Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2A-Smad2/3 in rats following myocardial infarction
Yue Xi,Meili Ho,b,Qioqin Ling,Yongxi Li,D-Wei Gong,Zhenjun Tin,*
a Institute of Sports and Exercise Biology,Shaanxi Normal University,Xi’an 710119,China
b School of Physical Education,Luoyang Normal University,Luoyang 471934,China
c School of Life Science,Shaanxi Normal University,Xi’an 710119,China
d Division of Endocrinology,Diabetes and Nutrition,Department of Medicine,University of Maryland School of Medicine,Baltimore,MD 21201,USA
Abstract Purpose:The aim of this study was to investigate the potential of dynamic resistance exercise to generate skeletal muscle-derived follistatin like-1(FSTL1),which may induce cardioprotection in rats following myocardial infarction(MI)by inducing angiogenesis.Methods: Male, adult Sprague-Dawley rats were randomly divided into 5 groups (n=12 in each group): sham group (S), sedentary MI group(MI), MI+resistance exercise group (MR), MI+adeno-associated virus (AAV)-FSTL1 injection group (MA), and MI+AAV-FSTL1 injection+resistance exercise group (MAR).The AAV-FSTL1 vector was prepared by molecular biology methods and injected into the anterior tibialis muscle.The MI model was established by ligation of the left anterior descending coronary artery.Rats in the MR and MAR groups underwent 4 weeks of dynamic resistance exercise training using a weighted climbing-up ladder.Heart function was evaluated by hemodynamic measures.Collagen volume fraction of myocardium was observed and analyzed by Masson’s staining.Human umbilical vein vessel endothelial cells culture and recombinant human FSTL1 protein or transforming growth factor-β receptor 1(TGFβR1)inhibitor treatment were used to elucidate the molecular signaling mechanism of FSTL1.Angiogenesis, cell proliferation, and disco interacting protein 2 homolog A (DIP2A) location were observed by immunofluorescence staining.The expression of FSTL1, DIP2A, and the activation of signaling pathways were detected by Western blotting.Angiogenesis of endothelial cells was observed by tubule experiment.One-way analysis of variance and Student’s t test were used for statistical analysis.Results:Resistance exercise stimulated the secretion of skeletal muscle FSTL1,which promoted myocardial angiogenesis,inhibited pathological remodeling,and protected cardiac function in MI rats.Exercise facilitated skeletal muscle FSTL1 to play a role in protecting the heart.Exogenous FSTL1 promoted the human umbilical vein vessel endothelial cells proliferation and up-regulated the expression of DIP2A,while TGFβR1 inhibitor intervention down-regulated the phosphorylation level of Smad2/3 and the expression of vascular endothelial growth factor-A, which was not conducive to angiogenesis.FSTL1 bound to the receptor, DIP2A, to regulate angiogenesis mainly through the Smad2/3 signaling pathway.FSTL1-DIP2A directly activated Smad2/3 and was not affected by TGFβR1.Conclusion:Dynamic resistance exercise stimulates the expression of skeletal muscle-derived FSTL1,which could supplement the insufficiency of cardiac FSTL1 and promote cardiac rehabilitation through the DIP2A-Smad2/3 signaling pathway in MI rats.
Keywords: Angiogenesis;Follistatin like-1;Myocardial infarction;Resistance exercise;Signaling mechanism
1.Introduction
Myocardial infarction (MI) leads to myocardial ischemia,blocked microcirculation, cardiomyocytes apoptosis, and necrosis in the infarcted area,thus making vicarious fibrosis of the heart occur.1Exercise intervention can stimulate the secretion of cytokines2and adipokines3in distant organs such as skeletal muscle.4Exercise also inhibits myocardial fibrosis and cardiomyocytes apoptosis, mobilizes the endothelial progenitor cells differentiation and improves coronary microcirculation,which are key steps in protecting the MI heart.
