Cytokine release assessment:a good de-risk approach to bi-specific T-cell engagers in non-clinical development
XIE Jin,ZHANG Hong-feng,LIU Hua-chun,PAN Xin-hong,Renke DAI
(1.Guangzhou Center for Disease Control and Prevention,Guangzhou 510440,China;2.Guangdong Ruigu Biotech Corporation,Qingyuan 511500,China;3.Guangzhou Medical University,Guangzhou 511436,China)
Abstract:Bi-specific T-cell engagers(BiTEs)show great clinical outcomes for anti-cancer purposes.However,potential cytokine release syndrome(CRS)is notorious to all BiTEs.The mechanism underlying CRS is still not fully known,even though such toxicities are considered to be cytokine release related.Assessment of CRS is a key to non-clinical de-risk programs for BiTEs therapeutic development.In the present review,possible mechanisms are discussed,especially factors contributing to CRS development.T cell activation may be just an initiation of the CRS cascade,and other cell types can greatly contribute to CRS,such as a chain reaction triggered by downstream B-cells,monocytes,and endothelium cells.A non-clinical de-risk program can be designed based on these components in the CRS cascade.Combination of in vitro cytokine release assay,and in vivo mouse and non-human primates studies should be reliable enough to predict and mitigate CRS risk in the clinics.Further more,a good de-risk program should be able to provide ranking for candidates for further development and provide enough confidence to select a first-in-human dose.
Key words:bi-specific T-cell engagers;single-chain of variable fragments;cytokine release syndrome
Bi-specific T-cell engagers(BiTEs)are a new class of artificial monoclonal antibodies consisting of two single-chains of variable fragments(scFvs)of two different antibodies for anti-cancer purposes.In general,one scFv binds to CD3 in T cells,while the other binds to a cancer antigen or tumor associated antigen(TAA)to link together T cells and tumor cells,which in killis tumor cells by T cells[1].As with CAR-T technology,this pathway of T cell activation is independent of major histocompatibility complex(MHC)by bypassing the traditionalantigen presenting process[2].Construction of BiTE with double antibodies is shown in Fig.1.

Fig.1 Bi-specific T-cell engager(BiTE)structure containing double antibodies.BiTEs are composed of an anti-CD3 antigen-binding domain,[IgG1single-chain of variable fragments(scFv)],and an anti-tumor associated antigen(TAA)antigen-binding domain(IgG2scFv)through a linkage peptide.
With the potential to outperform and complement CAR-T therapy,BiTEs have become a hot area in today′s pharmaceutical field,and many BiTEs are being developed in different stages.BiTEs interact with both T cells and tumor cells(Fig.2).However,only blinatumomab(Blincyto®)has been approved by US Food and Drug Administration(US FDA)(2014)to date[3].

Fig.2 BiTE mediates the interaction between T cell and cancer cell.BiTE induces the activation and targeting cytotoxicity of T cells to interact with cancer cells through the specific binding with CD3 of T cells and TAA of cancer cells.
Although good clinical outcomes are shown in many trials with BiTEs,potential cytokine release syndrome(CRS),similar to CAR-T therapy,is notorious to all BiTEs,which greatly limits their clinical benefits[4].After retrospectively reviewing and analyzing 17 investigational new drug(IND)submissions of CD3 bi-specific antibodies in 2017,FDA suggested that the most common toxicities in animals and patients were those related to cytokine release[5].Therefore,non-clinical de-risk strategies including assessment of CRS have become necessary and a critical step during early-and mid-stage development of BiTEs.This may help to increase predictivity for clinical outcomes,to reduce potential CRS,and to speed up pharmaceutical development of better BiTEs.
Assessment of cytokine release by cell-based studies,in combination with animal studies,has become a general practice to de-risk and select a better candidate for further development.CRS assessment can be started in early development while pharmacology or anticancer effects are evaluatedinvitro,in mouse models,or nonhuman primate(NHP)studies.
1 POSSlBLE MECHANlSM OF CRS
CRS was first described in 1989,a flu-like syndrome after infusion of anti-CD3 monoclonal antibody OKT3[6].CRS later became more common after approval of rituximab and a number of monoclonal antibodies for cancer treatment[7].It was not until a life-threatening clinical trial of TGN1412(CD28 agonist monoclonal antibody)in 2006 that CRS became a serious relevant to the drug development community,in which severe CRS was reported six healthy adults needed to be treated in intensive care units(ICUs)after first-in-human(FIH)dose of TGN 1412[8].Since,then CRS assessment in preclinical setting has been mandatory by US FDA for therapeutic antibodies,particularly for BiTEs before FIH[9].
