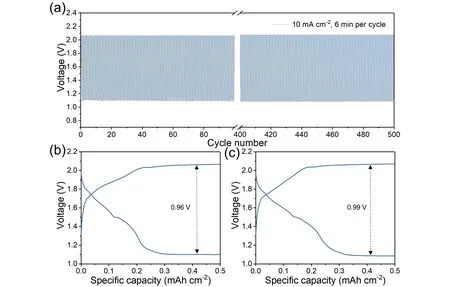
Figure 6.The cycling stability of the hybrid Zn battery with the Ag/Co3O4 electrode at 10 mA·cm-2,3 min discharge and 3 min charge:(a)the overall charge and discharge curves during 500 cycles;(b and c)the selected curves during the cycle:(b)the 1st and (c)the 500th cycle.
4 Conclusions
In summary,an Ag nanoparticle-decorated Co3O4electrode was synthesized by mixing the Ag powder onto the surface of the Co3O4electrode.Through the addition of Ag nanoparticles,the utilization ratio of active material and the ORR activity are improved simultaneously.With this electrode,a hybrid Zn battery shows five voltage plateaus of 1.85,1.75,1.6,1.55,and 1.3 V at 1 mA·cm-2,indicating the multiple voltage plateaus after the composition of Ag.Significantly,it delivers a high utilization ratio of 18% for the Zn-M reaction region.When operated under different current densities from 1 to 2,5,10 mA·cm-2,the battery shows the small discharge and charge voltage gaps for the Zn-air reaction of 0.69,0.76,0.89,and 0.97 V,respectively.Besides,good cycling stability is also exhibited.After a 500 cycle test at 10 mA·cm-2,the voltage gap only increases 0.03 V from 0.96 to 0.99 V.Hence,this work obtains an ultra-high performance hybrid Zn-Co/air battery with both the high utilization ratio of active material and small voltage gap,indicating the potential application in the future.
Acknowledgments
This work is supported by Anhui Provincial Natural Science Foundation (2008085ME155),USTC Research Funds of the Double First-Class Initiative (YD2090002006),Joint Laboratory for USTC and Yanchang Petroleum (ES2090130110),and USTC Tang Scholar (KY2090000065).
Conflictofinterest
The authors declare no conflict of interest.
Authorinformation
ShangWenxuPhD candidate.Research field:Electrochemical energy storage systems.
YuWentaoPhD candidate.Research field:zinc electrode and zinc-air flow batteries.
MaYanyiPhD candidate.Research field:electrode materials of hybrid Zn-air batteries.
TanPengCorresponding author,Professor.Research field:Advanced energy storage systems.
- 中国科学技术大学学报的其它文章
- Vocational interests and informal learning in the workplace:The mediating role of goal orientation
- Corporate resistance to pandemic:Evidence from China
- The performance analysis on a novel purified PV-Trombe wall for electricity,space heating,formaldehyde degradation and bacteria in activation
- Estimation based approximating control for wireless networked control systems
- 深度视觉目标跟踪进展综述

