Paragorgia papillata sp.nov.,a new bubblegum coral(Octocorallia:Paragorgiidae) from a seamount in the tropical Western Pacific*
Yang LI ,Zifeng ZHAN ,Kuidong XU ,
1 Laboratory of Marine Organism Taxonomy and Phylogeny, Shandong Province Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
2 Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai 519082, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract A new species of bubblegum coral,Paragorgia papillata sp.nov.,discovered from a seamount located on the Caroline Ridge at the water depth of 858 m,is studied using morphological and molecular approaches.The new gorgonian is white-colored,uniplanar with prominent autozooids,and measures about 670-mm high and 690-mm wide.The genetic distance and phylogenetic analysis showed that P.papillata sp.nov.was closely related to P.coralloides Bayer,1993,but the former differs morphologically from the latter by its prominent calyces (diameter 2.0–4.0 mm and height 1.5–3.0 mm vs.both diameter and height about 1 mm),white cortex (vs.pink),regular 8-radiates in surface cortex (vs.mostly 8-radiate derived globular radiates) and highly ornate medullar spindles (length 185–400 μm vs.no more than 150 μm).P.papillata sp.nov.is the third known white-colored species of the genus,and the fifth species found in the tropical Western Pacific.
Keyword:Paragorgia;gorgonian;Scleraxonia;Cnidaria;new species;seamount
1 INTRODUCTION
Paragorgiidae Kükenthal,1916,one of the nine families of the octocoral suborder Scleraxonia Studer,1887,comprises members having dimorphic polyps and an axial medulla formed by accumulation of unfused sclerites (Sánchez,2005;Li et al.,2020).To date,the family contains 18 known species within the genusParagorgiaMilne-Edwards and Haime,1857 and four species within the genusSibogagorgiaStiasny,1937;all members have restricted distributions in deep waters (Sánchez,2005;Herrera et al.,2010;Herrera and Shank,2016;Li et al.,2017;Horvath,2019).The two genera are differentiated by the large canals in the medulla of terminal branches and the sclerites of autozooid tentacles (Sánchez,2005;Herrera et al.,2010;Horvath,2019).
During the survey on a seamount biodiversity in the tropical Western Pacific in 2019,we collected a whitecolored individual ofParagorgiaat the water depth of 858 m.The specimen was inhabited by a basket star(probably of the echinoderm family Gorgonocephalidae Ljungman,1867),five ophiuroids of the order Euryalida de Lamarck,1816,and a scale worm of the polychaete family Polynoidae Kinberg,1856.Based on the morphological and molecular phylogenetic analyses,the species is described herein asParagorgiapapillatasp.nov.FollowingP.splendensThomson and Henderson,1906,P.coralloidesBayer,1993,P.sibogaeBayer,1993,andP.rubraLi et al.,2017,the new species becomes the fifth species of the genus found in the tropical Western Pacific.
2 MATERIAL AND METHOD
2.1 Specimen collection
Specimen was collected by the submersible remotely operated vehicle (ROV)Faxian(Discoveryin Chinese) from a seamount in the tropical Western Pacific during the cruise of the R/VKexue(Sciencein Chinese) in 2019.The seamount is located on the Caroline Ridge (named unoffi cially as“M6”).The specimen was photographed in situ before being sampled by the ROV.Post collection,the specimen was immediately photographed and small branches were stored at -80 °C for DNA Extraction.The rest of colony was fixed in 70% ethanol for morphological studies and permanent preservation.The holotype is deposited in the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS) at Qingdao,China.
2.2 Morphological examination
Micromorphology was observed and photographed by means of a stereo dissecting microscope (Zeiss SteREO Discovery.V12).Sclerites from the autozooid tentacles,surface cortex,inner cortex,and medulla were isolated respectively by digestion of these tissues in sodium hypochlorite,and then were washed with distilled water and pure ethanol.To investigate the ultrastructure,sclerites were transferred to carbon double adhesive tape,air-dried and coated for the Scanning Electron Microscope (SEM).SEM scans were obtained using Hitachi TM3030Plus SEM at 15 kV and the optimum magnification for each target sclerite.About 40 sclerites from each sample were photographed covering as many shape types,ornament variations,and size range as possible,and 20 of them were randomly chosen for measurement(Li et al.,2020).Terminology follows Bayer et al.(1983).