Cardiogenic follistatin like-1 (FSTL1) is derived from cardiomyocytes,endothelial cells(ECs),and smooth muscle cells.Pressure overload,ischemia-reperfusion injury,and MI lead to increased levels of FSTL1 in myocardial tissue and serum.5FSTL1 is highly expressed in skeletal muscle and is closely related to angiogenesis.6Muscle-specificfstl1overexpression attenuates neointimal formation in response to arterial injury,and the level of FSTL1 in plasma is mainly affected by the expression level of skeletal muscle.7Evidence has shown that exercise can promote the transcription offstl1in skeletal muscle and increase the level of circulating FSTL1 protein.8We have previously shown that after exogenous recombinant human FSTL1 (rhFSTL1) protein intervention, the cardiac vascular ECs proliferated significantly,the number of new vessels was increased and the cardiac function was improved in MI rats.4Therefore, as one of the mediators of crosstalk between heart and skeletal muscle,FSTL1 can play an important role in cardioprotection after MI.
In the previous study, we found that different exercise modes had different effects on stimulating the expression of FSTL1 in myocardium, and resistance exercise was found to have the most significant effect.9Exercise stimulated skeletal muscle-derived FSTL1 to reach the heart through blood circulation, which promoted cardiac angiogenesis and inhibited pathological remodeling of the MI heart, thus improving cardiac function.10But whether skeletal muscle-derived FSTL1 is more effective in protecting damaged hearts after exercise stimulation is still unknown, and the molecular mechanism by which FSTL1 protects MI hearts has not been clarified.Our hypothesis was that the secretion of FSTL1 in skeletal muscle could be more effectively mobilized by resistance exercise,which promotes angiogenesis through the disco interacting protein 2 homolog A (DIP2A)-Smad2/3 (DIP2A-Smad2/3) signaling mechanism in MI hearts.To test our hypothesis, the FSTL1 adeno-associated viral (AAV) vector and a resistance exercise intervention were applied in MI rats.Human umbilical vein ECs (HUVECs) were cultured and treated with inhibitor and recombinant protein,and tubules experiments were used to elucidate the molecular mechanisms of FSTL1 in promoting angiogenesis.The results of our study provide a theoretical support for clinical rehabilitation strategies of MI patients.
2.Methods
2.1.Animals,MI surgical procedure,and exercise protocol
The 70 male Sprague-Dawley rats (200 ± 20 g (mean ±SD), 8 weeks old, specific pathogen-free level) used in this study were from the Laboratory Animal Centre of Xi’an Jiaotong University.Animal studies in our research were performed in accordance with the Guiding Principles for Research Involving Animals and Human Beings.11After 1 week of adaptive feeding, 58 of the rats underwent weight measurements and isoflurane inhalation anesthesia.MI was induced by using the established method of left anterior descending (LAD) coronary artery ligation.12During the surgery 3 rats died,and 2 rats died 2 h after surgery.After excluding 5 rats with no abnormal electrocardiogram results, 48 rats showed the same degree of ST-segment elevation and were classified into the experimental groups.
After MI surgery, the rats were randomly divided into 4 groups (n=12): sedentary MI group (MI), MI+resistance exercise group (MR), MI+AAV-FSTL1 injection group(MA), and MI+AAV-FSTL1 injection+resistance exercise group (MAR).Sham-operation control (S) rats underwent the operation procedure without left anterior descending coronary artery ligation(n=12).
Exercise intervention was performed using a previously described MI rat resistance exercise method.9A ladder with a height of 1.1 m was used, with the interval between each step being 2.0 cm and the slope angle being 85°.One week after infarction,the rats underwent 1 week of no load-adaptive training before having 4 weeks of normal exercise with incremental load.The weights hung from the rats’ tails were increased from 0%to 75%of bodyweight,and was increased by 10%in every 2 days.The exercise protocol was performed 1 h a day,5 days a week for 4 weeks.The daily bodyweight of each rat was used to determine the carrying load, and each rat performed the same number of climbs(≥60)per session.None of the rats died in training.
