Planktonic ciliate trait structure variation over Yap,Mariana,and Caroline seamounts in the tropical Western Pacific Ocean*
Chaofeng WANG ,Haibo LI ,Yi DONG ,Li ZHAO ,Gérald GREGORI ,Yuan ZHAO ,,Wuchang ZHANG ,,Tian XIAO
1 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 Aix-Marseille University, Toulon University, CNRS, IRD, Mediterranean Institute of Oceanology UM110, Marseille 13288,France
Abstract Trait structure is increasingly used in plankton ecology to understand diversity and biogeography.However,our knowledge of microzooplankton (e.g.planktonic ciliates) trait structure and its variation with hydrography is limited.In this study,we analyzed planktonic ciliate trait structure in waters with different hydrography and deep Chlorophyll a maximum (DCM) layers over three seamounts:Yap,Mariana,and Caroline seamounts.Mariana seamount had a lower surface temperature than the Yap and Caroline seamounts.DCM layers over Mariana and Caroline seamounts were deeper than Yap seamount.There was a weak upwelling in upper 50 m around top of Mariana seamount.The ciliate distribution showed bimodal pattern (high abundance appeared in the surface and DCM layers) over three seamounts.At surface layer,the large size-fraction (>30 μm) abundance proportion to aloricate ciliate over Yap seamount (44.4%)was higher than Mariana (32.8%) and Caroline (36.1%) seamounts.For tintinnid abundance proportion to total ciliate,Mariana (12.0%) and Caroline (11.5%) seamounts at about 100-m depth were higher than that of Yap seamount (6.4%).Vertically,tintinnid could be divided into 4 groups over the three seamounts.At 30-m depth,group I (species occurring from surface to 100 m only) was dominant component over Yap and Caroline seamounts,while group Ⅳ (species occurring at every depth) changed into dominant component over Mariana seamount,the weak upwelling might be the reason.Salpingella faurei was the top dominant species,which corresponded to deeper DCM layers over Mariana and Caroline seamounts.Our results showed that the upwelling and the deeper DCM could influence the planktonic ciliate trait structure.
Key word:planktonic ciliate;upwelling;seamount;vertical distribution;Western Pacific Ocean
1 INTRODUCTION
Planktonic ciliates (Ciliophora,Spirotrichea,Oligotrichia,and Choreotrichia) are small (size range of 10–200 μm) protists with cilia around the body,including aloricate ciliates and tintinnids (Lynn,2008;Zhang et al.,2012,2015).Planktonic ciliates are ubiquitous and important consumers of pico-(0.2–2 μm) and nano-(2–20 μm) sized phytoplankton,and are important food sources for metazoan and fish larvae (Stoecker et al.,1987;Dolan et al.,1999;Gómez,2007).As the dominant component of the microzooplankton,the marine planktonic ciliate compartment is a key medium through microbial food web to classical food chain,which plays an important role in material circulation and energy flow in the marine ecosystem (Azam et al.,1983;Pierce and Turner,1992;Calbet and Saiz,2005).
Trait based approaches was proposed to describe plankton communities in a simple manner (Litchman et al.,2013;Kiørboe et al.,2018).The existence of lorica provide some protection as well as additional weight to drag for tintinnids (Capriulo et al.,1982).Tintinnids had a higher proportion in total ciliates in turbulent waters (Yu et al.,2016) and waters with higher chlorophyll concentration (Wang et al.,2020).Size classes of ciliate are important trait as in other plankton (Pomerleau et al.,2015;Brun et al.,2016).Arctic waters had a higher proportion of larger cells of aloricate ciliates and of tintinnids with larger lorica opening diameter (LOD) (Wang et al.,2020).LOD size classes were also used to estimate tintinnid functional redundancy (Dolan et al.,2016).Plankton in different depth experiences different temperature,food items,and metabolic rate and,therefore,shows different functional traits (Prowe et al.,2019;Teuber et al.,2019).Several types of vertical distribution pattern were reported for tintinnids (Kršinić,1982;Wang et al.,2019a).
In the Western Pacific,the trait structures of aloricate size fraction,tintinnid proportion,tintinnid vertical distribution groups,and LOD size-classes were studied by Wang et al.(2019a,2020).However,we still know very little about their variations in different hydrographic situations.Previous studies showed that seamount with abrupt structure could alter hydrological conditions at surrounding stations than that off sea areas,such as upwelling (Rogers,1994,2018;Ma et al.,2019).The response of planktonic ciliate trait structure to these hydrological variations needs to be elaborated.In this paper,we present the variation of planktonic ciliates trait structure in relation to the hydrological characteristics such as the depth of deep Chlorophyllamaximum(DCM) layers,surface temperature,and upwelling during three cruises in the tropical Western Pacific Ocean.
2 MATERIAL AND METHOD
Sampling was conducted during three cruises performed from December 3,2014 to January 8,2015(Yap seamount,transects A and B,winter),March 4 to April 3,2016 (Mariana seamount,transects C and D,spring) and August 3 to September 7,2017 (Caroline seamount,transects E and F,summer) aboard R/VKexue(Sciencein Chinese) (Fig.1) in the Western Pacific Ocean where depth was about 5 000 m.The depths at the top of the three seamounts were 300,30,and 50 m over Yap,Mariana,and Caroline seamounts,respectively.For each seamount,the sampling stations were aligned into two approximately perpendicular transects (Fig.1).
