Mitochondrial phylogenomics reveal the origin and adaptive evolution of the deep-sea caridean shrimps (Decapoda:Caridea)*
Shao’e SUN ,Zhongli SHA ,,Yanrong WANG
1 Deep Sea Research Center, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract The deep-sea is considered as the most extensive ecosystem on the Earth.It is meaningful for elucidating the life origins by exploring the origin and adaptive genetic mechanisms of the large deepsea organisms.Caridean shrimps have colonized and successfully adapted to deep-sea environments.They provide an ideal model to analyze the origin and adaptive evolution of modern deep-sea fauna.Here,we conducted the phylogenetic analyses of mitochondrial genomes (mitogenomes) from carideans,including 11 newly sequences reported in this investigation to explore the habitat origins,divergence times,and adaptive evolution of deep-sea (seamounts and hydrothermal vents) caridean shrimps.The results showed that the species of deep-sea Caridea formed a monophyletic group.Phylogenetic analysis supported that the deepsea caridean shrimps may originated from shallow sea.The hydrothermal vents alvinocaridid shrimps and Lebbeus shinkaiae from Thoridae underwent a second range expansion from seamounts to vent ecosystems.Estimates of divergence time showed that the caridean shrimps invaded into deep-sea at 147.75 Ma.The divergence of most of the modern seamount and hydrothermal vent species are in the late Cretaceous/early Tertiary.This may associate with the geological events of the Western Pacific,the climate change,and the global deep-water anoxic/dysoxic events during this period.Twenty-two potentially important adaptive residues were detected in the deep-sea shrimp lineage,which were located in atp6,atp8,cox1,cox3,cytb,nad2,nad4l,and nad5.This investigation adds our understanding of the evolutionary history of deep-sea caridean shrimps,and provides insights into the mitochondrial genetic basis of deep-sea adaptation in this group.
Keyword:hydrothermal vents;seamounts;Caridea;mitochondrial genome;phylogenetic analysis;evolutionary history
1 INTRODUCTION
Deep-sea ecosystems usually refer to the waters and sediments of the ocean below 200 m,representing the largest and most remote biome of the world(Herring,2002).With the development and improvement of technology,the deep-sea exploration has discovered diverse habitats and ecosystems,including seamounts,ridges,deep-water coral reefs,hydrothermal vents,cold seeps,trenches,and so on(Corinaldesi,2015).Compared with shallow water species,deep-sea species survive in harsh environmental conditions,such as low temperatures,low oxygen level,scarce food,a lack of sunlight,and high pressure (Sanders and Hessler,1969).Despite recent major advances in biological research,only 5%of the deep-sea has been explored in detail so far,and less than 0.001% deep-sea organisms has been sampled and described in detail (Danovaro et al.,2014).It is meaningful and important to elucidate life origin by exploring the origin and adaptive genetic mechanisms of the large deep-sea organisms.
As complete organelle genome,mitochondrial genome (mitogenome) shows several advantages,such as relatively high nucleotide substitution rates,lack of extensive recombination,and conserved gene content.The mitogenomes have been proved to be a useful tool for phylogenetic and phylogeographic analyses of animal taxa (Moritz and Brown,1987;Boore,1999;Curole and Kocher,1999).The mitochondrial genomes provide more phylogenetic signals,making more accurate results than analyses of one or few mitochondrial DNA (mtDNA) markers.Mitogenomic analyses are useful for phylogenetic reconstruction in different invertebrate groups,such as molluscs (e.g.,Mikkelsen et al.,2018;Kong et al.,2020),insects (e.g.,Yuan et al.,2015),and annelidans(e.g.,Li et al.,2015).Many phylogenetic studies using the complete mitochondrial genome are available for crustaceans (Shi et al.,2012;Shen et al.,2013;Ji et al.,2014).In addition,mitochondria provide 95% cell energy through the oxidative phosphorylation (OXPHOS),playing a crucial role in aerobic respiration and energy metabolism (Das,2006).The extreme environments of deep-sea may have affected the evolution of mitogenome,as well as the energy production of vent faunas,because several components of the electron transport chain are encoded by mitochondrial genes (Ki et al.,2009).