2.3 DNA extraction and sequencing
Genomic DNA was extracted from the polyps using DNeasy Blood and Tissue Kit (Qiagen,Hilden,Germany) following manufacturer’s instructions.To amplify the mitochondrial regionmutShomolog(mtMutS),the primers AnthoCorMSH (5ʹ-AGGAGAATTATTCTAAGTATGG-3ʹ;Herrera et al.,2010)and Mut-3458R (5ʹ-TSGAGCAAAAGCCACTCC-3ʹ;Sánchez et al.,2003) were used.PCR reactions were conducted following Xu et al.(2020).PCR purification and sequencing were performed by TsingKe Biological Technology (TsingKe Biotech,Beijing,China).
2.4 Genetic distance and phylogenetic analyses
The mtMutS as the most variable mitochondrial gene in octocorals,was selected for genetic distance and phylogenetic analyses.The mtMutS sequences of all availableParagorgiaassociated with published articles and twoSibogagorgiaspecies as the outgroup were downloaded from GenBank,and those not identified to species level were omitted from the molecular analyses.The sequences were aligned using MAFFT v.7 (Katoh and Standley,2013) with the G-INS-i algorithm.The nucleotide alignment was trimmed to equal length using BioEdit v7.0.5 (Hall,1999).Genetic distances between species/populations were calculated with MEGA 6.0 using uncorrected“p”model (Tamura et al.,2013).
For the phylogenetic construction,when conspecific sequences were identical,one was chosen randomly for analysis.The best-fitted model TPM2uf+I was selected with the Akaike information criterion in jModeltest2 (Darriba et al.,2012).Maximum likelihood (ML) analysis was performed using PhyML-3.1 (Guindon et al.,2010) with the TPM2uf+I model.Node support came from a majority-rule consensus tree of 1 000 bootstrap replicates.Following Hillis and Bull (1993),the ML bootstraps <70%,70%–94%,and ≥ 95% were considered as low,moderate,and high,respectively.Bayesian inference (BI) analysis was carried out using MrBayes v3.2.7a (Ronquist and Huelsenbeck,2003) with GTR model.Priors were fixed for substitution rate (“revmat”) and stationary nucleotide frequencies (“statefreg”) as output by jModeltest2.Posterior probability was estimated based on 10 000 000 Monte Carlo Markov Chain (MCMC)generations (×4 chains) sampling every 1 000 generations (burn-in=25%).Convergence of the MCMC was assessed using Tracer 1.4.1 (Rambaut and Drummond,2007).Following Alfaro et al.(2003),the Bayesian posterior probabilities <0.95 and≥ 0.95 were considered as low and high,respectively.The GenBank accession numbers of the sequences were listed next to the species names in the phylogenetic trees.
3 RESULT
3.1 Systematics
Class Anthozoa Ehrenberg,1834
Subclass Octocorallia Haeckel,1866
Order Alcyonacea Lamouroux,1812
Family Paragorgiidae Kükenthal,1916 GenusParagorgiaMilne-Edwards and Haime,1857Paragorgiapapillatasp.nov.(Figs.1–3)
Material examined Holotype:MBM286408,collected from M6 seamount at the station FX-Dive 219 (10°06.78ʹN,140°14.53ʹE) with the water depth of 858 m (temperature 5.04 °C;salinity 34.5) on 7 June 2019,rocky bottom.
DescriptionGrowth form and size:Colony uniplanar overall (terminal branches often overlapped but without anastomoses),dichotomous,approximately 670-mm high and 690-mm wide in preservation (Fig.1).Holdfast irregularly circular,detached from colony.The main stem compressed,cross-section 33×46 mm in width,approximately 60-mm long before a broken large branch arising.Two large branches arising about 40-mm long before the first branch,one measuring about 19-mm wide and 33-mm deep,the other one 23-mm wide and 27-mm deep.Terminal branches mostly 10–90 mm in length,2–5 mm in diameter exclusive of autozooid clumps,each clump with 8–9 autozooids (Fig.1).Transversal cross-section of the main stem full of canals,from which fixation liquid can flow out when the colony is placed in air (Fig.2a).Medulla in the terminal branches usually perforated by 4–7 canals,and subsurface perforated usually by 15–20 boundary canals around the medulla (Fig.2b &c).
