Mitigation of hydrogen permeation into steel by bacteria:a new research proposal*
Yanliang HUANG
1 CAS Key Laboratory of Marine Environmental Corrosion and Bio-fouling, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Open Studio for Marine Corrosion and Protection, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract A new research proposal was introduced aiming at solving the fundamental theory for reducing the risk of hydrogen embrittlement (HE) in high-strength steels by utilizing hydrogen-consuming microorganisms.The superior performance of high-strength steel can meet the material strength requirements for remote deep-sea marine engineering development.Due to the heavy corrosive marine environment,steel structures must be protected by cathodic protection.However,high-strength steel is sensitive to stress corrosion cracking and HE,and cathodic protection can promote hydrogen permeation into steel.Hydrogen-consuming microorganisms are widespread in the natural environment and they utilize the energy of hydrogen oxidation to survive.If we could make use of the hydrogen-consuming function of microorganisms to consume the hydrogen generated during the cathodic protection process,then the potential for cathodic protection can be reasonably lowered,ideally protecting the steel and simultaneously reducing the possibility of HE.
Keyword:hydrogen-consuming bacteria;high-strength steel;hydrogen permeation;hydrogen embrittlement(HE);mitigation
1 INTRODUCTION
The goal of remote deep-sea development presents new requirements for marine engineering materials.High-strength steel has the advantages of good mechanical properties,material savings,and reduced structural weight and is capable of meeting these requirements.Its application in marine engineering has gradually expanded,and the demand is increasing.However,the ocean is also a harsh and corrosive environment.Marine corrosion must be addressed to develop and utilize marine resources effi ciently.Cathodic protection is a commonly used method for mitigating marine corrosion.However,high-strength steel is sensitive to stress corrosion cracking (SCC) and hydrogen embrittlement (HE),and cathodic protection may promote hydrogen permeation into steel,thus increasing the hydrogen content and the brittleness of high-strength steel.With increasing strength requirements,the risk of SCC and HE could gradually increase,which has restricted the development of off shore engineering projects (Luo et al.,2015).Generally,increasing the strength of high-strength steel increases its sensitivity to SCC and HE (Hirth,1980;Katano et al.,2001;Tsay et al.,2006;Akiyama et al.,2010;Li et al.,2010;Ge et al.,2020).The mere presence of water in the environment can cause SCC.For example,SCC can occur in a humid atmosphere,even during product manufacturing,testing,and storage processes (Chen et al.,2001;Li et al.,2004).The HE failure of high-strength steel parts has occurred;a high-strength steel bolt broke during use.A failure analysis showed that the reason for the fracture was HE (Lin and Yang,2009;Tang,2015).
At present,although the resistance of high-strength steel to HE has been improved in terms of material structure,HE in high-strength steel has not been solved completely in the development and application of marine resources.In practical engineering applications,the potential of cathodic protection is usually too negative,which increases the risk for HE in materials,manifesting as reduced or failed performance.In the marine environment,negative cathodic protection potential leads to the brittle fracture of materials,which not only causes serious economic losses but also leads to serious accidents.If a material is less sensitive to HE due to a lower hydrogen diffusion rate,the cathodic protection potential can be appropriately lowered.However,it is commonly understood that to prevent an obvious change of material performance under cathodic protection,the potential used is generally higher than the ideal protection potential.In fact,without ideal protection,the corrosion process still occurs on the surface of the material.If hydrogen that produced during the cathodic protection potential application on the steel surface can be consumed in some way to prevent it from diffusion to the inside of the material,the cathodic protection potential can be properly lowered so that the steel can obtain an ideal protection level that minimizes the corrosion rate and avoids HE,which could play an important role in the safe application of high-strength steel in the marine environment.Hydrogen-consuming microorganisms that are engaged in hydrogen oxidation as a life activity exist widely in the natural environment.If the hydrogen consumption function of these hydrogenconsuming microorganisms can be utilized to consume the hydrogen produced during the cathodic protection process and reduce the hydrogen permeation into the metal,the cathodic protection potential can be appropriately lowered,and the threat of HE can be simultaneously reduced along with the corrosion rate.