Caridean shrimps (Decapoda:Caridea) are the second most diverse group amongst the decapods,which are widespread from tropical to polar regions of the world,in both marine and freshwater habitats(Li et al.,2011).Furthermore,some caridean shrimps adapt to deep-sea environments.Species from the families Thoridae (Komai et al.,2019),Pandalidae(Yang et al.,2017),Oplophoridae (Cardoso and Young,2005),Acanthephyridae (Li,2015) inhabit in deep-sea seamounts.Species from the family Bathypalaemonellidae have been collected in a deepsea coral reef habitat (Cardoso,2010).The alvinocarid shrimps (Bresilioidea:Alvinocarididae) are ventendemic species,which comprise the predominant faunal biomass of various hydrothermal ecosystems(Hernández-Ávila et al.,2015).Alvinocarislongirostrisis a species of alvinocarid shrimp codistributed in deep-sea hydrothermal vent and cold seep environments (Hui et al.,2018).Shrimps of the genusLebbeusfrom the family Thoridae have also been reported from various deep-sea hydrothermal vents and cold seep sites (Komai et al.,2012;Chan and Komai,2017).Caridean shrimps are good models to test the hypotheses regarding the origin and adaptive evolution of the deep-sea organisms.However,limited studies have focused on these matters because of the diffi culty of sampling deep-sea species.Scientists have only focused their attention on habitats of deep-sea hydrothermal vents (Wang et al.,2017;Sun et al.,2018a,b,2019a) and cold seeps(Hui et al.,2018;Xin et al.,2020),where life conditions are even more extreme.
In this study,we analyzed three complete mitogenomes of deep-sea hydrothermal vent alvinocarid shrimps and eight mitogenomes of deepsea seamount caridean shrimps.Our specific aims were to clarify (1) where did the deep-sea(hydrothermal vent and seamount) caridean shrimps originate;(2) when did the caridean shrimps colonized deep-sea habitat;(3) how did the caridean shrimps adapted to the deep-sea environment in the perspective of mitochondrial genome.
2 MATERIAL AND METHOD
2.1 Sampling and DNA extraction
In this study,the deep-sea caridean shrimps were collected from both seamount and hydrothermal vent habitats.The hydrothermal vent alvinocaridid shrimps were captured from Manus Basin,Western Pacific.The seamount specimens of the other caridean families were captured from Yap Seamount,Western Pacific.Both the hydrothermal vent and seamount specimens were collected using the remotely operated vehicle (ROV) and then preserved in 95% ethanol until DNA extraction.Total genomic DNA of each species was isolated using the DNeasy tissue kit(Qiagen) according to the manufacturer's instructions.
2.2 Illumina sequencing,mitogenome assembly,and annotation
The mitogenomes were sequenced using Illuminabased whole genome shotgun sequencing.Sequencing libraries were generated using NEBNext®Ultra™ DNA Library Prep Kit for Illumina (NEB,USA) following manufacturer’s recommendations,which was sequenced on an Illumina HiSeq 2500 platform.The reads with average quality scores less than 20 were trimmed from further analysis.Clean data were then assembled using CLC Genomics Workbench v.11.0.64 (http://www.clcbio.com/products/clcgenomics-workbench/) and SOAP denovo (Li et al.,2010) (k-mer=55).De novo assembled contigs were blasted against the NCBI nr database using“BLAST”tool implemented in the CLC Genomics Workbench to identify contigs of mitochondrial origin (E-value=1.0E-15).The putative“mitochondrial DNA”contigs were aligned with the available complete mitogenomes of the Caridea.In order to obtain a circular mtDNA,the contigs identified as mitogenome sequences were examined for repeats at the beginning and end of the sequence using SeqMan of the DNASTAR software package (http://www.dnastar.com).
ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and BLASTx were used to annotate the protein-coding genes (PCGs) using the invertebrate mitochondrial genetic code.The software ARWEN(Laslett and Canbäck,2008),DOGMA (Wyman et al.,2004),and MITOS Web Server (Bernt et al.,2013)located the tRNA genes using the invertebrate mitochondrial genetic code.The rRNA genes were inferred by BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST/) against the available caridean sequences.
2.3 Phylogenetic analysis
Phylogeny of the Caridea was inferred based on 44 available complete mitogenomes.Available caridean mitogenomes were expanded with 11 newly determined complete sequence from the families Alvinocarididae,Thoridae,Pandalidae,Oplophoridae,Nematocarcinidae,Acanthephyridae,and Bathypalaemonellidae.Five penaeoid species were selected as outgroup taxa (Supplementary Table S1).
The phylogenetic relationships were reconstructed based on the concatenated nucleotide sequences of 13 PCGs.The nucleotide sequences for each PCG were aligned with MAFFT (Katoh et al.,2005) using default settings.Ambiguously aligned and variable areas were recognized using the program Gblocks(Talavera and Castresana,2007) (default setting) and excluded from the analyses.A single alignment for phylogenetic analyses was conducted by concatenating all nucleotide sequences of the 13 PCGs.The best-fit nucleotide substitution models were selected by jModelTest (Posada,2008).
Phylogenetic trees were built by both Maximum Likelihood (ML;Felsenstein,1981) and Bayesian inference (BI;Huelsenbeck and Ronquist,2001)methods.ML trees were constructed using RAxML Black-Box webserver (http://phylobench.vital-it.ch/raxml-bb/index.php;Stamatakis et al.,2008).The reliability of the tree topology was evaluated using bootstrap support with 1 000 replicates.RAxMLoptimized parameters of the GTR model for nucleotide sequences.BI was conducted with PhyloBayes MPI (Lartillot et al.,2013) with the CAT site-heterogeneous mixture model (Lartillot and Philippe,2004,2006;Lartillot et al.,2007).Two independent Markov chain Monte Carlo (MCMC)runs chains were run.Tracer v 1.6 (Rambaut et al.,2014) was used to assess stationarity and appropriate burn-in (1 000).Chains were considered to converge well when the maxdiff value was less than 0.1,rel_diff value was less than 0.3,and the minimum effective sample size was greater than 50,respectively,which were measured by bpcomp and tracecomp (Lartillot et al.,2013).