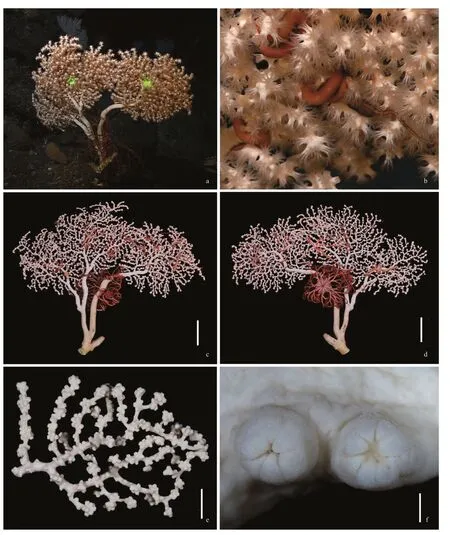
Fig.1 The holotype of Paragorgia papillata sp.nov.
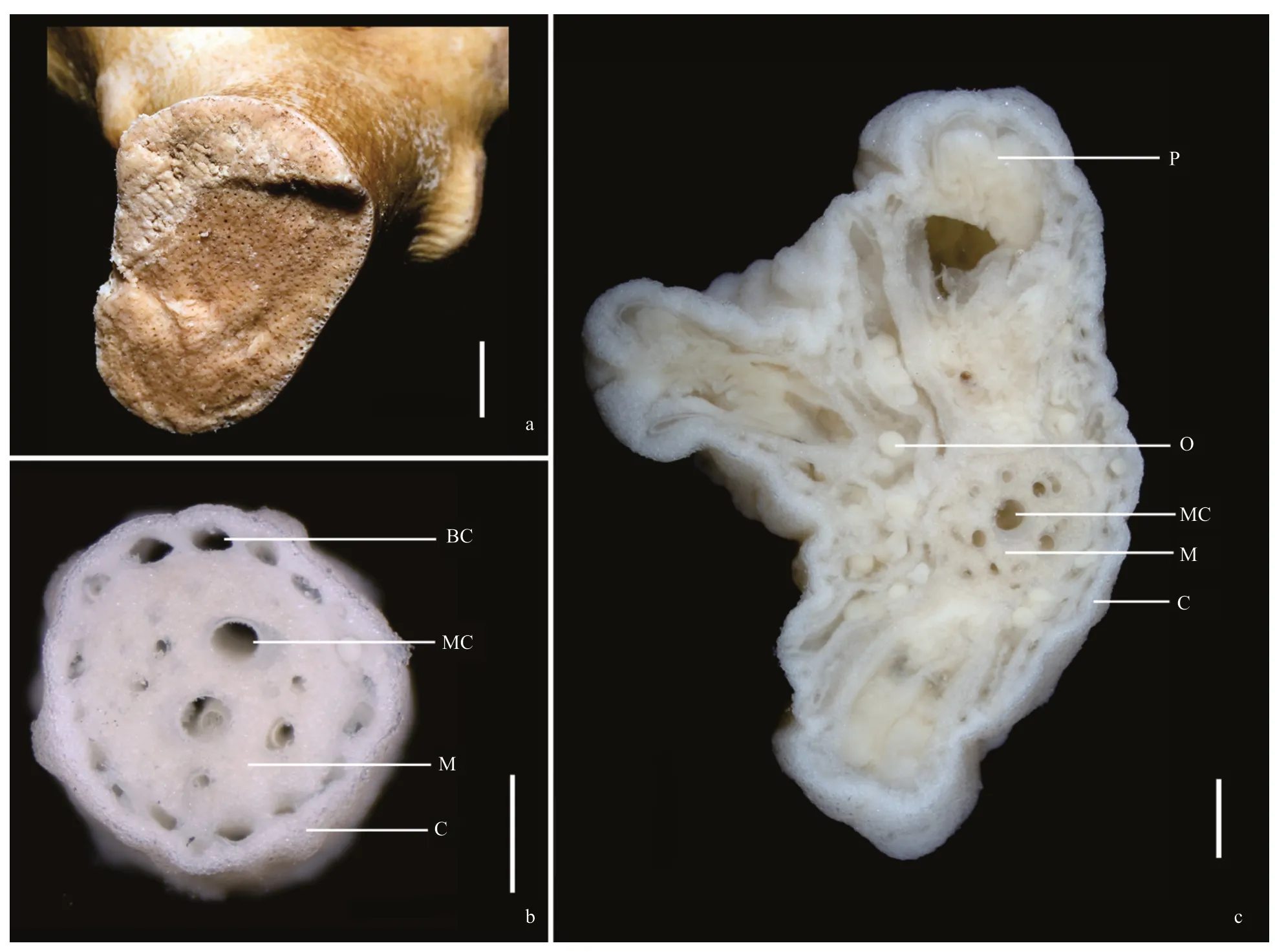
Fig.2 Cross-section of Paragorgia papillata sp.nov.