2.2.The construction and application of the AAV-FSTL1 vector
The construction method we used was the same as used in a previous study.10Briefly, pAAV-muscle creatine kinase promoter (MCK)-FSTL1-3xflag-internal ribosome entry site 2(IRES2)-enhanced green fluorescent protein (EGFP) was obtained by amplification, enzyme digestion, and Gibson Cloning.After the co-transfection of pAAV-MCK-FSTL1-3xflag-IRES2-EGFP,pAAV-helper,and pAAV-8 vector was conducted by the polyethylenimine method,13the AAV vector was obtained by collection and purification.The titer was 3.66×1012vg/mL.The expression of the GFP reporter gene was confirmed(Fig.1).
Fig.1.The expression of the GFP reporter gene after PEI transfection in cultured HEK-293 cells.GFP=green fluorescent protein; HEK=human embryonic kidney;PEI=polyethylenimine.
After MI surgery but before the rats awakened, the AAV-FSTL1 vector was injected into the tibialis anterior muscle of the left hind limb at a dose of 1.0×1010vg.14
2.3.Cardiac function evaluation
The cardiac hemodynamic measurement has been described previously.12The rats were anesthetized with pentobarbital sodium (30 mg/kg body weight) after 4 weeks of exercise training.A pressure transducer catheter was inserted retrograde from the right carotid artery to the left ventricular (LV)cavity.The LV systolic pressure(LVSP,mmHg),LV end-diastolic pressure (LVEDP, mmHg), heart rate, and maximum LV pressure increasing/decreasing rate (± dp/dt max) were measured by using Powerlab 8/30 (ML 870; AD Instruments,Castle Hill,Australia)to evaluate cardiac function.
2.4.Histological staining
Heart samples were taken after hemodynamic measurement and fixed in ice-cold 4% paraformaldehyde for 24-48 h, and then dehydrated in a concentration gradient of ethanol,embedded in paraffin and sectioned(5 μm)for histopathologic examination.The slices were stained with Masson’s trichrome, and the results were observed and photographed with an optical microscope.The fibrosis area of each heart was measured with>20 randomly selected squares of field located in the infarcted-free wall area of the LV(1 mm2)and calculated as a percentage of total area.The collagen volume fraction (CVF)was defined as the sum of all the connective tissue areas of the selected fields divided by the sum of all the myocardial areas.12,15CVF was measured using Image-Pro Plus software(Version 6.0; Media Cybernetics, Silver Spring, MD, USA).CVF(%)=collagen area/tissue total area×100%.
2.5.Immunofluorescence staining
After deparaffination and antigen, retrieval heart sections were incubated overnight at 4°C with the rabbit polyclonal antibody von Willebrand factor(vWF,1:50;Merck Millipore,St.Louis, MO, USA), mouse monoclonal antibody proliferating cell nuclear antigen(PCNA,1:50;Cell Signaling,Danvers,MA, USA), and rabbit polyclonal antibody α-smooth muscle actin (α-SMA, 1:50; Abcam, Cambridge, MA, USA).After paraformaldehyde fixation, phosphate buffer saline (PBS)-Ttriton treatment and goat serum blocking, HUVECs were incubated overnight at 4°C with rabbit polyclonal antibody DIP2A (1:50; GeneTex, Irvine, CA, USA) or PCNA (1:50;Cell Signaling).Tetramethylrhodamine (TRITC)-conjugated goat anti-mouse/rabbit IgG(1:100;Jackson ImmunoResearch,West Grove, PA, USA) and fluorescein-conjugated goat antirabbit IgG (1:100; Jackson ImmunoResearch) were used as secondary antibodies.The nucleus was stained with diaminophenylindoles (DAPI, 1:800; US EVERBRIGHT, Suzhou,China).
2.6.Cell culture and treatment
After the cell density reached 85%, 500 nmol/L of LY215729916(TGFβR1 inhibitor;Selleck Chemicals,Houston,TX, USA) was used to treat the HUVECs for 24 h.Then,100 ng/mL of rhFSTL117(ProSpec,East Brunswick,NJ,USA)was used as a treatment for 48 h after the low serum culture medium was changed.