CRS include fever,fatigue,hypotension,tachycardia,nausea,capillary leak,and multiorgan(cardiac,pulmonary,renal and hepatic)dysfunction,along with release of pro-inflammatory cytokines in the blood stream systemically and locally in the central nervous system(CNS).Severe CRS is a medical emergency which can result in significant morbidity or mortality[4].Although CRS is associated with all BiTEs,the underlying mechanism is not fully understood,and the scale of CRS varies with different BiTEs.The cause of CRS is considered to be associated with several factors,including T cell action,monocyte activation,myeloid cell activation,and endothelium activation.A number of other factors may also contribute to development of CRS,such as density of the TAA,disease burden,and TAA in normal tissues(on-target off-tumor effect)[4,10-12].
CRS cascade is considered to be initiated by T cell activation at the TAA site,which can be in tumor or off-tumor or both.Activated T cell releases cytokines,tumor necrosis factor-α (TNF-α),and inteferon-γ (IFN-γ)for direct target cell lysis.These cytokines trigger a chain reaction in downstream events including activation of macrophages,monocytes and endothelial cells,which release more cytokines to profound a systemic effect[4,12].Godbersenetal[13]reported that in an immunocompetent tumor bearing mouse model,IFN-γ produced by T cells in the spleen changed both T cells and myeloid populations at thebeginning of the CRS cascade.RAG deficiency,IFN-γ deficiency,or depletion of either CD4 or CD8 T cells might prevent toxicity,whereas perforin deficiency,granulocyte-macrophage colony-stimulating factor(GM-CSF)deficiency,or modulation of the myeloid population through clodronate-mediated depletion showed a partial reduction of toxicity.Although T cells are required for the initiation of toxicity,whether IFN-γ is the first cytokine to mediate the CRS cascade is still not clear.
In animals and patients,CRS is associated with consistent elevation in key cytokines such as interleukin(IL)-2,IL-6,IL-10,TNF-α and IFN-γ in serum.Elevated IFN-γ can cause fever,chills,headache,dizziness,and fatigue directly.IFN-γ can also induce activation of macrophages.The activated macrophages and monocytes produce excessive amounts of additional cytokines such as IL-6,TNF-α and IL-10.Like IFN-γ,TNF-α can produce not only fever,chills,and fatigue but also diarrhea,vascular leakage,cardiomyopathy,lung injury,and synthesize acute phase proteins.Activation of macrophages and monocytes can cause inflammatory response systemically and locally,as well as vasculature and organ damage.Severe CRS can even cause life-threatening multi-organ failure[4,12].
IL-6 is considered to play a key role in downstream development of CRS.In both animal studies and clinical trials,IL-6 is always at the highest level or may be considered the most sensitive cytokine in response to CRS or beginning of CRS.IL-6 is thought to contribute to development of severe CRS,which,in turn,can cause vascular leakage,activation of the complement system and coagulation cascade,inducing disseminated intravascular coagulation[14-18].In addition,IL-6 likely contributes to cardiomyopathy that is often observed in patients with CRS by promoting myocardial dysfunction[14].A simple diagram of the possible mechanism for CRS is displayed in Fig.3.
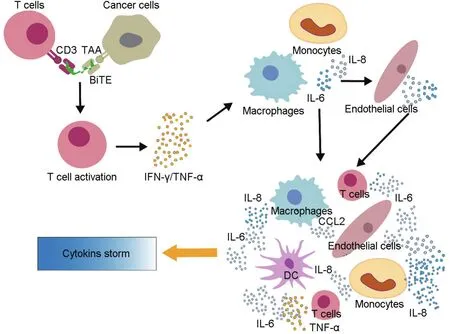
Fig.3 Mechanism of action of BiTE mediated cytokine storm.BiTE mediates T cell activation and target cell lysis over the time,leading to massive release of cytokines,such as tumor necrosis factor-α (TNF-α)and interferon-γ (IFN-γ).These cytokines would also activate the innate immune cells,including macrophages,monocytes,endothelial cells,and dendritic cells to further produce excessive cytokines(such as IL-6,IL-8 and TNF-α,etc.)and then to generate cascading amplification reactions through feedback regulation,eventually leading to cytokine storm.
In the case of blinatumomab,neurotoxicity is considered independent of systemic CRS.Klingeretal[19]and Helwicketal[20]proposed that the cause of neurotoxicity was due to increase in adhesiveness of vascular endothelium,particularly the in CNS,by T cell activation,which results in targeting B-cells,and releases cytokine in CNS to attract monocytes into the CNS to cause local inflammation and neurotoxicity.
Hayetal[11]demonstrated that endothelium activation biomarkers,such as angiopoietin-2(Ang-2)and von Willebrand Factor(VWF),were elevated in severe CRS in a study of 133 patients treated by CAR-T,which may suggest that endothelium activation plays a role in developing sever CRS.Further more,severe CRS is also accompanied by high serum concentrations of endothelium-activating cytokines,such as IL-6 and IFN-γ.Together,these suggest that endothelium activation indeed plays a key role in the develop ment of severe CRS.