Polyps:Autozooids expanded and exhibited yellowish-white color in life.In preservation,calyces prominent,usually clustered in distinct nodules both at branch tips and along branches,giving colony and branches tuberculate appearance (Fig.1).Autozooids retractile,most fully withdrawn into calyces,measuring 2.0–4.0-mm wide and 1.5–3.0-mm exsert,and distributed somewhat more in one side of the colony than the other.Siphonozooids tiny,distributed on all sides of branches.
Sclerites:Autozooid tentacles densely armed with ornate ovals that have conical protuberances and an indistinct waist,ranging 69–123 μm in length (average 98± 13.4 μm,n=20),and 1.7–3.0 times (average 2.27)longer than wide (average 43.2 ± 4.5 μm,n=20)(Fig.3a).Surface cortex mostly 8-radiates,ranging 40–79 μm in length (average 57± 8.6 μm,n=20),and 1.4–1.9 times (average 1.60) longer than wide (average 35± 3.4 μm,n=20) (Fig.3b).Radiates usually asymmetrical as differentiation of rays that are mostly smooth and lobulated (Fig.3b).Subsurface sclerites include ornate and smooth spindles,up to 195 μm in length (Fig.3c).Medullar sclerites mainly ornate spindles having high tubercles with prominent thorns,ranging 185–400 μm in length (average 279± 56 μm,n=20),and 2.6–6.8 times (average 4.35) longer than wide (average 66± 11.8 μm,n=20) (Fig.3d).
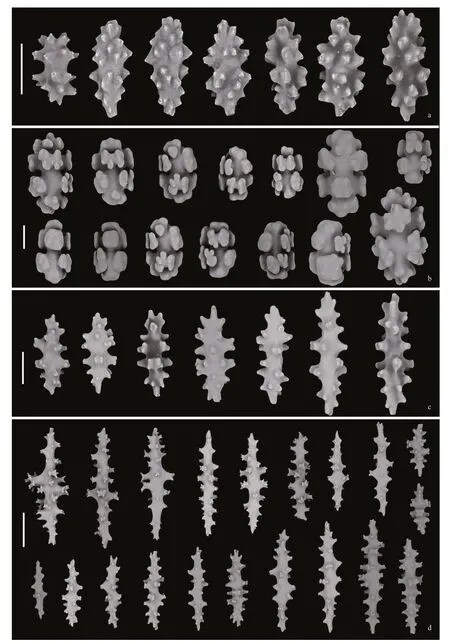
Fig.3 Sclerites of Paragorgia papillata sp.nov.
Color:Cortex white to yellowish in vivo and in freshly collected specimen.In ethanol preservation,upper cortex milk white,medulla and cortex of lower stems yellowish (Fig.1).Sclerites whitish or colorless under transmitted light.
EtymologyThe Latin adjectivepapillata(papillate) refers to the autozooid shape of the species.
Distribution and habitatFound on a seamount(M6) located on the Caroline Ridge in the tropical Western Pacific,where the water depth was 858 m,temperature was 5.04 °C,and salinity was 34.5.In the field,the holotype was attached on rocky bottom,and was inhabited by five individuals of the ophiuroid order Euryalida de Lamarck,1816,a basket star(probably belonging to the family Gorgonocephalidae Ljungman,1867) and a scale worm of the family Polynoidae Kinberg,1856.