2.4 Divergence time estimation
BEAST 1.8.1 (Drummond et al.,2012) was applied to estimate divergence times of deep-sea clades of Caridea with the relaxed uncorrelated lognormal clocks,random starting trees,and the Yule speciation model.Two independent MCMC runs were executed for 10 million steps,sampling every 1 000 steps.Convergence of the chains was checked using the program Tracer v 1.6 (Rambaut et al.,2014) to ensure that effective sample sizes (ESSs) were above 200,and discard the initial 50% as burn-in.The remaining samples were combined and the maximum-cladecredibility tree was obtained using TreeAnnotator in BEAST.
2.5 Analysis of selective pressure
CodeML implemented in the PAML package(Yang,2007) was used to explore the patterns of natural selection and identify the positive selection sites in each of the thirteen PCGs.The overall mean ratio of nonsynonymous (Ka) to synonymous (Ks)substitution rates (ω) for 13 PCGs was calculated with the“one-ratio (M0)”model.The deep-sea caridean shrimps were labeled the foreground branch,and all other caridean shrimps were labeled the background branch.The“two-ratio (M2)”model was used to computeωratio for the foreground (ω1) and background (ω0) branches.Likelihood ratio tests(LRTs) were performed to test whether the M2 fits the data significantly better than the M0.The branch-site model (Zhang et al.,2005) was used to test for positive selection on the foreground branch.The Model A(MA) assumes either purifying selection (0≤ω0≤1) or neutrality (ω1=1) on all branches,and positive selection (ω2>1) along the foreground branch.The null model (MA0) is constrained thatω2=1.When the likelihood ratio tests were significant,we used the Bayes Empirical Bayes (BEB) method (Yang et al.,2005) to identify sites under positive selection by calculating the posterior probabilities.
3 RESULT
3.1 General features of the new mitochondrial genomes
From the eleven new mitochondrial genomes(mitogenomes),10 were complete and 1 was partial(Supplementary Table S2).The complete mitogenomes ranged in size from 15 618 to 17 168 bp,with length variations mainly in the A+T-rich regions.Most of these mitogenomes harboured a typical set of 37 genes and an identical gene arrangement as previously reported for other caridean mitogenomes.While thetRNA-Ala,tRNA-Val,andtRNA-Tyrgenes were absent fromBathypalaemonellasp.mitogenome,andtRNAIlegene was absent fromParalebbeusjiaolongimitogenome.In order to verify if the tRNA absence in these species is a methodological fail,we diagnosed the targeted tRNA sequences by means of the polymerase chain reaction (PCR).However,thetRNA-Ala,tRNA-Val,andtRNA-Tyrgenes were still not found inBathypalaemonellasp.mitogenome,andtRNA-Ilegene was still absent fromP.jiaolongimitogenome.The partial sequence ofLebbeusshinkaiaemitogenome lacks the region betweennad5andnad4.According to the annotation of the sequenced caridean mitogenomes,we inferred that partial sequences of thenad5andnad4genes,and the tRNA genetRNA-Hislocate in the unamplified portion ofL.shinkaiaemitogenome.
3.2 Phylogenetic reconstruction and divergence time estimation
Maximum Likelihood (ML) and Bayesian inference(BI) inferred similar topological structures with varying levels of support.Therefore,we showed the nodal supports obtained from the two methods together on the ML tree (Fig.1).At the first level,the families Rhynchocinetidae and Crangonidae from shallow water were separated from all other taxa and placed as a basal clade of the tree.The other caridean families included in the analysis separated into three clades.The first clade was solely comprised of Palaemonidae species from both shallow water and freshwater,and the second clade contained only Atyidae species from freshwater.The third major clade included all the remaining families (Thoridae,Bathypalaemonellidae,Pandalidae,Nematocarcinidae,Acanthephyridae,Oplophoridae,and Alvinocarididae) from seamount and hydrothermal vent environments.The hydrothermal vent alvinocaridid shrimps were situated at a more evolved position within the infraorder Caridea.They have the closest relationship with the seamount caridean shrimps (Fig.1).