Density of TAA,or/and disease burden,as well as affinity to CD3 were also contributed to CRS.The denser TAA or heavier disease burden,the more likely CRS will be,compared to lower density of TAA or patients who are almost cured.The higher affinity to CD3,the more potential for CRS.As a result,more and more designs of new generations of BiTEs are using mild or weak affinity of Fv arm to CD3 activation[21-23].
In order to find out if a lower affinity CD3 BiTE,and/or a lower affinity to TAA can result in less CRS,Kamperschroeretal[24]tested a group of BiTEs in combination with lower and higher affinity variants for both the CD3 and the human epidermal growth factor receptor 2(HER2).The lower affinity CD3 variant was found to be well tolerated in mice and cynomolgus monkeys,while the higher affinity CD3 variant would increase CRS,weight loss and systemic inflammatory responses.Staflinetal[25]also found that the higher affinity anti-HER2 variant was not well tolerated in animals with higher systemic levels of IL-6 and IFN-γ and histopathologic effects in HER2-expressing tissues.These results suggest that high affinity to CD3 or/and to TAA can cause more CRS.
Since TAA is also often expressed in normal tissues,binding to the TAA in normal tissue can result in on-target-off-tumor toxicities.Toxicity studies with healthy NHPs may only address the risk of on-target-off-tumor CRS,which may not fully predictthe risk in patientswho have more TAA in tumor sites in addition to normal tissues.Therefore,these is the possibility that NHP studies may underpredict the potential CRS risk in patients[26].
2 ASSESSMENT TO CRS
Since the life-threatening trial with TGN1412 in 2006,assessment of CRS has become a must for all the therapeutic antibodies before entering FIH.There is no single study or assay which can address CRS issue for this purpose.The current approach in the industry is to employ a battery of studies for de-risk strategy and for better prediction of clinical outcomes for therapeutic antibodies during development,particularly for BiTEs[24].
Invitrocytokine release assay(CRA)is usually the first step in the de-risk strategy.CRA can be conducted in several formats,by using either human whole blood cells(WBs),or human peripheral blood mononuclear cells(PBMCs)from heathy donors.When TAA is not in WBs or PBMCs,cells containing TAA may be added as a target for antitumor effect of the tested BiTEs[24].WBs or PBMCs are often pooled from several or many donors to increase the sensibility of the assay.In general,it is thought that assay format with an immobilized antibody in the wells of tissue culture microplate can more sensitively detect CRS,but there is no confirmed information that aqueous format or other formats are less sensitive for detection.Red blood cells are thought to interfere with CRA in format using WBs[24,26-27].Iwataetal[28]showed PBMCs together with an immobilized format of CRA is the most sensitive method to detect potential CRS for TGN1412.Iwata even showed the most sensitive biomarker was IL-8 in this assay format.
As for BiTEs,there is no confirmed study results or consensus as to which format is the best to assess CRSinvitro.However,methods by WBs or PBMCs with added TAA expressing cells showed great correlation to CRS detection[29].WCs in some dilution with or without TAA cells in an aqueous format of BiTEs are also used for this purpose,and the results are comparable to those with an immobilized format.But CRA without appropriate TAA may not be physiologically relevant and may not be predictable[30-31].Some pharmaceuticals may use PBMCs from patients directly to conduct CRA study.When TAA is expressed in PBMCs,and results are useful for risk-ranking purposes[24].
The overall understanding suggests that CRA may be a useful approach to risk assessment,but varied assay formats may yield different outcomes.As is part of the pharmacology of BiTEs,cytokine release is already an endpoint of pharmacology and toxicology studies.A standaloneinvitroCRA is not necessary for regulatory purposes,but it can still provide good information as part of de-risk strategy during preclinical development[24].As US FDA suggested in a review of 17 IND submission of BiTEs,cytokine release is the most common toxicity in animals and patients.Invivoassessment of CRS in mice and NHP is extremely valuable for de-risk in preclinical development[5].
However,invivomouse models are poor models for predicting CRS,although they may be used to understand basic questions of how molecular properties affect cytokine production(or other T-cell functions)in certain models[24].Because CRS by BiTEs is also dependent on CD3 affinity as well as target(ieTAA)density and tumor burden,tumor bearing mouse models can shed light on target mediated CRS.Immunocompromised NOD-SCID IL-2 receptor gamma null(NSG),human TAA knock-in models,and immunocompetent mouse models have been widely used as pharmacological models for BiTE preclinical development.While the main purpose of these studies is to test the antibody′s ability to reduce tumor growth volume for efficacy assessment,CRS is a common endpoint in the studies.However,CRS in mouse models is considered not sensitive enough,and not a good predictor in the clinic[24].