2 THE CHARACTERISTICS OF HYDROGEN EMBRITTLEMENT AND RECENT DEVELOPMENTS IN MATERIAL IMPROVEMENT
The characteristics of HE include the following:(1) a deterioration in mechanical properties,especially a significant reduction in elongation and fractured area;(2) a change in fracture mechanism:with the increased hydrogen concentration in the material,the fracture mode changes from ductile dimple fracture to brittle cleavage or intergranular fracture;and (3)fractures occurring suddenly without obvious portents.Therefore,the prevention of hydrogeninduced brittle fractures in high-strength steel has been an important research interest.To solve the problem of HE in high-strength steels,many studies have examined the alloying (Sandoz,1972;Wu et al.,1984;Magdowski and Speidel,1988;Li et al.,1993a;Wang et al.,2005;Zhong et al.,2010),heat treatment(Li et al.,1991,1993b,1996;Gao et al.,1993;Liu et al.,1993),microstructures (Li et al.,1991,1993b,1996;Gao et al.,1993;Liu et al.,1993;Zan et al.,2016),SCC and HE mechanisms (Gerberich and Chen,1975;Bandyopadhyay et al.,1983;Lin et al.,1988;Li et al.,2001,2004),and evaluation methods(Hui et al.,2001).For example,the theory of yield strength control (Gerberich and Chen,1975;Li et al.,2001) and the theory of segregation control of impurity elements at the original austenite grain boundary (Bandyopadhyay et al.,1983;Lin et al.,1988) were examined earlier in terms of the SCC/HE mechanisms of high-strength steels.Because SCC/HE resistance generally decreases with increasing yield strength,the view of yield strength control holds that the yield strength of steel determines the level of hydrostatic stress at the crack tip,thus affecting the degree of hydrogen accumulation at the crack tip and determining the SCC/HE resistance.According to the theory of impurity segregation control,the segregation of impurity elements in the original austenite grain boundary creates embrittlement in the SCC environment,resulting in low stress fractures along the grain,and the degree of impurity segregation controls the fracture sensitivity.According to their research results,Li et al.(2004) indicated that carbides on the original austenite grain boundary control SCC and HE sensitivity,which was verified by experiments.There exists a thin layer of carbide on the original austenite grain boundary with tempered martensite as the main structure,which makes the steel prone to low stress intergranular fractures in an SCC environment.The main reason for the increase in carbon content leading to the increase in low stress intergranular fractures is the increase in carbides on the austenite grain boundary.After high-temperature tempering,the coarsening of carbides on the grain boundary and the precipitation of carbides in the grain lead to a decrease in intergranular fracture tendency and an increase in fracture resistanceKISCC.According to this point of view,if these carbides accumulated in the original austenite grain boundary during hightemperature austenitizing and quenching can be effectively reduced and eliminated by alloying or hot working,the stress corrosion fracture resistance of steel can be significantly improved while maintaining its high strength.Based on the existing research achievements,the SCC/HE resistance of highstrength steel has been greatly improved,but the safety problems of high-strength steel in the development and application of marine resources have not been meaningfully addressed.Due to the strong corrosiveness of the marine environment,cathodic protection is an effective method for preventing the corrosion of steel materials in seawater immersion zones and seabed zones and has been studied extensively.