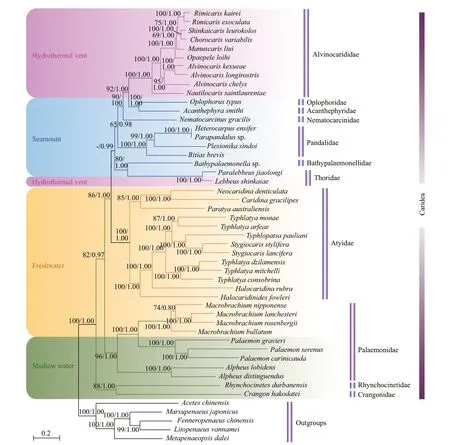
Fig.1 Phylogenetic trees derived from ML and BI analysis of caridean species based on nucleotide sequences of 13 PCGs
The divergence times and the 95% highest posterior density (HPD) intervals are presented for each node(Fig.2).The time to the most recent common ancestor(MRCA) of seamount and hydrothermal vent caridean shrimps was estimated at 147.75 Ma (95% HPD:111.44–185.73).The MRCA of the hydrothermal vent alvinocaridid shrimps were estimated at 61.39 Ma(95% HPD:24.31–102.01) at the Cretaceous-Tertiary boundary.In the family Thoridae,divergence between hydrothermal vent shrimpL.shinkaiaeand its seamount relativeP.jiaolongiwas estimated to have occurred 20.09 Ma (95% HPD:4.22–40.50).The divergence of most of the modern deep-sea species are in the late Cretaceous/early Tertiary.
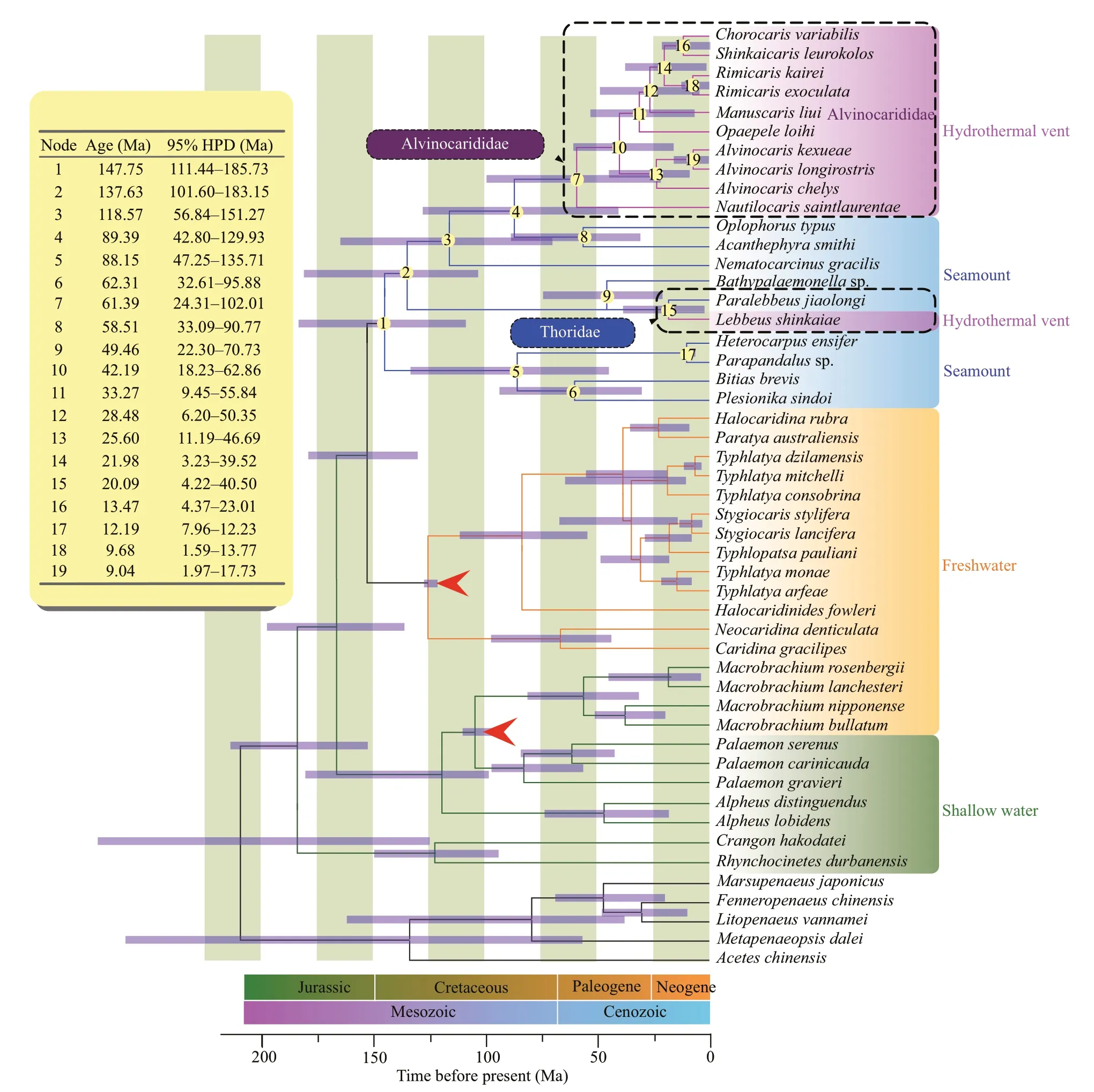
Fig.2 Caridea divergence time estimated using the Bayesian relaxed-molecular clock method
3.3 Natural selection test
The one-ratio (M0) model analyses of 13 PCGs showed that theωvalues for each gene were significantly less than 1 (Table 1),implying that these genes have experienced constrained selective pressure to maintain their function.The result showed thatcox1gene (0.013) experienced the strongest purifying Pressure,whileatp8gene (0.064) experienced the weakest purifying pressure.Based on the two-ratio(M2) model,we found that all the PCGs of deep-sea caridean shrimps showed a higherωvalue than other caridean shrimps,except fornad1,which had a slightly reducedωvalue in alvinocaridid shrimps branch.The LRT tests showed that the M2 model fits the data significantly better than the M0 model at five genes (atp6,cox1,cytb,nad2,andnad5),suggesting a divergence in selective pressure between deep-sea and other caridean shrimps.