NHPs,particularly cynomolgus monkeys,are the relevant species for non-clinical studies,because BiTEs,more often,do not cross-react in other species.NHPs are thought to have a similarinvivocytokine release pattern to humans.Therefore,using NHPs becomes a common path for non-clinical development of BiTEs,particularly for early toxicology and IND enabling toxicology studies if the antibody has cross-reactivity to NHP.InvivoCRS is often assessed in exploratory and short-term NHP studies,which are usually correlated withinvitroand mouse studies.CRS is usually observed within the first and second days after dosing in NHPs,and clinical signs may include emesis,decreased activity,inappetence,diarrhea,increased body temperature,together with elevated key cytokines,such as IL-2,IL-6,IL-10,IFN-γ and TNF-α.
It has been showed that in NHP studies,CRS is detected in a dose-dependent manner to increase circulating cytokines,while IL-6 is observed in higher concentrations well correlated with mortality[24].Clinical pathology is also well correlated with elevation of cytokines,monocyte infiltration in multiple tissues,and tissue damage in organs expressing TAA.Repeated dosing can reduce CRS effect,while step-up dosing with lower doses at the beginning before higher dosing is applied can reduce dose-limiting CRS,too[24].Effective management of the initial CRS response in NHP can help to establish an approach to FIH and to reduce the potential clinical CRS risk.Correct and precise assessment of CRS bothinvitroandinvivois critical and beneficial,so this can help to understand target expression,density,T cell activation,on-target-off-tumor toxicity,as wellas behavior manners of the BiTEs in both animal species and humans.
3 CONCLUSlON
CRS is a common toxicity and side effect to BiTEs in the clinic,which can greatly limit the potential benefits to the patients.Mitigation and management of CRS has become a critical step in pharmaceutical development.To establish a predictable approach and strategy is by far the most important goal for the industry in non-clinical development.IninvitroCRA studies or ininvivoanimal studies,an overall strategy for CRS de-risk programs,should be formulated and a successful de-risk program should be correlated well enongh bothinvitroandinvivoto be eventually translated into the clinic.Different CRA formats,in combination with different animal study results,should be used as an overall approach to the de-risk program,and design of studies and interpretation of study results should be scientific and appropriate.There is no general rule as to which study is necessary,or the most important or preferable.There is the need to understand underlying characteristics and mechanisms of the assessed antibody and to understand how it behaves ininvitroandinvivoand how similar physiologically and pathologically the tested animal models are to human patients.TAA density,affinity to targets both CD3 and TAA,disease burdens,etc.are some basic consider ations before design and a preclinical study[32].
Although NHP is used widely for both pharmacology and toxicology studies for BiTEs in non-clinical settings,NHP is not a default model unless there is cross-reactivity to NHP.If TAA is in solid tumor,and expressed in normal tissues,NHP study result may mislead the potential CRS risk,and more study in more control setting may be needed to correctly predict the clinic outcomes[33].Overall,appropriateinvitroCRA,together within vivoanimal studies,should be able to provide a good de-risk program in preclinical settings,and the overall result should be able to predict clinical outcomes.Also,the result should be able to provide some CRS ranking for candidates,prioritize a safer antibody for further development,and provide enough confidence to select an FIH dose.
Although CRS can be a life-threatening event for BiTEs,many risk mitigation steps are available to minimize the toxic effect of treatment.Ascending dose or step-up dose method has proned effective to reduce CRS in animals and patients[34-35].Moreover,change of the administration route can effectively reduce CRS from intravenous to subcutaneous administration[36].Change of the isotype is also a practical way to reduce CRS,IgM isotype is shown to reduce CRS greatly in animals[37].
Laboratory techniques of analysis to cytokine concentration is also a key point to consider.There are currently two methods to analyze cytokines,ELISA and multiplex.But the results of these two methods are of the different,and multiplex is thought to be more sensitive and accurate for analysis[38-39].In the comparison of study results,it may be wise to compare the results either from the ELISA or multiplex method,but not mutually.
Although the mechanism of CRS by BiTEs is not fully known,it may be similar to CRS induced by CAR-T and corona virus disease 2019(COVID-19),all of which involving activation of T cells.Release of T cell specific cytokines,such IFN-γ and TNF-α,together with monocyte specific IL-6,is reasonably believed to play a key role in development of CRS.Studies show that higher levels of cytokines such as TNF-α,IL-6 and IFN-γ are associated with COVID-19 patients in the ICU,and these may lead to development of CRS to cause pulmonary consolidation and edema,which may advance to acute respiratory depress syndrome and fatal multiorgan failure[40-41].Interestingly,the present studies also showed and proposed that IL-6 plays a key role in development of CRS with COVID-19,and anti-IL-6 antibody,tocilizumab,also approved to treat CRS by CAR-T,offers benefit to severe COVID-19 infection patients[42-44].As such,anti-IL-6 antibodies may also offer the benefit to BiTE related CRS.