3 RELATIONSHIP BETWEEN HYDROGEN EMBRITTLEMENT AND CATHODIC PROTECTION
In the seawater environment,the corrosion of steel structures and components can be prevented by cathodic protection with appropriate potential.However,in actual engineering applications,an overly negative cathodic protection potential usually leads to HE,resulting in the degradation or loss of material performance.In the marine environment,the occurrence of HE failure because of overly negative cathodic protection potential not only causes serious economic losses but also leads to catastrophic accidents.Tan et al.(1988) studied the environmental HE of ZC-120 steel in a seawater environment caused by cathodic protection.Their results showed that the environmental HE of ZC-120 steel in a seawater environment is closely related to the current density of cathodic protection.The more negative the applied cathodic protection potential is,the greater the current density and the greater the environmental HE sensitivity of ZC-120 steel.Zucchi et al.(2006)studied the effect of cathodic protection on the HE of duplex stainless steel in seawater containing sulfides.When the applied cathodic protection potential was-900 mV (vs.saturated calomel electrode (SCE)),the HE of duplex stainless steel occurred in acidic artificial seawater;when the potential was shifted negatively to -1 000 mV (vs.SCE),the sensitivity to HE increased.Yang et al.(2009) studied the effect of cathodic polarization on 907 steel in a seawater environment.Their results also showed that the material’s HE sensitivity increased with the negative shift of polarization potential.When the potential polarization reached -1 160 mV,brittle fracturing occurred.Jang et al.(2009) obtained the cathodic protection potential range of three kinds of stainless steel—STS304,STS316,and STS630—in a seawater environment through the cathodic polarization curve test and determined the hydrogen evolution potential of these three materials as -912 mV (vs.SCE),-912 mV (vs.SCE),and -1 070 mV (vs.SCE).Du and Sun (2010) studied the cathodic polarization behavior of 410 stainless steel in natural seawater using a potentiodynamic polarization curve and potentiostatic polarization method and finally determined that -700–-900 mV (vs.SCE) is the protection potential range for 410 stainless steel.When the applied potential is more negative than-900 mV (vs.SCE),the steel will susceptible to HE.Zhang et al.(2011) studied the influence of cathodic polarization potential on the sensitivity to HE for X70 steel in seawater.When the polarization potential is lower than -1 050 mV (vs.SCE),a tensile test fractured surface of the X70 steel appeared to be quasi-cleavage.Qiu et al.(1992) studied the appropriate cathodic protection potential range of 16Mn steel in a 3% NaCl solution by means of the weight loss and slow strain rate test (SSRT) method.HE had already occurred in the 16Mn steel at the applied potential with no obvious hydrogen evolution.It was only at a further negative potential that obvious hydrogen evolution occurred.Finally,-775–-900 mV(vs.SCE) was determined as the appropriate cathodic protection potential range.Wen (2014) studied the cathodic polarization of marine steels(ZG06Cr13Ni4Mo cast steel,304L stainless steel,and Q235 steel) in a 3.5% NaCl solution by means of electrochemical impedance and potentiodynamic polarization experiments.The experimental results showed that the best protective potential of ZG06Cr13Ni4Mo cast steel,304L stainless steel and Q235 steel was approximately -0.75 V,-0.85 V,and-0.75 V respectively,while the hydrogen evolution potential was -1.00 V.The experimental results of hydrogen permeation and hydrogen content measurements showed that hydrogen had already entered the metal material when the applied potential did not reach the hydrogen evolution potential.The amount of hydrogen entering the material increased with a further negative shift of the cathodic polarization potential.The brittle fracture of ZG06Cr13Ni4Mo steel had already begun in the absence of obvious hydrogen evolution.Zeng et al.(2009) studied the diffusion behavior of hydrogen in 304 stainless steel under different cathodic polarization potentials by the electrochemical hydrogen permeation method.The comparatively lower HE sensitivity of 304 stainless steel could be attributed to the smaller apparent diffusion coeffi cient.
4 NEW METHODS USING MICROORGANISMS FOR HYDROGEN PERMEATION MITIGATION
It can be seen in the above studies that lower sensitivity to HE is due to the smaller diffusion rate of hydrogen into the material,and the cathodic protection potential can be appropriately lowered.However,it is commonly understood that to prevent the obvious deterioration of material performance under cathodic protection conditions,the cathodic protection potential is usually higher than the ideal protection potential(not fully protected).In fact,corrosion still occurs on the material surface.If the hydrogen produced by applying cathodic protection potential on the steel surface can be consumed in some way so that the hydrogen does not diffuse to the inside of the material,then the cathodic protection potential can be lowered so that the steel can obtain ideal protection and the corrosion rate can be minimized without the risk of HE.The realization of this goal could play an important role in the safe application of high-strength steel in marine environments with broad application prospects.