The branch-site model was performed to detect positive selection in individual codons.Eight genes(i.e.,atp6,atp8,cox1,cox3,cytb,nad2,nad4l,andnad5) were found to be under positive selection in the deep-sea shrimp lineages,where LRTs of the branchsite model (MA vs.MA0) were statistically significant(Table 2).In these eight genes,total 22 positive selection sites showed BEB values >0.95 using branch-site models.
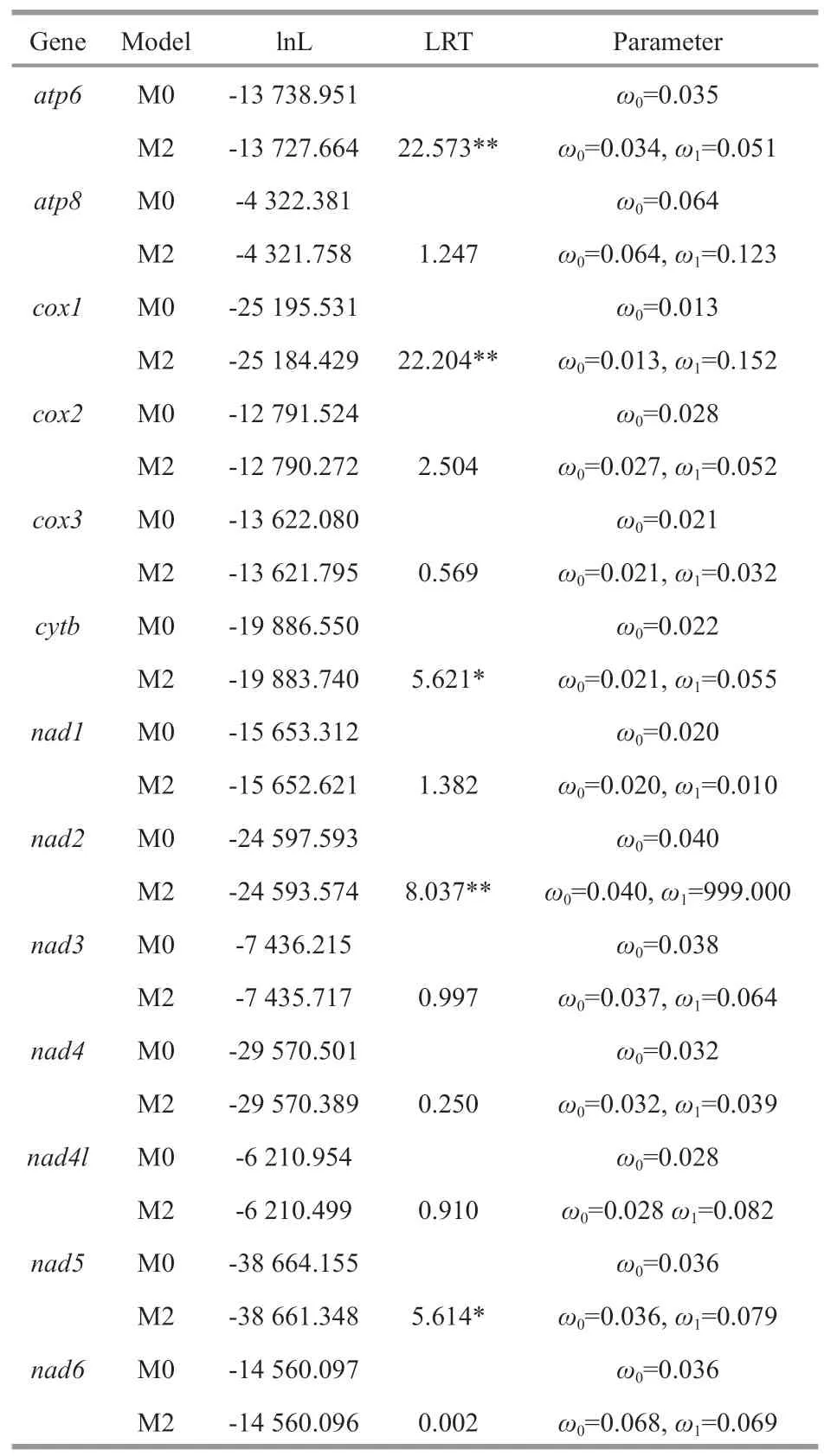
Table 1 Likelihood ratio tests of selective pressures on mtDNA genes of the deep-sea caridean shrimps
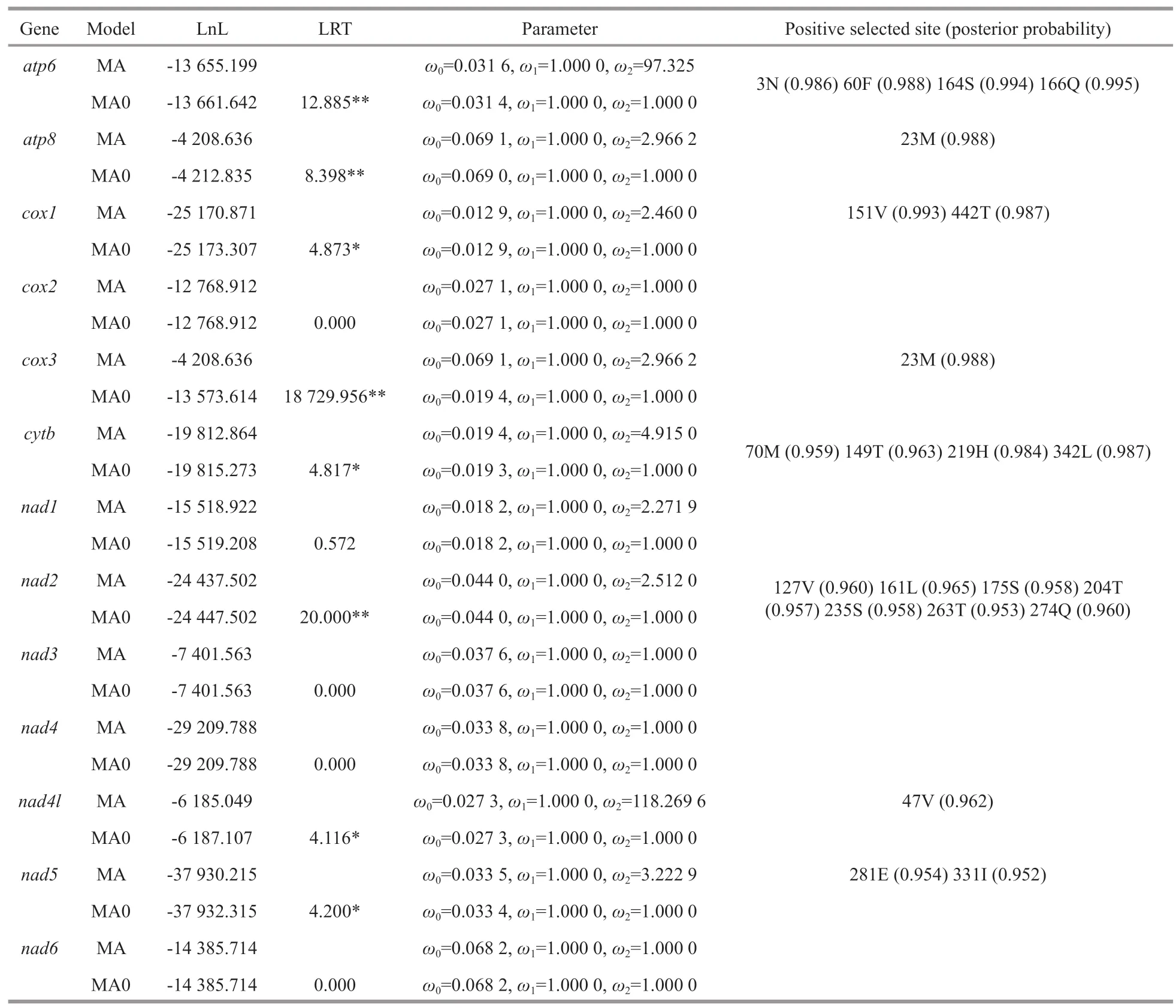
Table 2 CodeML analysis of the mitochondrial protein-encoding genes and positively selected sites (branch-site model)among atp6,atp8,cox1,cox3,cytb,nad2,nad4l,and nad5 genes in deep-sea caridean shrimps
4 DISCUSSION
4.1 Habitat origins of deep-sea caridean shrimps
The evolutionary origin of the deep-sea fauna has been debated for a long time.The study of different taxa has revealed a variety of different colonization patterns based on the zoogeographic data.The analysis of the distribution and taxonomy of deep-sea molluscan,echinoderms,and asellote isopods suggested that all the faunas are probably shaped by invasions from adjacent shallow-water regions (Allen,1979;Jablonski and Bottjer,1990;Raupach et al.,2009;Woolley et al.,2016).In the cases of stylasterid corals,it has been suggested that abyssal forms are most primitive,while those in the shallow waters are more recently evolved (Lindner et al.,2008).The microfossil analyses of Foraminifera from both deepsea and shallow Antarctic indicate that migration of foraminiferal species may has occurred in both directions (Lipps and Hickman,1982;Hayward,2001).