2.7.Western blotting
After treatment, 50-60 mg of myocardial tissue from the LV infarction border area (5 mm) or HUVECs was homogenized.The total protein extraction, concentration determination and sodium dodecyl sulfate polyacrylamide gel electrophoresis were in accordance with previously described research methods.4The dilution rates of antibodies was as follows: FSTL1 (1:1000; GeneTex), DIP2A (1:1000; GeneTex),vascular endothelial growth factor-A (VEGF-A, 1:1000; Cell Signaling), TGFβ1 (1:2000; Cell Signaling), Smad2/3(1:1000;Cell Signaling),phosphorylated Smad2/3(p-Smad2/3,1:1000;Cell Signaling),and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:5000; Bioworld, Bloomington, MN,USA).Horseradish peroxidase-conjugated secondary antibody(1:500; Jackson ImmunoResearch) and an enhanced chemiluminescence substrate (Bio-Rad, Hercules, CA, USA) were incubated, and the protein bands were visualized using the ChemiDocTMMP Imaging System(Bio-Rad).
2.8.Tubular formation observation
The Matrigel(BD,San Jose,CA,USA)was slowly thawed at 4°C and evenly spread in a 24-well plate.After solidification in 5%CO2and 37°C,the HUVECs were digested and the density was adjusted to 2.0×105cells/mL.A total of 400 μL of cell suspension was added to each well,and the plate was cultured in a 5%CO2and 37°C for 20 h,36 h,and 48 h.
2.9.Data analysis
The number of positive cells, density means of random views and diameter and density of tubules were calculated by Image-Pro Plus (Media Cybernetics).The grayscale values of protein bands were calculated by image processing and analysis in Image Processing and Analysis in Java(Image J,Version 1.48;https://imagej.nih.gov/ij/).All data are presented as mean±SD in this study.The histogram was plotted using GraphPad Prism(Version 6.01;GraphPad Software,La Jolla,CA,USA).SPSS (Version 19.0; IBM Corp., Armonk, NY, USA) was used to verify the normality of the data.One-way analysis of variance and paired-samples Student’sttest were used for group comparison.Apost hocleast significant difference multiple comparison test was conducted to complement analysis of variance.Apvalue of<0.05 was considered significant.
3.Results
3.1.Resistance exercise stimulated FSTL1 secretion in skeletal muscle to protect the MI heart
vWF is a marker of vascular ECs,and PCNA is a marker of proliferative nuclear antigen.Dual-color fluorescent labeling of vWF and PCNA could show the proliferation of ECs,which indirectly reflect the level of angiogenesis.Immunofluorescence staining results showed that vWF was labeled green,PCNA was labeled red, and the nucleus was blue (Fig.2A).Compared with the S group,the number of vWF+/PCNA+cells increased significantly in the MI group (p< 0.001), and was further increased in the MR group(p=0.001).The vWF+/PCNA+cells in MAR group were not only more numerous (p< 0.001)compared to the MA group, but also surrounded each other to form primary vascular structures(Fig.2A and 2D).