Hydrogen-consuming microorganisms that engage in hydrogen oxidation as life activities exist in the natural environment.Collet et al.(2005) studied the effect of hydrogen-consuming microorganisms on the metabolism ofClostridiumthermolacticum.The results showed that the production of acetic acid was increased threefold by the hydrogen-consuming microorganismsMethanothermobacterthermoautotrophicusandM.thermoautotrophica.WhenC.thermophilusmetabolizes lactose,it produces acetic acid along with hydrogen,carbon dioxide,lactic acid and other byproducts.M.thermoautotrophicusplays an important role during this process.It is an effective hydrogen remover that can significantly reduce the partial pressure of hydrogen in the system and convert hydrogen into methane.M.thermoautotrophicaconverts hydrogen,lactic acid,and carbon dioxide into acetic acid.Hoehler et al.(2001) noted a kind of hydrogenconsuming microorganismMethanogenicarchaeain the study of the minimum apparent free energy required by microorganisms in anoxic marine sediments.Homoacetogenicbacteria are also hydrogen-consuming microorganisms.Kotsyurbenko et al.(2001) studied the competitive behavior for hydrogen withMethanogenicarchaeain an anaerobic environment.Lin et al.(2014) showed that basalt aquifers on the sea floor could support hydrogendriven ecosystems.Feng et al.(2014) and Zhao(2012) carried out an experimental study on enhanced methane production in the anaerobic digestion process of sludge by adding 0 valent iron.The reason for the enhancement was that the hydrogenconsuming bacteria utilized hydrogen produced by iron corrosion.Mori et al.(2010) isolated 26 kinds of microorganisms from petroleum facilities.Among them,hydrogen-consuming microorganisms had no strong promoting effect on the corrosion of iron,while another kind of nonhydrogen-consuming microorganism causes serious iron corrosion.This is also the only study on hydrogen-consuming microbial corrosion.Nybo et al.(2015) reviewed the work of usingChemolithoautotrophicbacteria to fix greenhouse gases during the production of fuel and chemical products.These autotrophic microorganisms also consume hydrogen.There are also published studies (Giblin et al.,2000;Libert et al.,2011) on the use of hydrogen-consuming microorganisms in microbial engineering.Hydrogen-consuming microorganisms also exist in the deep-sea environment (Boyle,2011).Hydrogen-consuming microorganisms are widespread in nature.If the function of these hydrogen-consuming microorganisms can be used to consume the hydrogen produced during the cathodic protection process and reduce the diffusion of hydrogen into metallic materials,the cathodic protection potential can be appropriately lowered,and the probability of HE occurring can be reduced while simultaneously reducing the corrosion rate.This is very important for the safe application of high-strength steels in marine environments and has broad application prospects.However,this work has not been fully carried out,and studies are still in preliminary stage.Therefore,it is necessary to carry out research on the fundamentals and mechanisms of marine hydrogenconsuming microorganisms on the cathodic protection of high-strength steels to provide theoretical support for the future applications of hydrogen-consuming microorganisms and the development of biomimetic technology to improve the safety and reliability of high-strength steel applications in the marine environment.