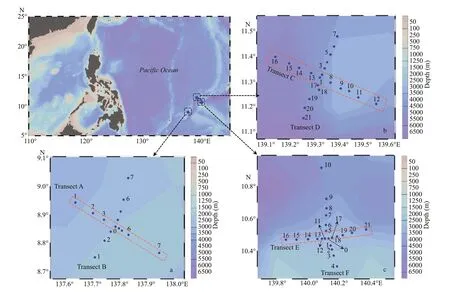
Fig.1 Survey stations and transects over Yap (a),Mariana (b),and Caroline (c) seamounts in the tropical Western Pacific Ocean
For each station,vertical profiles of temperature,salinity (conductivity),and chlorophyllain vivo fluorescence were obtained from the surface down to 200-m depth (or bottom with depth <200 m) using a conductivity-temperature-pressure sensor (CTD;Sea-Bird Electronics,Bellevue,WA,USA).Water samples were collected at 3 to 7 depths using 12-L Niskin bottles attached to a rosette.The depths sampled were 3 m (surface),30 m,50 m (not for Yap seamount),75 m,100 m,150 m,and 200 m (or bottom with depth<200 m).Around the DCM,the sampling depths were adapted to sample the DCM ifit was within 10 m of any neighbor sampling depth.All water samples (1 L)were fixed with 1% acid Lugol’s iodine and stored at<4 °C in the dark.
Each water sample was subsequently concentrated to about 100 mL by gently siphoning out supernatant water after at least a 48-h sedimentation in the laboratory.The settling and siphoning processes were repeated to concentrate each sample to a final volume of 20 mL (concentrated sample),then settled in an Utermöhl counting chambers (Utermöhl,1958) for at least 24 h.
For each concentrated sample,the entire volume was examined using an Olympus IX 71 inverted microscope (100× or 400×).The statistical method of counting according to Lund et al.(1958),and the counting of 10–15 individuals in a sample volume of 1 L implies a counting error higher than 50% (Lund et al.,1958).Some loricae might have been empty when sampled (Kato and Taniguchi,1993;Dolan and Yang,2017).Because mechanic and chemical disturbance associated with collection and fixation procedures could provoke detachment of the protoplasma from the loricae (Paranjape and Gold,1982;Alder,1999),empty loricae of tintinnids were considered as living cells in our study.
For each species,size (length,width according to the shape) of the cells (for aloricate ciliates) or of the loricae (for tintinnids,especially length and oral diameter) were measured for at least 20 individuals when possible.According to lorica morphology and size,tintinnids were identified to the species level according to references (Kofoid and Campbell,1929;Lynn,2008;Zhang et al.,2012;Wang et al.,2019a).Ciliate volumes were estimated by using appropriate geometric shapes (cone,ball,and cylinder).Tintinnid carbon biomass was estimated using the equation:C=Vl(μm3,lorica volume)×0.053+444.5 (Verity and Lagdon,1984).The conversion factor of carbon biomass for aloricate ciliates used in this study was 0.19 pg C/μm3as defined by Putt and Stoecker (1989).
The size of the aloricate ciliates was divided into fractions of 10 μm according to the longest length,according to Lessard and Murrell (1996),Sohrin et al.(2010),and Wang et al.(2020).The size-fractions were further clustered into small (10–20 μm),medium(20–30 μm),and large (>30 μm) (Sohrin et al.,2010).The vertical distribution of tintinnid species was defined according to Kršinić (1982).According to the sampling depth,four groups were classified:group I(species in waters from 0-to 100-m depth),Ⅱ (species in waters from 50-to 200-m depth),Ⅲ (species in waters deeper than 100 m) and Ⅳ (species throughout the water column,which belong to group V in Kršinić(1982)).The biogeography of tintinnid genera (e.g.neritic,cosmopolitan,and warm water types) were derived from Dolan et al.(2013).The tintinnids LOD were divided into different size classes,which are 4-μm apart (12–16 μm,16–20 μm,and so on)according to Dolan et al.(2016).Redundant tintinnid species and proportion of redundant species calculation also followed Dolan et al.(2016).
3 RESULT
3.1 Hydrography
Hydrological characteristics over the three seamounts were different (Fig.2).Horizontally,surface waters over Mariana seamount had a lower temperature (27.7–28.6 °C,average 28.1±0.2 °C) and a higher salinity (34.5–34.7,average 34.7±0.1) than surface waters over Yap (temperature 28.9–29.3 °C,average 29.1±0.1 °C,salinity 33.2–34.0,average 33.8±0.2) and Caroline (temperature 29.8–30.1 °C,average 30.5±0.2 °C,salinity 33.0–33.8,average 33.6±0.2) seamounts.The depths of 28 °C isotherm appeared at upper 100 m,30 m,and 50 m over the Caroline,Mariana,and Yap,respectively.The chlorophylla(Chla) in vivo fluorescence values were less than 0.1 at surface layers over the three seamounts (Fig.2).