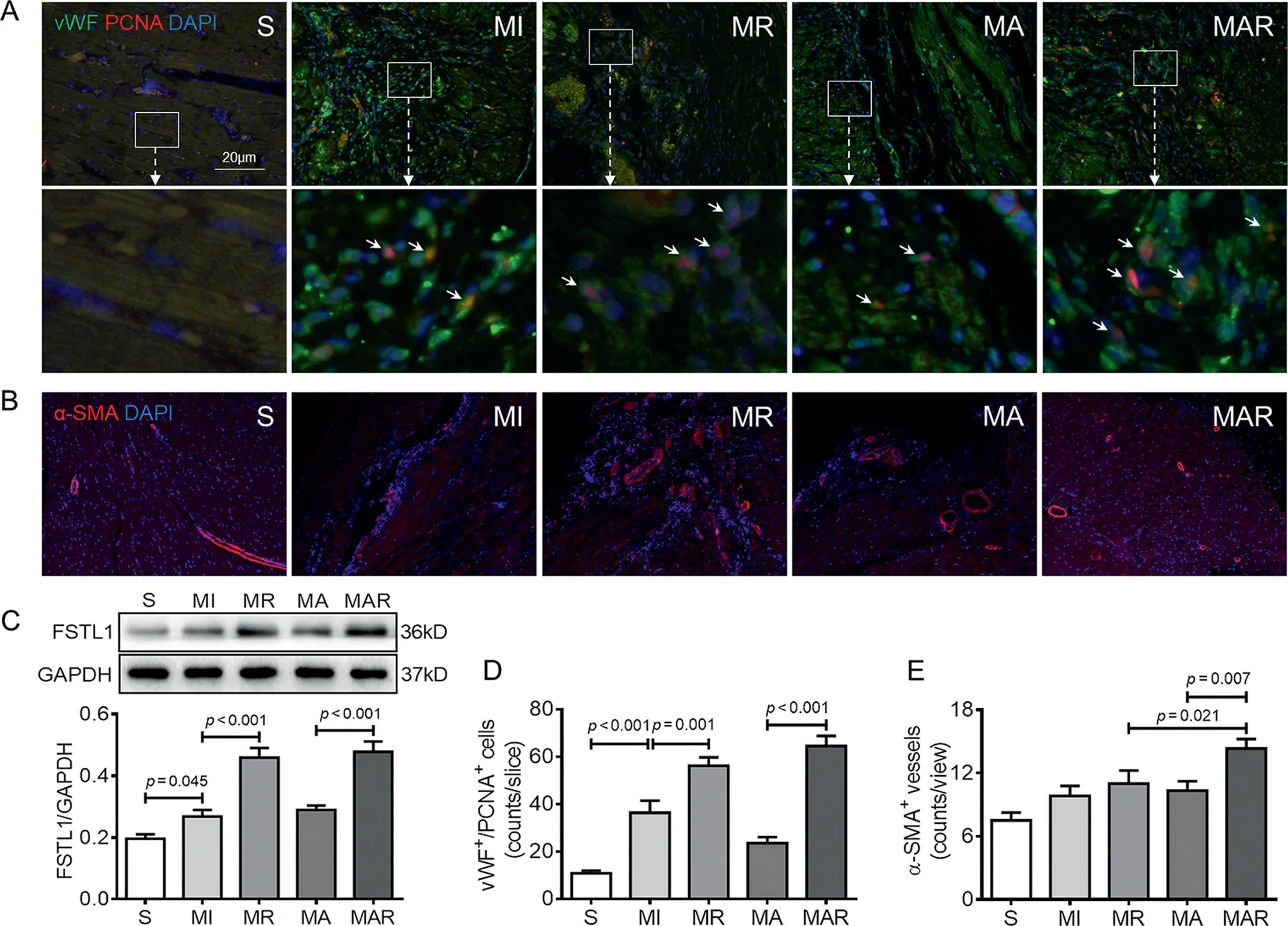
Fig.2.Resistance exercise and AAV-FSTL1 injection improved the angiogenesis and FSTL1 level in the MI heart.(A)Paraffin heart sections from each group were stained with vWF(green),PCNA(red),and DAPI(blue).The lower image is an amplification of the upper white box.The vWF+/PCNA+cells are indicated by white arrows.(B)Heart sections from each group were stained with α-SMA(red)and DAPI to show the vessel density.(C)The myocardial FSTL1 protein level was quantified by Western blotting.(D,E)The quantitative results of immunofluorescence staining.Data presented are mean±SD(n=6).One-way ANOVA with post hoc LSD multiple comparison test was used (C-E).AAV=adeno-associated virus; ANOVA=analysis of variance; DAPI=diaminophenylindoles;FSTL1=follistatin like-1; GAPDH=glyceraldehyde-3-phosphate dehydrogenase; LSD=least significant difference; MA=MI+AAV-FSTL1 injection group;MAR=MI+AAV-FSTL1 injection+resistance exercise group;MI=sedentary myocardial infarction group;MR=MI+resistance exercise group;PCNA=proliferating cell nuclear antigen;S=sham-operation control;SMA=smooth muscle actin;vWF=von Willebrand factor.