5 WORK TO BE CONDUCTED AND TECHNIQUES TO BE USED
5.1 Effects of hydrogen-consuming microorganisms on the cathodic polarization processes of low-alloy high-strength steels
Studying the effects of hydrogen-consuming microorganisms on the cathodic polarization processes of low-alloy high-strength steel will provide necessary electrochemical experimental parameters for future research.Cathodic polarization curves,electrochemical impedance (EIS),and potentiostatic cathodic polarization measurements can be used in the study of hydrogen-consuming microorganisms on the cathodic polarization behavior of low-alloy highstrength steels.Cathodic polarization curve measurement is a common electrochemical method used to study the corrosion process of metallic materials.The influence of hydrogen-consuming microorganisms on the cathodic protection potential range and hydrogen evolution potential of a material can be obtained through the measurement of the cathodic polarization curve.A schematic of the measuring device is shown in Fig.1.Unlike a normal electrochemical cell,the device is specifically designed to incorporate the bacteria culturing function.The cultured solution is introduced into the experimental cell before measurement.The electrochemical workstation can be used to measure the cathodic polarization curve with a three-electrode test system.A short cylindrical sample is suggested as the working electrode,with a platinum (PT) electrode as the auxiliary electrode and a SCE as the reference electrode.The EIS test starts from the open circuit potential and is conducted at every certain potential interval in the direction of cathodic polarization.The test results can be analyzed and processed by relevant data processing software.EIS is effective at obtaining the electrochemical characteristics under different polarization conditions,and the electrochemical parameters from the equivalent circuit reflect the cathodic process.In addition,a long-term potentiostatic cathodic polarization experiment needs to be carried out to determine whether the presence of hydrogen-consuming microorganisms has an impact on the cathodic protection effi ciency at the same potential.
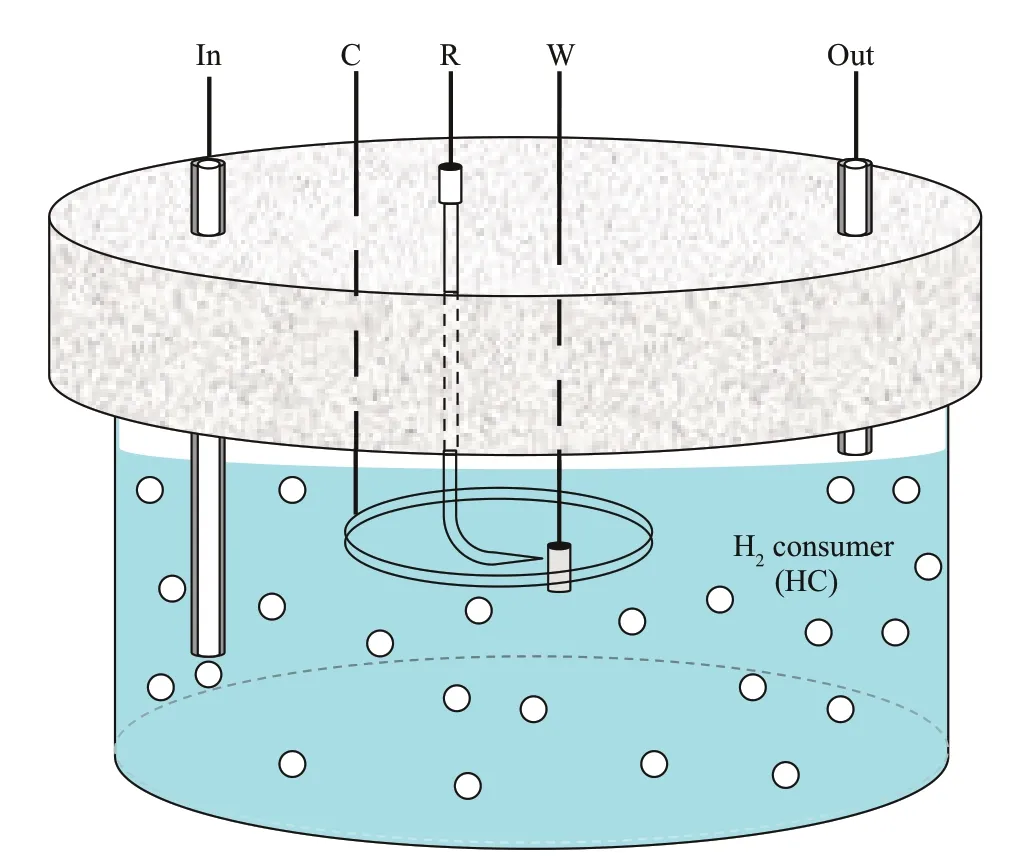
Fig.1 Schematic of the device for electrochemical polarization measurements in an environment containing hydrogen-consuming microorganisms