3.2 Genetic distance and phylogenetic analyses
The GenBank accession number of the mtMutS sequence fromParagorgiapapillatasp.nov.is MW139970,and its length is 701 bp.The alignment dataset comprised 610 nucleotide positions.The genetic distances betweenP.papillatasp.nov.and the group includingP.coralloidesandP.regalisKX008439 were 0.16% (corresponding to one nucleotide difference),and those betweenP.papillatasp.nov.and the rest congeners were in range of 1.48%−3.77%.
The ML and BI phylogenetic trees of the mtMutS gene were nearly identical in topology except for the position ofP.papillatasp.nov.(Figs.4 &5).In the both trees,P.kaupekaSánchez,2005 andP.rubraLi et al.(2017) formed a sister clade,branching early with full node support.Excluding this basal clade,P.papillatasp.nov.fell outside of the clade including all the restParagorgiaspecies with high support (ML 93%) in the ML tree.While there was low support (BI 0.50) forP.papillatasp.nov.,P.coralloidesandP.regalisKX008439 forming a clade that was sister to the remaining congeners in the BI tree.Hence,we elucidated the phylogenetic position ofP.papillatasp.nov.based on the ML tree.
4 DISCUSSION
In the both phylogenetic trees,ParagorgiaregalisNutting,1912 populations were separated into four divergent clades,among whichP.regalisKX008439 clustered withP.coralloidesBayer,1993 (Figs.4 &5).Furthermore,Li et al.(2017) proposed that theP.regalispopulations might represent a species complex based on the genetic distance analysis,which accords with the morphological confusion suggested by Sánchez (2005).Hence,a taxonomic reexamination is needed for these populations.Morphologically,P.papillatasp.nov.differs distinctly from the holotype ofP.regalisby its prominent calyces (very exserted vs.totally retracted into nodules) and white cortex (vs.salmon pink),though both species have similar sclerite types and sizes (Nutting,1912;Sánchez,2005).P.papillatasp.nov.is also closely related toP.coralloidesbased on the genetic distance and phylogenetic analysis.Nevertheless,P.papillatasp.nov.differs distinctly fromP.coralloidesBayer,1993 by its prominent calyces (diameter 2.0–4.0 mm and height 1.5–3.0 mm vs.both diameter and height about 1 mm),white cortex (vs.pink),regular 8-radiates in surface cortex (vs.mostly 8-radiate derived globular radiates) and highly ornate medullar spindles (length 185–400 μm vs.no more than 150 μm) (Bayer,1993;Sánchez,2005).In fact,the calyces ofP.papillatasp.nov.are more exserted than those of all other congeners.
Paragorgiapapillatasp.nov.is similar toP.stephencairnsiSánchez,2005 andP.yutlinuxSánchez,2005 in the whitish cortex color,while the other members of the genus are pink or red.The new species can be easily differentiated fromP.stephencairnsiandP.yutlinuxby the sclerite forms and sizes.The surface sclerites ofP.papillatasp.nov.are mostly 8-radiates,while those ofP.stephencairnsiare mostly 7-radiates,and those ofP.yutlinuxare mostly 6-radiates;the medullar spindles ofP.papillatasp.nov.are up to 400-μm long,while those ofP.stephencairnsiandP.yutlinuxare less than 300 μm and 250 μm,respectively (Sánchez,2005).The data available showed thatP.papillatasp.nov.andP.stephencairnsiare distantly related,indicating that the whitish cortex ofParagorgialikely evolved several times.
5 CONCLUSION
A new bubblegum coral species collected from a seamount on the Caroline Ridge in the tropical
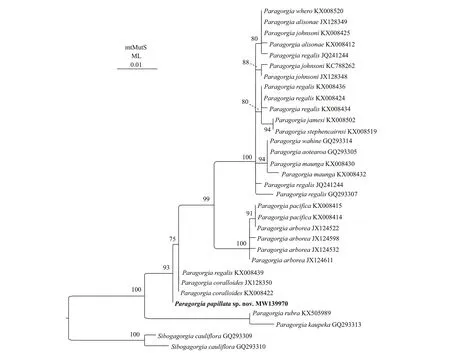
Fig.4 Maximum likelihood (ML) tree constructed by mtMutS showing phylogenetic relationships among Paragorgia sequences
Newly sequenced species are in bold.The GenBank accession numbers of the mtMutS sequences are listed next to the species names.Western Pacific is described and illustrated asParagorgiapapillatasp.nov.The new gorgonian is characterized by the prominent autozooids,rarely known white cortex color,8-radiates in surface cortex and highly ornate medullar sclerites.Within the genusParagorgia,P.papillatasp.nov.is the third species discovered with white cortex and the fifth species found in the tropical Western Pacific.The genetic distance and phylogenetic analysis showed thatP.papillatasp.nov.was closely related toP.coralloidesBayer,1993,but the two species differ distinctly in morphology.