α-SMA is a marker of vascular smooth muscle.To evaluate the effect of angiogenesis, immunofluorescence staining was used to visualize the α-SMA+area and show the vessel density.The number of α-SMA+vessels increased in the MI group compared with the S group, and resistance exercise caused a significant increased to 11.00 ± 3.03 counts/view in the MR group.Compared with the MA group, the number of myocardial α-SMA+vessels in the MAR group was increased by 38.71%(p=0.007;Fig.2B and 2E).
Western blotting results showed that, compared with the S group, the FSTL1 protein expression was significantly increased in the MI group(p=0.045),and further increased in the MR group(p<0.001).Compared with the MA group,the FSTL1 protein level was much higher in rats of the MAR group (p< 0.001; Fig.2C).This finding was consistent with statistical results of immunofluorescence staining(Fig.2D).
Cardiomyocytes were red, myocardial interstitial collagen was blue, and cell nucleus was brown after Masson’s trichrome staining.The myocardial tissue was replaced by a large amount of collagen,which caused severe fibrosis and significantly increased CVF in the MI heart (p< 0.001; Fig.3A and 3B).CVF was decreased by 38.45%in the MR group compared with the MI group (p=0.001), which indicates that the pathological remodeling of myocardium was inhibited effectively by exercise.The MAR group had much more myocardial tissue and fewer interstitial collagen in the infarcted area,as well as a significantly reduced CVF% (p< 0.001; Fig.3A and 3B).Moreover, the differences in CVF% between groups were more significant in the non-perivascular area than in the perivascular area (Fig.3C and 3D), which indicates that cardiac tissue was more conserved after the intervention of AAV-FSTL1 and exercise.
Statistical results of cardiac hemodynamic parameters showed that LVSP (p=0.001) and ± dp/dt max (p< 0.001)were reduced,while LVEDP was elevated after MI(p<0.001).Each cardiac function parameter was improved in the MR group.Compared with the MA group,both LVSP(p=0.02)and± dp/dt max (p< 0.001) were elevated, and LEVDP was reduced significantly in the MAR group(p=0.005;Fig.3E).
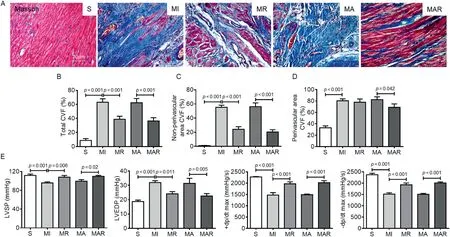
Fig.3.Resistance exercise and AAV-FSTL1 injection reduced myocardial fibrosis and improved heart function.(A)Masson staining of heart sections for fibrosis evaluation.Collagen was stained blue and cardiomyocyte was stained red.(B)Total fibrosis level was indicated by quantification of CVF.(C)The CVF quantification in non-perivascular areas.(D) The CVF quantification in perivascular areas.(E) The measurement of heart hemodynamics.Data presented are mean ± SD(n=6).One-way ANOVA with post hoc LSD multiple comparison test was used (B-E).AAV=adeno-associated virus; ANOVA=analysis of variance;CVF=collagen volume fraction;FSTL1=follistatin like-1;LSD=least significant difference;LVEDP=left ventricular end-diastolic pressure; LVSP=left ventricular systolic pressure;MA=MI+AAV-FSTL1 injection group;MAR=MI+AAV-FSTL1 injection+resistance exercise group;MI=sedentary myocardial infarction group;MR=MI+resistance exercise group;PCNA=proliferating cell nuclear antigen;S=sham-operation control;±dp/dt max=maximum left ventricular pressure increasing/decreasing rate.
These results indicate that angiogenesis of the MI heart occurred in a compensatory way, and resistance exercise can facilitate cardiac angiogenesis after MI.Overexpression of FSTL1 in skeletal muscle had no significant effect on the vascularization of the infarcted area without exercise intervention.Resistance exercise stimulated the secretion of skeletal muscle-derived FSTL1 and up-regulated the FSTL1 level in the MI heart in order to improve the myocardial angiogenesis and inhibit the development of myocardial pathological remodeling.