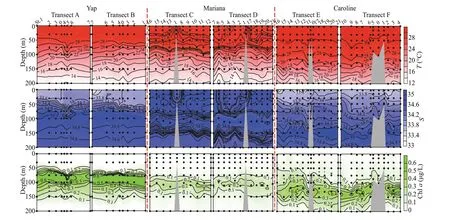
Fig.2 Vertical distribution of temperature (T),salinity (S),and chlorophyll a (Chl a) in vivo fluorescence from surface downto 200-m depth
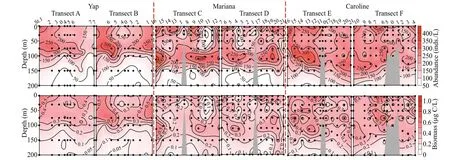
Fig.3 Vertical distribution of ciliate abundance and biomass from the surface down to 200-m depth
Water column was stratified according to temperature,salinity,and Chlavertical distribution(Fig.2).There was a weak upwelling in Mariana seamount at the top 50 m with temperature higher than 27.7 °C,salinity lower than 34.7.The halocline and DCM layers were in about the same depths over three seamounts (Fig.2).Compared to the DCM depths,Caroline (ranged from 120–150 m,average 126.7±8.3 m) and Mariana (80–110 m,average 107.5±5.6 m) seamounts were deeper than Yap (60–90 m,average 76.1±3.6 m) seamount (Fig.2).The depth of weak upwelling around the top of Mariana seamount was shallower than 50 m,which had no effect on the DCM depths (average 107.5±5.6 m).
3.2 Ciliate abundance and biomass
The vertical profiles of ciliate average abundance showed bimodal (in the surface and at the DCM layers) patterns over Yap,Mariana,and Caroline seamounts (Figs.3–4).However,there were some differences in some details.
Ranges of ciliate abundance and biomass at surface(130–323 inds./L,0.24–0.57 μg C/L) and DCM layers(130–331 inds./L,0.17–0.64 μg C/L) over Yap seamount were lower than Mariana (surface 103–384 inds./L,0.11–0.87 μg C/L,DCM 161–405 inds./L,0.17–0.72 μg C/L) and Caroline (surface 174–380 inds./L,0.23–1.06 μg C/L,DCM 141–405 inds./L,0.14–0.77 μg C/L) seamounts.The integrated abundance (the upper 200 m or bottom when depth<200 m) over Yap ((71.7–196.3)×103inds./m2,average (147.1±32.7)×103inds./m2) was lower than Mariana ((109.6–254.6)×103inds./m2,average(163.3±36.4)×103inds./m2) and Caroline ((150.5–238.7)×103inds./m2,average (195.3±25.7)×103inds./m2) seamounts (Table 1),but the integrated biomass over Yap (0.18–0.33 mg C/m2,average 0.26±0.05 mg C/m2) seamount was similar to Mariana (0.17–0.41 mg C/m2,average 0.26±0.06 mg C/m2) seamount,which both lower than Caroline (0.23–0.41 mg C/m2,average 0.33±0.05 mg C/m2) seamount (Table 1).
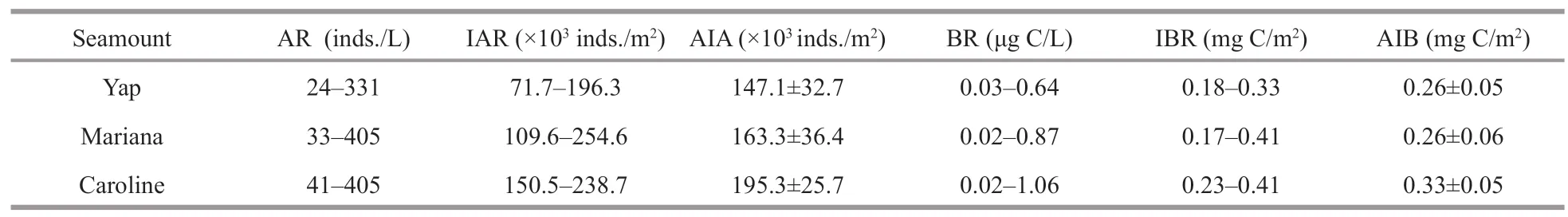
Table 1 Ciliate integrated abundance and biomass from surface down to 200-m depth over Yap,Mariana,and Caroline seamounts
Vertically,the highest tintinnid abundance appeared at DCM layers over Yap and Mariana seamounts.While over Caroline seamount with the deepest DCM(average 126.7±8.3 m),the highest tintinnid abundance occurred at 100-m depth instead of the DCM layer.Yap seamount had the lowest value of tintinnid average abundance proportion to total ciliates (average 4.8%±2.9%) compared to Mariana(average 6.6%±3.9%) and Caroline (average 6.4%±3.1%) seamounts.Although the highest tintinnid abundance proportion to total ciliates appeared at around 100-m depth over all three seamounts,this value over Mariana (12.0%) and Caroline (11.5%) seamounts were about twice higher than Yap (6.4%) seamount (Fig.4).