5.2 Effect of hydrogen-consuming microorganisms on the hydrogen permeation behavior of low-alloy high-strength steel at different polarization potentials
The purpose of this study is to obtain the relationship between the hydrogen content and the polarization potential of materials in the environment with different concentrations of hydrogen-consuming microorganisms.The hydrogen permeation behavior of low-alloy high-strength steel under different polarization potentials can be measured by Devanatan-Stachurski’s double-cell technique (Devanathan et al.,1963) and then compared with the results obtained with and without hydrogen-consuming microorganisms.The experimental device is shown in Fig.2.The hydrogen detection cell used in Devanatan-Stachurski’s technique is a three-electrode system.The inner wall of the cylindrical sample is the working electrode for hydrogen permeation detection,with both ends being sealed.The cylinder is filled with a NaOH solution.Nickel or palladium is plated on the inner side of the cylindrical sample to contact with the NaOH solution.Hg/HgO is used as the reference electrode,and platinum is used as the auxiliary electrode.The hydrogen content of low-alloy highstrength steel can be measured by electrochemical or thermal desorption spectrum (TDS) methods in environments with or without hydrogen-consuming microorganism media under different polarization potentials.Hydrogen content measurement by an electrochemical method records the hydrogen permeation current over time when the polarization experiment is finished for a specified time.By integrating the area under the permeation currenttime curve,the concentration of hydrogen in the sample can be estimated.These two experimental methods are now commonly used by scientists engaged in hydrogen permeation research (Zhang et al.,2019;Akiyama et al.,2010).
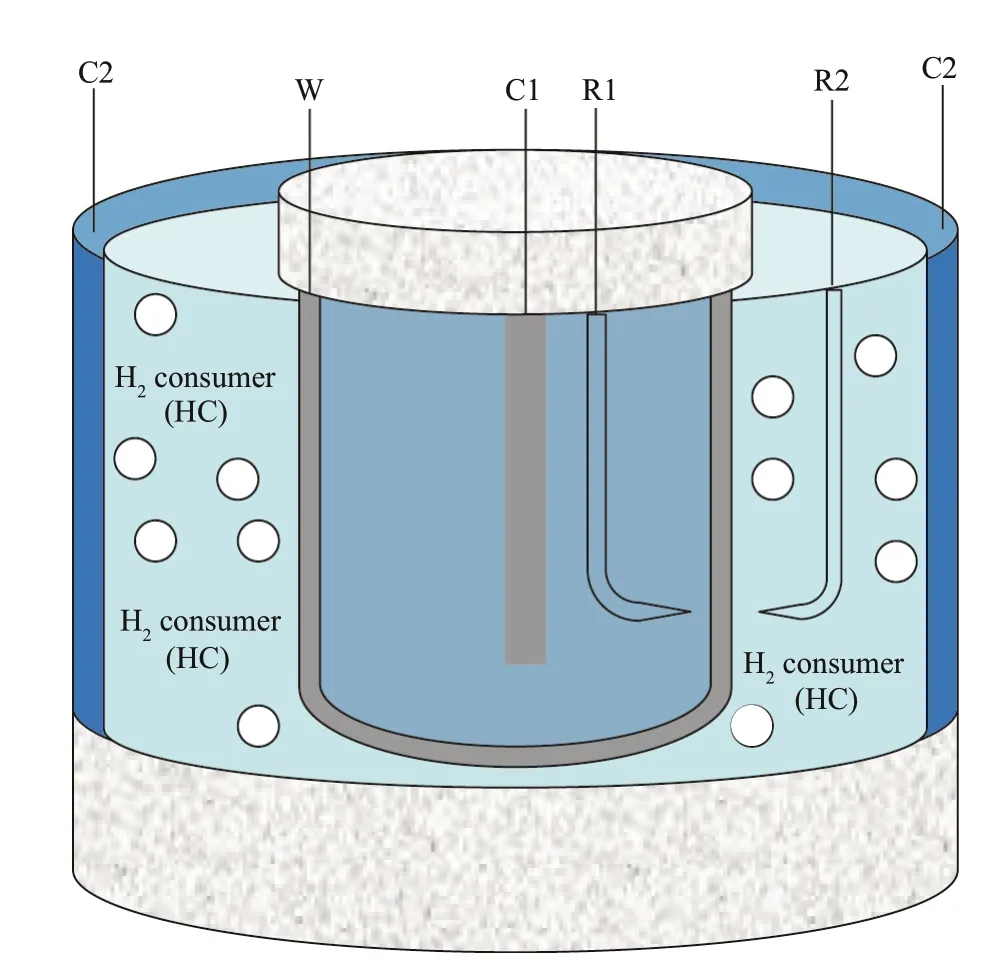
Fig.2 Schematic of the device used for hydrogen permeation measurements under cathodic polarization conditions
5.3 Effect of hydrogen-consuming microorganisms on the mechanical behavior of low-alloy highstrength steel at different polarization potentials