In this context,our phylogenetic analyses showed that the deep sea might be initially colonized with caridean shrimps by the migration events from the shallow water.Transitions from shallow (onshore) to deep (off shore) environments has already been documented in many other decapod crustaceans (e.g.Chan et al.,2009;Palero et al.,2009;Tsang et al.,2009;Tsoi et al.,2011;Yang et al.,2012,2015).These studies suggested that the invasion of deep-sea habitats might drive the early diversifications in diverse groups of decapods (Yang et al.,2012).
Furthermore,our investigation revealed that the Alvinocarididae and Thoridae species may underwent a second range expansion from the seamount to the hydrothermal vent habitats.These observations suggested that the vent-endemic species perhaps migrated from the surrounding deep-sea environments,i.e.seamount,instead of the remnants of ancient hydrothermal species,which agree with the extinction/repopulation hypothesis of vent taxa (Jacobs and Lindberg,1998).The extinction/repopulation hypothesis posited that the global deep-water anoxic/dysoxic events during the Late Cretaceous and Early Tertiary resulted in the extinction of nearly all contemporary vent species,and the hydrothermal vent species later migrated from non-hydrothermal environments (Jacobs and Lindberg,1998).The hydrothermal vents are the typical extreme deep-sea environment,which present some of the most physically and chemically challenging habitats to evolutionary invasion (Van Dover,2000).The hydrothermal vent environment is full with plentiful chemoautotrophic bacteria,producing a rich food source.Simultaneously,this environment has the least predation pressure as predators must also have specialized adaptation to these extreme habitats,and very few appear to have evolved these adaptations(Fisher et al.,2007).Thus,the hydrothermal vents also act as environmental filters that promote the evolution and distribution of species with specialized adaptation and limits of tolerance to oxygen,temperature,and sulfide (Tunnicliffe et al.,2003;Takai et al.,2006;Fisher et al.,2007).The widespread persistence of hydrothermal vent environments in earth’s geologic history (Shock et al.,1995) may have provided an important element enabling the independent colonization by alvinocaridid shrimps.
4.2 When did caridean shrimps invade deep sea?
Although the time to the MRCA of deep-sea caridean shrimps was in the late Jurassic,the MRCA of hydrothermal vent alvinocarid shrimps and the divergence time of the most modern seamount species were in the late Cretaceous/early Tertiary.These times are comparable to the estimates of origin and radiation in other deep-sea taxa,e.g.radiation of hydrothermal vent barnacles at ca.68 Ma (Herrera et al.,2015);origin of siboglinid tubeworms at ca.60 Ma(Chevaldonné et al.,2002);origin of bythograeid crabs at 48.4–55.9 Ma (Yang et al.,2013);radiation of mytilid mussels at ca.45 Ma (Lorion et al.,2013).The period of the Late Cretaceous/Early Tertiary was marked by the continuous separation of continents(Parker and Gealey,1985).During this period,the back-arc systems of Southeast Asia and northeast Australia were opened,forming the main back-arc basins of the Western Pacific.This tectonic and oceanographic transition provided habitats for the caridean species colonization in the deep sea.Moreover,the global deep-water anoxic/dysoxic events occurred in the late Cretaceous/early Tertiary were thought to lead to a massive extinction in deepsea benthic organisms (Jacobs and Lindberg,1998),providing opportunities for invasion of the modern taxa (Jacobs and Lindberg,1998).In addition,this period coincided with a series of other remarkable events including global warming,profound changes in carbon cycling,as well as the greenhouse gas concentrations (Norris et al.,2001).These events may have been attributed to increasing the disturbance in shallow marine environments,such as the evolution of new predators (Vermeij,1987) and more effective competitors (Vermeij,1995),promoting the off shore retreat.The global warming was followed by adeclined deep-water temperature beginning in the Middle (50–48 Ma) to Late (40–36 Ma) Eocene,associated with the initial glaciation of Antarctica(Zachos et al.,2001).The low water temperatures may decreased the metabolic rate of carideans larvae,increasing their longevity and enhancing their dispersal capability (Lorion et al.,2013).This may be an advantage for carideans diversifying in deep-sea habitats.