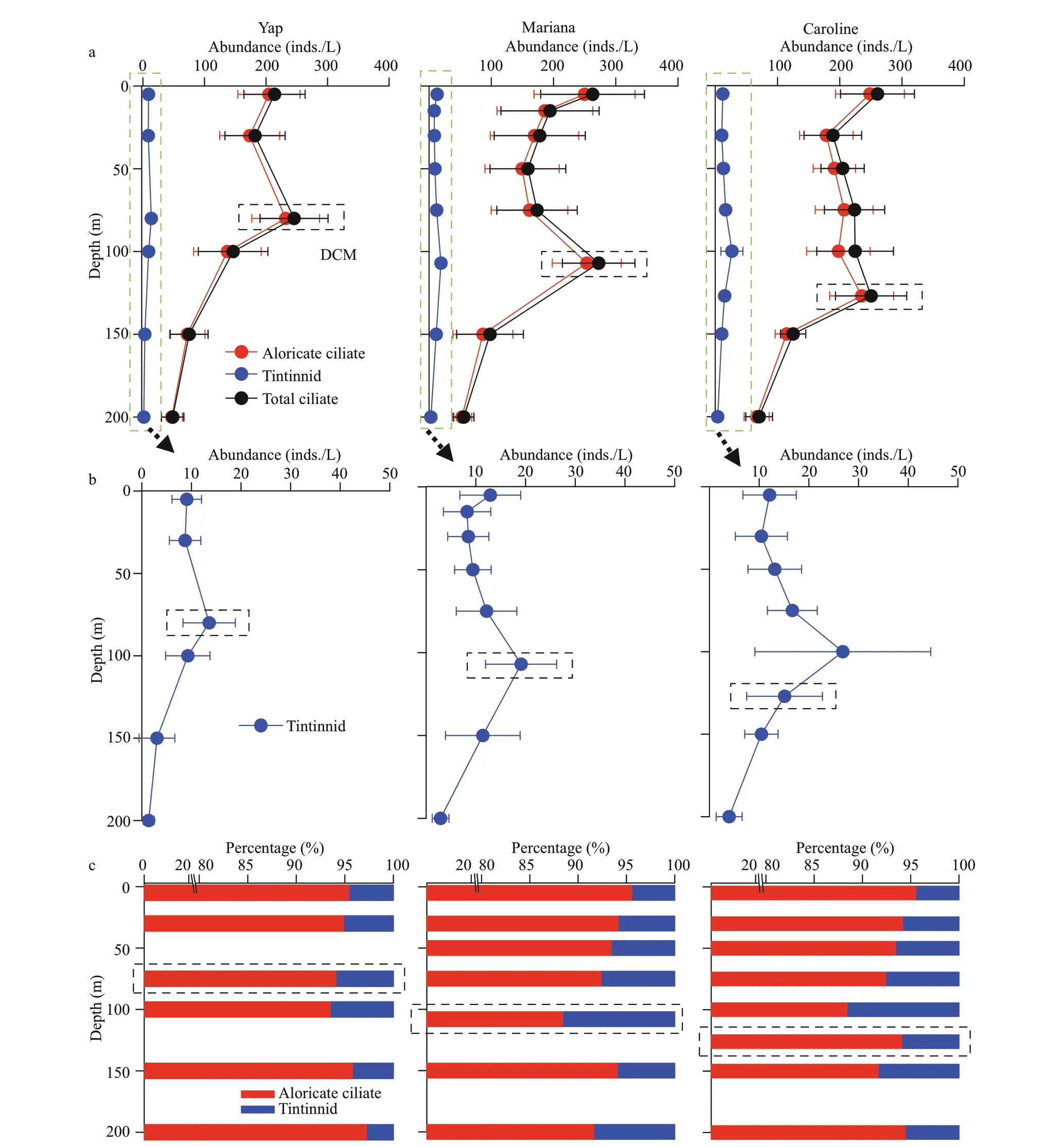
Fig.4 Vertical distribution of the average planktonic ciliate (aloricate ciliates and tintinnids) abundance in every sampling layers (a),with a zoom for tintinnids (b),and contribution of the two groups to the total ciliate abundance (c)
3.3 Aloricate ciliate size-fraction
Abundance proportion of different size-fractions of aloricate ciliates from surface to 200-m depth revealed that individuals of 10–20-μm size-fraction occupied more percentage (larger than 30% at each depth) over the three seamounts (Fig.5).Vertically,the abundance proportion of aloricate ciliates in the small sizefraction (10–20 μm) increased from surface down to 200-m depth,and the medium size-fraction (20–30 μm) abundance proportion was relatively constant throughout the water column over the three seamounts(Fig.5).Despite the above-mentioned similarities,there were also some dissimilarities.First,at the surface layer of Yap seamount,the large size-fraction(>30 μm) aloricate ciliate abundance proportion(44.4%) were higher than that of Mariana (32.8%)and Caroline (36.1%) seamounts.Second,at surface and 30-m depth of Yap seamount area,the large sizefraction (>30 μm) abundance proportion was larger than the small size-fraction (10–20 μm).While over both Mariana and Caroline seamounts,the large sizefraction (>30 μm) abundance proportion was lower than the small size-fraction (10–20 μm) (Fig.5).