3.2.FSTL1 facilitated proliferation of HUVECs and up-regulated DIP2A expression
DIP2A is a main receptor of FSTL1in vitro.18To confirm the increase in the expression of DIP2A in ECs after FSTL1 intervention and the effects of FSTL1 on EC proliferation,immunofluorescence staining was used to detect the expression of DIP2A and PCNA in HUVECs after rhFSTL1 treatment.The results showed that the expression of DIP2A on the surface of HUVECs was increased by 2.90 times after the rhFSTL1 treatment at 100 ng/mL for 48 h compared with PBS control(p<0.001;Fig.4A and 4B).Furthermore,the number of PCNA positive HUVECs was increased by 4.41 times compared with PBS control (p< 0.001; Fig.4C and 4D).These results indicate that FSTL1 up-regulated the expression of its receptor, DIP2A, on ECs.FSTL1 promoted ECs proliferation to facilitate angiogenesis, which was consistent within vivoexperimental results.
3.3.FSTL1 regulated the activation of the TGFβ1-Smad2/3 signaling pathway
TGFβ1-Smad2/3 acts as the key signaling pathway to regulate the tissue angiogenesis.19To confirm whether FSTL1 had a regulatory effect on the TGFβ1-Smad2/3 signaling pathway,LY2157299, the inhibitor of TGFβR1, was used to treat the HUVECs at a dosage of 500 nmol/L, followed by rhFSTL1 administration at the 100 ng/mL.The expression of FSTL1,DIP2A, TGFβ1, Smad2/3, p-Smad2/3, and VEGF-A were detected by Western blotting.Compared with the groups having no rhFSTL1 treatment (Lanes 2 and 4 in Fig.5A), the expression of FSTL1 and DIP2A were significantly increased in the rhFSTL1 treatment groups (p< 0.001; Lanes 1 and 3 in Fig.5A), which was not affected by LY2157299 (Lane 1 in Fig.5A-5C).The expression level of TGFβ1 was also elevated after rhFSTL1 treatment,and it was elevated more significantly in the-LY2157299/+rhFSTL1 group than in the+LY2157299/+rhFSTL1 group(p<0.001;Fig.5A and 5D).
It was noteworthy that although LY2157299 treatment significantly decreased the phosphorylation level of Smad2/3,the+LY2157299/+rhFSTL1 group showed a higher ratio of p-Smad2/3vs.Smad2/3 than the +LY2157299/-rhFSTL1 group.Compared with the control (no treatment) group, the phosphorylation level of Smad2/3 was significantly increased in the-LY2157299/+rhFSTL1 group(p<0.001;Fig.5A and 5E).The expression of VEGF-A was also down-regulated by LY2157299 and up-regulated by rhFSTL1 treatment(p=0.019;Figs.5A and 5F).The trend of VEGF-A expression was also same with Smad2/3 phosphorylation except in the-LY2157299/-rhFSTL1 group,which suggested that although Smad2/3 phosphorylation was affected by LY2157299, FSTL1 could act as an independent regulatory factor for TGFβ1-Smad2/3 signaling pathway activation and VEGF-A expression.