6 DATA AVAILABILITY STATEMENT
Sequence data analyzed during this study has been deposited in the GenBank.
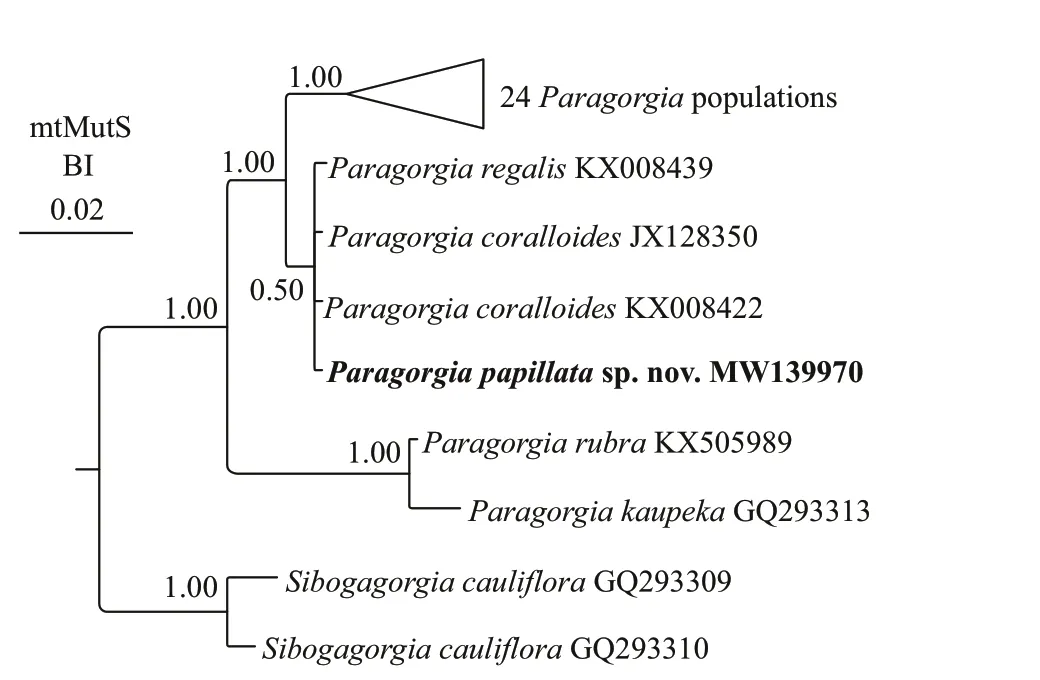
Fig.5 The Bayesian inference (BI) tree constructed by mtMutS showing phylogenetic relationships among Paragorgia sequences
7 ACKNOWLEDGMENT
We appreciate the crew of R/VKexueand ROVFaxianfor their assistance on sample and data collection,and we thank our colleague Mr.Shaoqing WANG for photographing freshly collected specimen onboard.We also appreciate Center for Ocean Mega-Science,Chinese Academy of Sciences,for organizing the expedition.
 Journal of Oceanology and Limnology2021年5期
Journal of Oceanology and Limnology2021年5期
- Journal of Oceanology and Limnology的其它文章
- Screening of stable internal reference genes by quantitative real-time PCR in humpback grouper Cromileptes altivelis*
- Morphology and multifractal features of a guyot in specific topographic vicinity in the Caroline Ridge,West Pacific*
- Geochemical characteristics and geological implication of ferromanganese crust from CM6 Seamount of the Caroline Ridge in the Western Pacific*
- Deep-sea coral evidence for dissolved mercury evolution in the deep North Pacific Ocean over the last 700 years*
- Physical oceanography of the Caroline M4 seamount in the tropical Western Pacific Ocean in summer 2017*
- Characteristics and biogeochemical effects of oxygen minimum zones in typical seamount areas,Tropical Western Pacific*