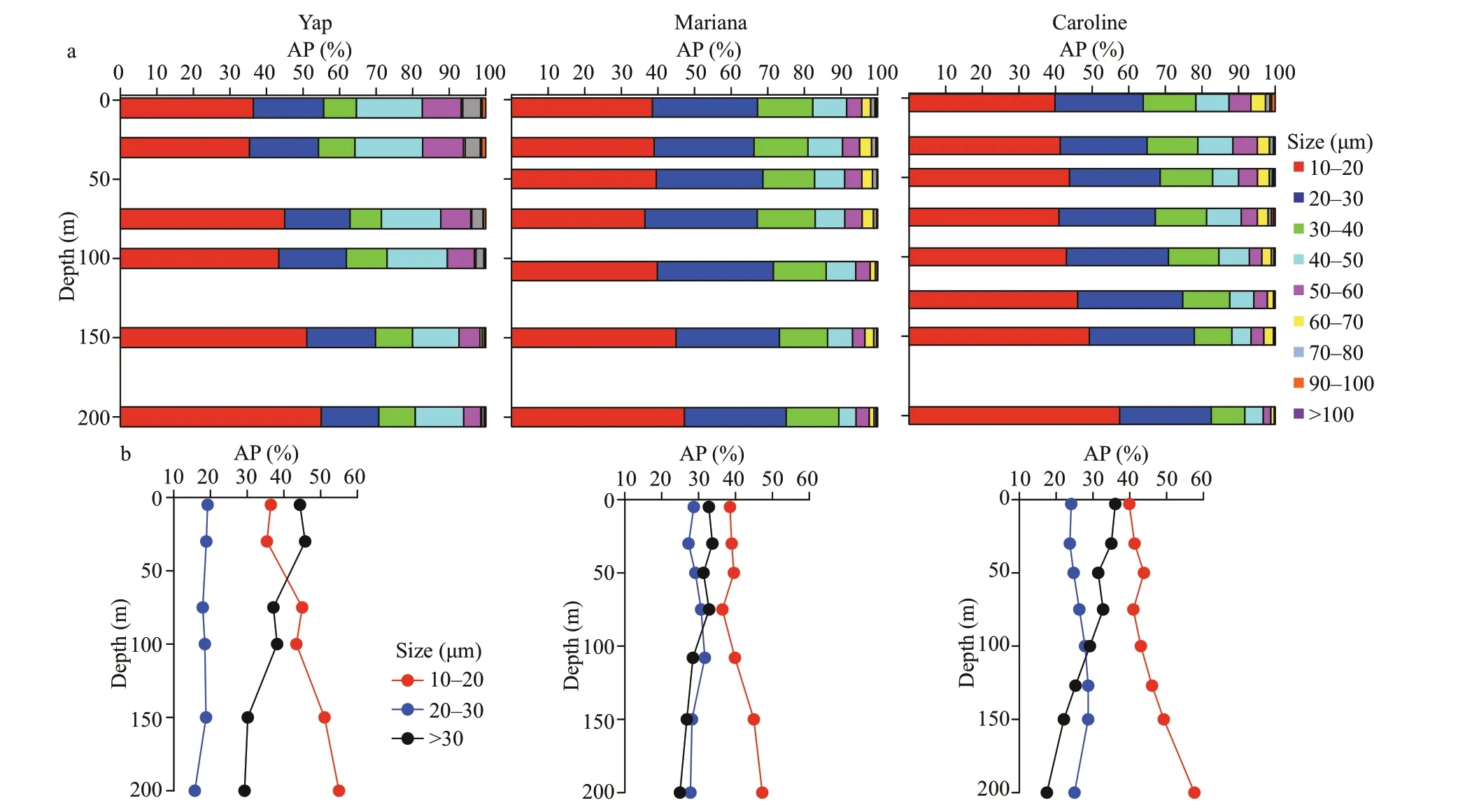
Fig.5 Average abundance proportion (AP) of the different sized aloricate ciliates in every sampling layers
3.4 Tintinnid assemblage
3.4.1 Tintinnid species composition
Range of tintinnid abundance over Yap (0–26 inds./L) seamount was lower than Mariana (0–33 inds./L) and Caroline (1–76 inds./L) seamounts.Totally,89 tintinnid species from 32 genera were found in all stations over three seamounts (Fig.6;Supplementary Fig.S1;Table 2).Among them,64 tintinnid species from 28 genera,73 tintinnid speciesfrom 31 genera and 76 tintinnid species from 33 genera were recorded over Yap,Mariana,and Caroline seamounts,respectively (Table 2).Fifty tintinnid species appeared in all three seamounts areas.Salpingella faureiwas the top dominant species over Mariana and Caroline seamounts.Abundance over Mariana (ranged from 0–11 inds./L) and Caroline(ranged from 0–5 inds./L) seamounts were higher than Yap (ranged from 0–3 inds./L) seamount.