The mechanical properties of low-alloy highstrength steel under different cathodic polarization potentials in environments with different concentrations of hydrogen-consuming microorganisms can be studied using a slow strain rate tensile test combined with scanning electron microscope (SEM) observations of fractured surfaces compared with a situation without hydrogen-consuming microorganisms.The inhibiting effects of hydrogen-consuming microorganisms on HE can be confirmed by obtaining the relationship between the mechanical properties of high-strength steel under constant cathodic polarization potentials and the concentration of hydrogen-consuming microorganisms.
5.4 Interaction model between hydrogenconsuming microbial films and metal surfaces under polarized conditions and the biomimetics of hydrogen-consuming microbial films
One must study the morphology and structural characteristics of a hydrogen-consuming microbial film on the surface of a material and analyze the interactions between the hydrogen-consuming microorganisms and a metal surface under polarized conditions by pH and potential distribution measurements of the microbial film to lay the foundation for the biomimetics of the hydrogenconsuming microbial film.The formation conditions of natural hydrogen-consuming microbial films are not easy to control,and there are side effects to hydrogen consumption.The study of the morphology,structural characteristics,and interactions between the film and metal surface can provide a theoretical basis for the biomimetic study of the film for the development of practical HE protection techniques.A schematic illustration of the mutual effects between hydrogen-consuming organisms and polarized steel surfaces is shown in Fig.3.
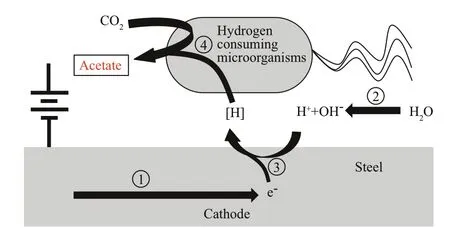
Fig.3 Schematic illustration of the mutual effects between hydrogen-consuming organisms and polarized steel surfaces
6 PROSPECT AND CHALLENGE
Hydrogen-consuming microorganisms exist widely in the natural environment,and their resources are abundant.The ultimate cause of HE is hydrogen entry as a result of generated hydrogen during the cathodic processes at free corrosion or cathodic polarization potentials.The generated hydrogen can provide energy for hydrogen-consuming microorganisms,which engage in hydrogen oxidation for survival.Ideally,integrating the actions of hydrogen consumption by microorganisms and cathodic polarization can create an effi cient means of combating HE of marine structures.