4.3 Positive selection of mitochondrial genes associated with deep-sea adaptation in caridean shrimps
Once shrimps invaded into deep-sea habitats,they began to face drastic environmental fluctuations,including low water temperature,high pressure,dark,low oxygen concentration,and so on (Katayama et al.,2012).Energy metabolism is an important aspect in adapt to different environments,which can be altered due to environmental conditions to best match energetic demands (da Silva-Castiglioni et al.,2010,2011;Guo et al.,2018).Mitochondrion is the main site of energy production,providing about 95% of the adenosine triphosphate (ATP) needed for the basic activities of life through the OXPHOS (Das,2006).The mitogenome can be characterized by its adaptations to the extreme living environments(Castellana et al.,2011).Previous researchers have found that one major adaptation of decapod crustaceans to harsh conditions is positive selection on mitochondrial genes involved in hypoxia response and energy metabolis,such as cave shrimps (Guo et al.,2018),hydrothermal vent squat lobsters (Sun et al.,2019a,b),hydrothermal vent alvinocaridid shrimps and crabs (Sun et al.,2019a).
In the present study,twenty-two residues,which were located in eight mitochondrial OXPHOS genes along the lineage of deep-sea caridean shrimps,were identified as positively selected sites.These mutations may have functional implications,as they are in components of the electron transport chain.At complex IV (cytochromecoxidase),about 95% of the molecular oxygen (O2) is consumed to form water(Ferguson-Miller et al.,2012;Koopman et al.,2013).Therefore,hypoxia is a major inhibitor for cytochromecoxidase (Cooper and Brown,2008).Previous study reported that the mutation of the structure and/or activity of cytochromecoxidase of the respiratory chain might help to hypoxia adaptation (Luo et al.,2008).Therefore,it is reasonable to assume that the positive selections detected incox1andcox3perhaps enhanced the ability of caridean shrimps to resist the hypoxia environment in deep sea.The nicotinamide adenine dinucleotide (NADH) dehydrogenase complex acts as a proton pump and mutations in this complex may influence the effi ciency of protonpumping,and then affect the metabolic effi ciency (da Fonseca et al.,2008).ATP synthase is the last enzyme complex in the respiratory,which participates in the oxidative phosphorylation after electrons have been transported and a proton gradient has been formed(Mishmar et al.,2003;Zhou et al.,2014;Zhang et al.,2017).When environmental oxygen is reduced,the ATPase activity will increase (Martinez-Cruz et al.,2011).According,mutations inatp6andatp8genes could influence the ATPase activity.In this study,most positive selected residues were identified in the NADH dehydrogenase complex,followed by ATP synthase.Thus,it is reasonable to assume that positive selections in the NADH dehydrogenase complex and ATP synthase perhaps enhanced the ability of deepsea caridean shrimps to resist the hypoxic deep-sea environment.These results provided a necessary molecular mechanism underlying the adaptation of the carideans to deep-sea environments at mitochondrial level,and revealed a modified and adapted energy metabolism in deep-sea species under extreme environments.
5 CONCLUSION
In this study,we newly determined 11 mitogenomes of deep-sea caridaen shrimps.The phylogenetic relationship,divergence times,and adaptive evolution of deep-sea caridean shrimps were explored.The origin analysis indicated that deep sea was initially colonized with caridean shrimps from shallow water at 147.75 Ma,and the hydrothermal vent shrimps underwent a second range expansion from deep sea to vent ecosystems at 61.39 Ma (shrimps of Alvinocarididae) and 20.09 Ma (shrimps of Thoridae),which support the extinction/repopulation hypothesis of vent taxa.The divergence of most of the modern deep-sea species are in the late Cretaceous/early Tertiary.Total twenty-two positively selected sites were identified in eight mitochondrial genes of deepsea species,indicating their deep-sea adaption.This investigation strengthen our understanding of origin and adaptive evolution in the mitogenome of deepsea shrimps.
6 DATA AVAILABILITY STATEMENT
The authors declare that all data supporting the findings of this study are available within the appendix sections.
7 ACKNOWLEDGMENT
We thank the crews of R/VKexuefor their help in sample collection.
 Journal of Oceanology and Limnology2021年5期
Journal of Oceanology and Limnology2021年5期
- Journal of Oceanology and Limnology的其它文章
- Screening of stable internal reference genes by quantitative real-time PCR in humpback grouper Cromileptes altivelis*
- Effect of fasting on protein metabolism in muscle tissue of Larimichthys crocea revealed by transcriptome and proteome*
- Comparison of fungal community composition within different intestinal segments of tilapia and bighead carp*
- C17-fengycin B,produced by deep-sea-derived B acillus subtilis,possessing a strong antifungal activity against Fusarium solani*
- Relationship between morphospecies and microcystinproducing genotypes of Microcystis species in Chinese freshwaters*
- Size-dependent spatio-temporal dynamics of eukaryotic plankton community near nuclear power plant in Beibu Gulf,China*