Fig.6 Photomicrographs of several tintinnid species found in this study
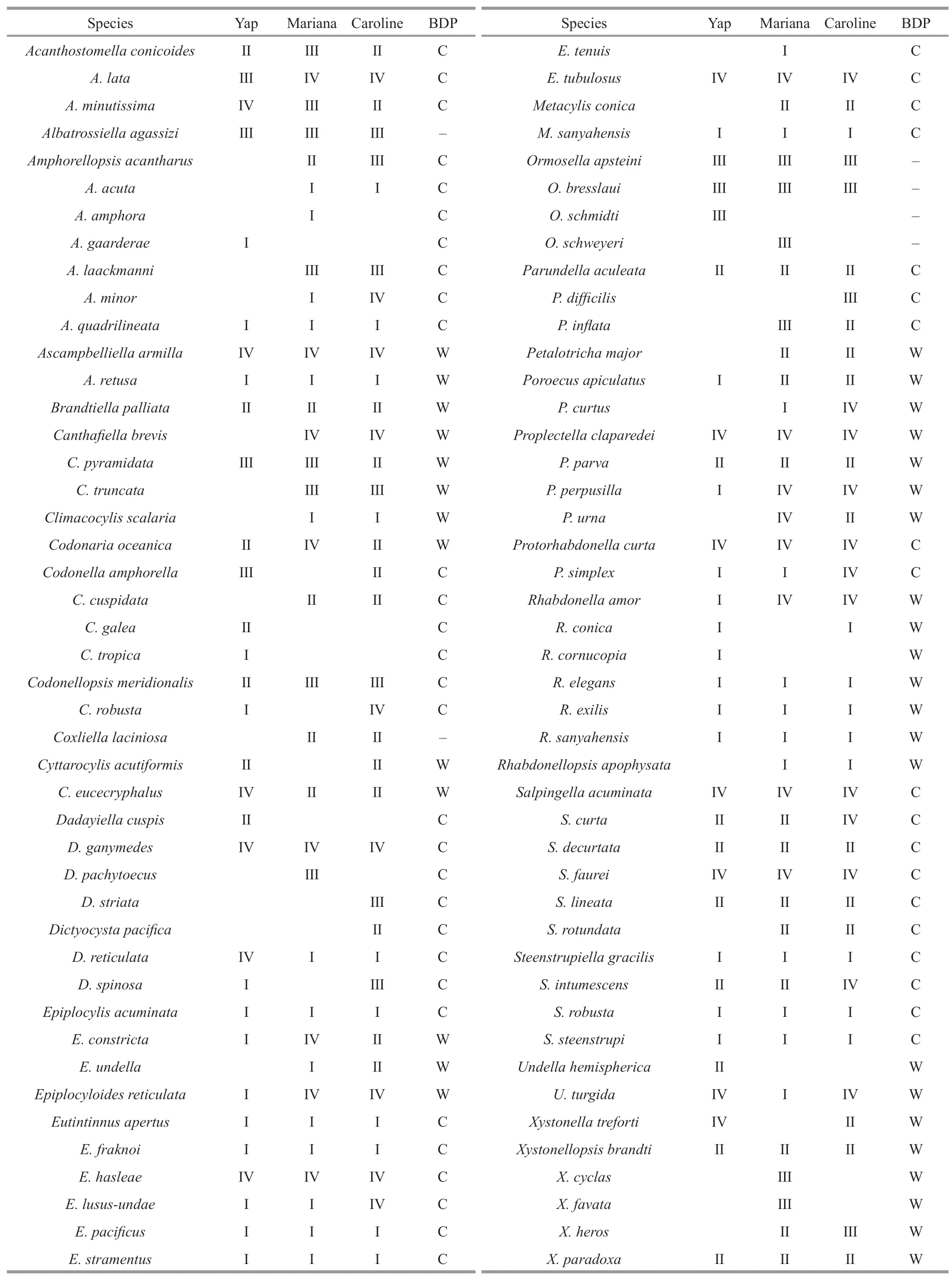
Table 2 Summary of all tintinnid species over Yap,Mariana,and Caroline seamounts
3.4.2 Tintinnid vertical distribution
Based on the vertical distribution of abundance,tintinnid species were divided into four groups over every seamount (Table 2).Groups I and Ⅳ were dominant component from surface to 100 m contributing to more than 60% of the total tintinnid abundance proportion over all three seamounts.Group I species abundance was high in surface,and then decreased to 100-m depth.Group Ⅳ species abundance increased from surface to DCM (Yap and Mariana seamounts) or 100 m (Caroline seamount),then decreased to 200-m depth (Fig.7).The group Ⅳabundance and abundance proportion at surface layer over Mariana (3.9 inds./L;36.9%) seamount were higher than Yap (1.6 inds./L;14.9%) and Caroline(3.2 inds./L;28.7%) seamounts (Fig.7).At 30-m depth,group Ⅳ species abundance was higher than group I over Mariana seamount (Fig.7),while over Yap and Caroline seamounts,group Ⅳ species abundance was lower than group I.
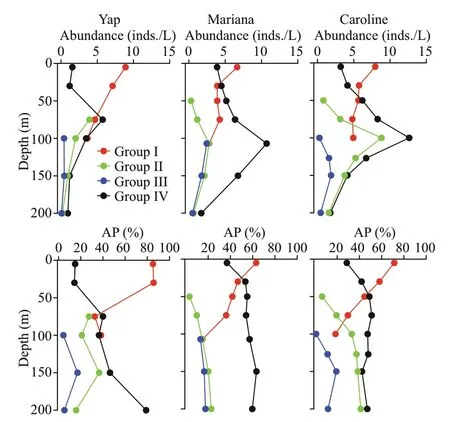
Fig.7 Vertical distribution of different types of tintinnid abundances and its abundance proportion (AP) in every sampling layers
3.4.3 Lorica oral diameter (LOD) size-classes and redundancy of tintinnid groups
The number of size-classes for the tintinnid species LOD over Yap (13),Mariana (15),and Caroline (16)seamounts were similar (Fig.8).The LOD size-class with a maximum species richness (28–32 μm) was also same over the three seamounts.Although the number of redundant species over Caroline seamount(61 species) was higher than Yap (50 species) and Mariana (59 species) seamounts,the proportion of redundant species was similar over Yap (79.4%),Mariana (79.7%),and Caroline (79.2%) seamounts.