This is a noble goal,but its realization is by no means straightforward.The metabolism of hydrogenconsuming microorganisms is a complex process.They not only consume hydrogen but also produce byproducts with side effects.For example,homoacetogen can grow autotrophically through the reduction of CO2to acetate with H2as an electron donor (Diekert and Wohlfarth,1994;Madigan et al.,2015).The presence of acetate can result in a decreased pH value of the corrosive medium near the specimen surface and dissolve the protective calcareous deposits(Little et al.,1988).When homoacetogen was inoculated,the hydrogen permeation effi ciency for an AISI 4135 steel specimen was reduced to 28% from 37% for a sterile medium at a potential of -0.85 V vs.SCE,showing the effect of its hydrogen entry mitigation (Liu et al.,2020).However,comparing the hydrogen permeation current densityjpvalues of steel specimens in a sterile medium and a homoacetogen inoculated medium,thejpvalue in homoacetogen inoculated medium remained higher than thejpin the sterile medium (Fig.4).This problem arises from the fact that the hydrogen-consuming effect and the side effect of producing acetate occur at the same time.
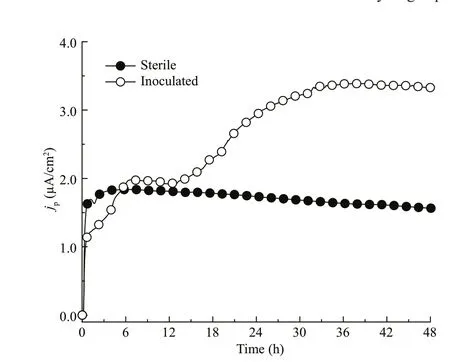
Fig.4 Hydrogen permeation current density-time curves of AISI 4135 steel in sterile and homoacetogeninoculated medium at a potential of 0.85 V vs.SCE
The homoacetogen can consume hydrogen for its growth,but the side effect of producing acetate can also occur at the same time in the inoculated medium.Yes,the acetate by microbes not only dissolves the protective calcareous deposits,the acidification of solution can directly corrode the steel as well especially under the biofilm.In some cases,the pH value is much lower on the partial surface of steel than the value on other parts,enhancing the corrosion of a metal.As shown in Fig.4,as a direct evidence,the hydrogen permeation current was reduced at about the first 6 h,indicating that the corrosion was not enhanced to a higher value while homoacetogen was consuming hydrogen,because the produced acetate needed time to accumulate on the surface of specimen to accelerate corrosion.Apparently,with the growth of the microbe,more acetate was accumulated in the inoculated media and the corrosion was enhanced,overcoming the inhibition of cathodic protection(CP),restricting the practical application of this novel method.
New ways to separate the hydrogen-consuming effect from the side effects will be helpful but not easy.Another approach would be to find a microorganism capable of converting byproducts into nonharmful byproducts.The formation of a microbial film on a steel surface is not easy to control,and biomimetic studies of these films will be useful.With the rapid development of biotechnology,such as genome editing and other relevant techniques,there is a high probability of realizing the goal of mitigating HE using microorganisms or biomimetic technology.
I hope the publication of this article will attract scientists of related disciplines to combat the problem by finding microbes without acid producing or finding way to avoid the side effects while consuming the hydrogen.
7 DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2021年5期
Journal of Oceanology and Limnology2021年5期
- Journal of Oceanology and Limnology的其它文章
- Screening of stable internal reference genes by quantitative real-time PCR in humpback grouper Cromileptes altivelis*
- Morphology and multifractal features of a guyot in specific topographic vicinity in the Caroline Ridge,West Pacific*
- Geochemical characteristics and geological implication of ferromanganese crust from CM6 Seamount of the Caroline Ridge in the Western Pacific*
- Deep-sea coral evidence for dissolved mercury evolution in the deep North Pacific Ocean over the last 700 years*
- Physical oceanography of the Caroline M4 seamount in the tropical Western Pacific Ocean in summer 2017*
- Characteristics and biogeochemical effects of oxygen minimum zones in typical seamount areas,Tropical Western Pacific*