The LOD size-class with the highest abundance proportion was 24–28 μm over the three seamounts(Fig.8).Steenstrupiella gracilis,Canthariella brevis,andAscampbelliella armillawere the main contributors to the higher abundance proportion of the 24–28-μm LOD size-class for the three seamounts (Fig.6).
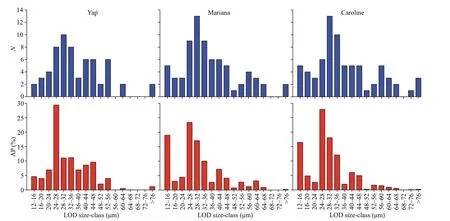
Fig.8 Number of species (N) and its abundance proportion (AP) for each lorica oral diameter (LOD) size-class of tintinnid species
The abundance proportion of 12–16-μm LOD sizeclass was much higher over Mariana and Caroline seamounts compared to Yap seamount.Salpingella faureiwas the main contributor of the 12–16-μm LOD size-class (Figs.6 &8).Average maximum abundance ofSalpingella faureiin DCM layer over Caroline (2.1 inds./L) seamount or at 150-m depth for Mariana (3.3 inds./L) seamount were higher than at Yap (0.9 inds./L,100-m depth) seamount.
3.5 Correlation between ciliate abundance and environmental factors
Different ciliate group (aloricate ciliates,tintinnids,and total ciliates) abundances had different correlation with environment factors (depth,temperature,salinity,and Chla) (Table 3).The total ciliate abundance had a positive correlation with temperature and Chlain vivo fluorescence,but negative correlation with depth over the three seamounts (Table 3).Ciliate abundance had negative correlation with salinity over Yap and Caroline seamounts,but a positive correlation over Mariana seamount.Aloricate ciliate and tintinnid abundance had significant positive correlation with temperature over the three seamounts (Table 3).Over Mariana seamount,aloricate ciliate and tintinnid had significant positive correlation with salinity,while it changed into a significant negative correlation over Caroline seamount (Table 3).Tintinnid abundance over Yap,Mariana,and Caroline seamounts had significant positive correlation with Chla(Table 3).
4 DISCUSSION
The ciliate vertical distribution patterns were the same over three seamounts with abundance peaks in surface and DCM layers.The food items of the ciliate might be the reason for the two groups:Prochlorococcusand picoeukaryotes had maximum abundance in DCM,Synechococcusand heterotrophic bacteria had maximum abundance in upper 50-m depth (Zhao et al.,2017,2020).
Although ciliates trait structure was proposed by previous studies (Kršinić,1982;Dolan et al.,2016;Yu et al.,2016;Wang et al.,2020),there were few studies on their variations.The variations of size classes and tintinnid proportion were compared through a large space scale in the Arctic,Subarctic,and tropical waters (Wang et al.,2020).The present study contributed to the study on ciliate trait structure variations in the tropical waters with different hydrological conditions.
4.1 Large ciliates in the surface layer over waters with shallow DCM depth
The DCM depth over Yap seamount was shallower than other two seamounts,and the integrated abundance over Yap seamount was lower than Mariana seamount,while the integrated biomass over Yap seamount was similar to Mariana seamount(Table 2).The large size-fraction (>30 μm) abundance proportion was also higher than Mariana and Carolineseamounts.For tintinnids,the 12–16-μm LOD sizeclass had low abundance proportion in the shallow DCM depth water over Yap seamount.These results indicate that the largest aloricate ciliates and tintinnids were dominant component over waters with a shallow DCM depth.This phenomenon also occurred over Cobb seamount where the ciliate trait structure showed a low abundance but a large size (Sime-Ngando et al.,1992).
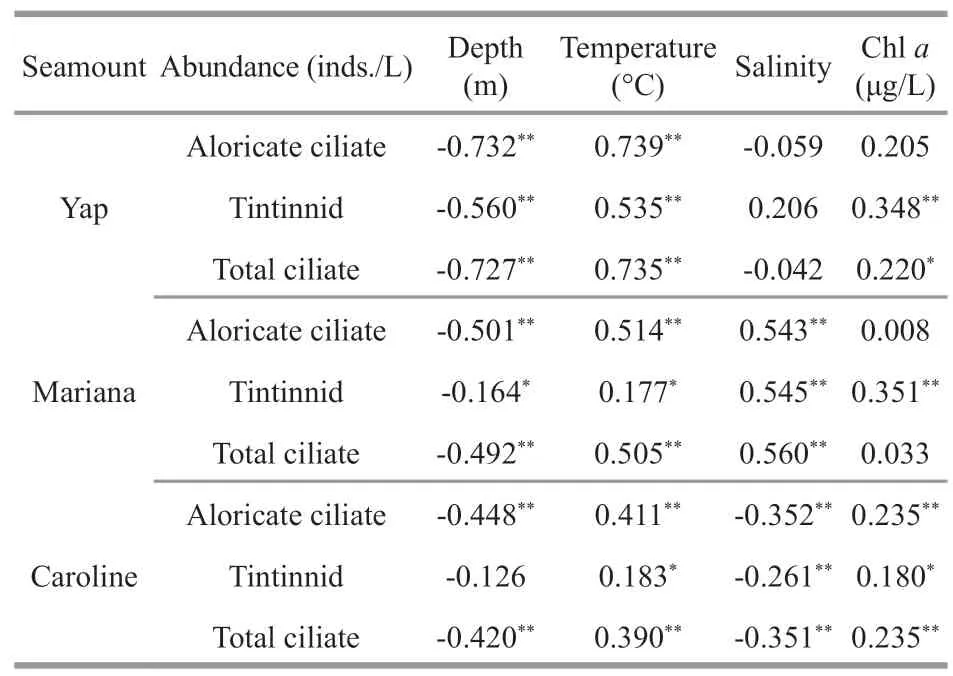
Table 3 Spearman’s rank correlation between the planktonic ciliate abundance and depth,temperature,salinity,and chlorophyll a (Chl a)in vivo fluorescence
4.2 Relationships between tintinnids and upwelling
A weak upwelling was put in evidence over Mariana seamount.Previous studies showed that nutrients (e.g.,NO3-N,dissolved inorganic nitrogen(DIN),PO4-P,and SiO3-Si)) had obvious uplifts around this seamount (Ma et al.,2019).However,no seamount effect was evidenced on phytoplankton(Zhao et al.,2017;Dai et al.,2020).The reason might be the shallowness of the weak upwelling.The nutrient concentrations were high in waters deeper than 100 m over three seamounts (Ma et al.,2019).Therefore,the weak upwelling in the upper 50 m could not cause a strong increase of nutrient concentration in surface water,which could not eventually enhance phytoplankton growth significantly.Aloricate ciliate group also did not have any obvious increase around the seamount compared to off seamount.For tintinnids,group Ⅳ (deep-water dweller) had a higher abundance proportion than group I (surface water dweller) at 30-m depth.This observation was different for Yap and Caroline seamounts,and along a transect in the tropical Western Pacific Ocean (Wang et al.,2019a).This might reflect a seamount effect over Mariana seamount.
4.3 Tintinnids and DCM depth
Our results showed that DCM depth influenced tintinnid trait structure.When the DCM depths were shallower than 100 m,the tintinnid highest abundances appeared at these DCM depths.While when DCM depths were deeper than 100 m,they were in about 100-m depth (Fig.4).This result was consistent with a transect in the tropical Western Pacific Ocean described in Wang et al.(2019a).The around 100-m depth might be a deepest boundary for tintinnid maximum abundance.In the tropical seas,DCM is a common feature,with depths varied from 50 to 150 m (Martin et al.,2006).Whether these relationships reoccur in different parts of the oceans need more investigations.
Salpingella faureiis a cosmopolitan deep water species and abundant when DCM was deeper than 100-m depth over Mariana and Caroline seamounts.This species distributed from tropical seas to Arctic Oceans (Dolan et al.,2013;Li et al.,2018;Wang et al.,2019a,b).In the Bering Sea and the Arctic Ocean,S.faureimainly distributed in the DCM layers.While at two stations with temperature and salinity well mixed in the Bering Sea,this species could also be brought to surface waters (Wang et al.,2019b).
5 CONCLUSION
In this study,we compared the planktonic ciliate trait structure over Yap,Caroline,and Mariana seamounts.We evidenced that weak upwelling and DCM depths influence the planktonic ciliate trait structure.At the surface layer with shallower DCM,the large size-fraction (>30 μm) abundance proportion to aloricate ciliates increased.The weak upwelling evidenced on Mariana seamount could take group Ⅳtintinnid species into surface waters,which lead to a higher abundance of this group compared to group I at 30-m depth.The depths of maximum tintinnid abundance were around 100 m even when DCM deeper than 100 m.Despite of the depth variation of DCM,tintinnid abundance proportion to total ciliate was highest at about 100-m depth.Our results represent a snapshot influence of the upwelling and the deeper DCM to ciliate trait structure.It constitutes a baseline for further comparative studies on the temporal and spatial environmental influence on ciliate in other seas.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
Special thanks to the great eff orts of the captain and crew of R/VKexueduring the cruises over Yap,Mariana,and Caroline seamounts.
 Journal of Oceanology and Limnology2021年5期
Journal of Oceanology and Limnology2021年5期
- Journal of Oceanology and Limnology的其它文章
- Screening of stable internal reference genes by quantitative real-time PCR in humpback grouper Cromileptes altivelis*
- Morphology and multifractal features of a guyot in specific topographic vicinity in the Caroline Ridge,West Pacific*
- Geochemical characteristics and geological implication of ferromanganese crust from CM6 Seamount of the Caroline Ridge in the Western Pacific*
- Deep-sea coral evidence for dissolved mercury evolution in the deep North Pacific Ocean over the last 700 years*
- Physical oceanography of the Caroline M4 seamount in the tropical Western Pacific Ocean in summer 2017*
- Characteristics and biogeochemical effects of oxygen minimum zones in typical seamount areas,Tropical Western Pacific*