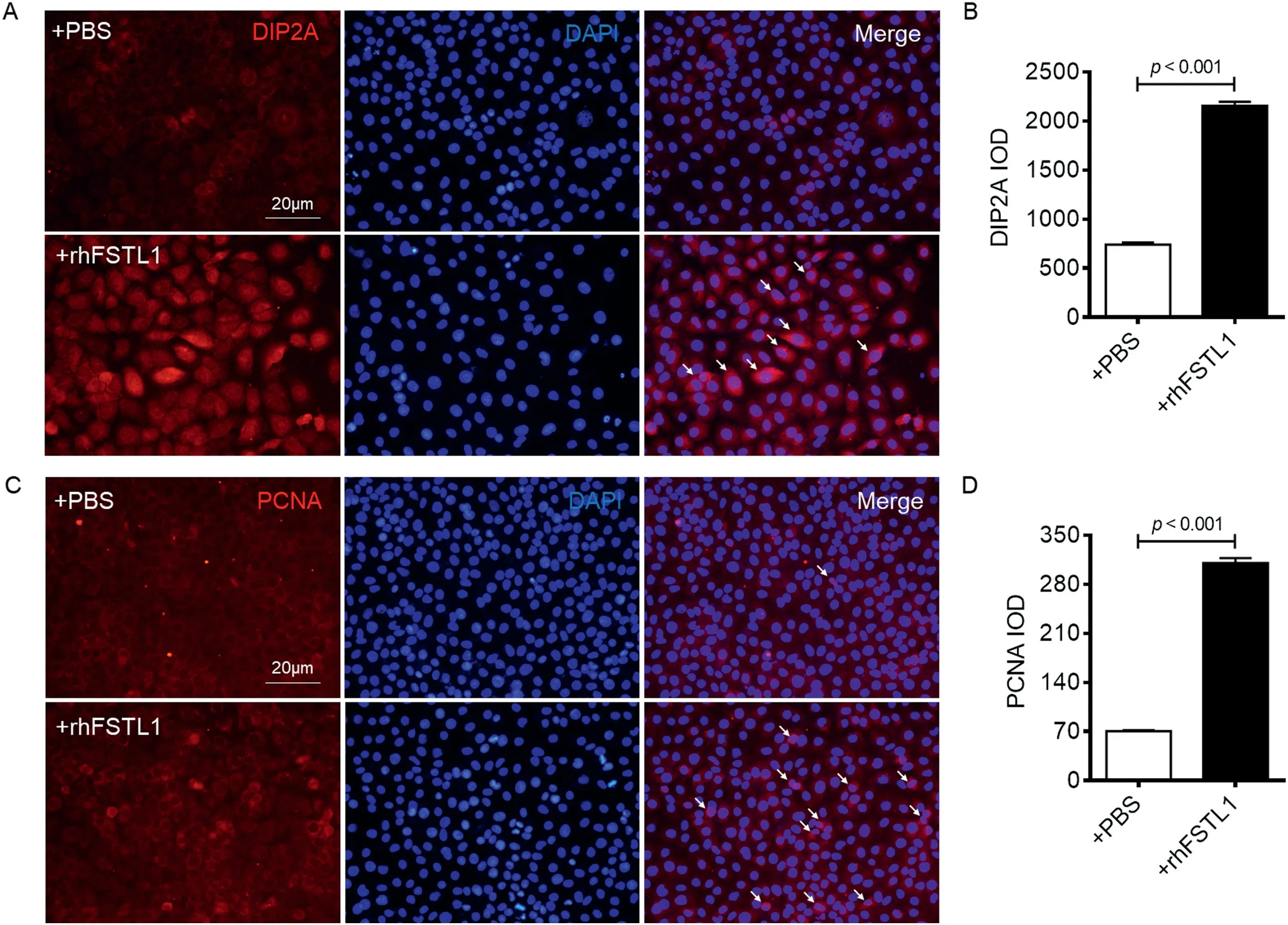
Fig.4.rhFSTL1 elevated DIP2A and PCNA level in cultured HUVECs.(A) HUVECs grew on glass coverslips and stained with DIP2A (red)and DAPI (blue).The third column of is a merged image of DIP2A with DAPI,indicating that DIP2A was expressed around the nucleus.(B)Quantification showed rhFSTL1 treatment increased the DIP2A level.(C)HUVECs stained with PCNA(red)and DAPI.PCNA was expressed in the nucleus.(D)Quantification showed that rhFSTL1 treatment increased the PCNA level.Typical DIP2A+ and PCNA+ cells are indicated by white arrows.Data presented are mean ± SD (n=6).Paired-samples Student’s t test was used(B and D).DAPI=diaminophenylindoles;DIP2A=disco interacting protein 2 homolog A;HUVECs=human umbilical vein endothelial cells;IOD=integral optical density;PBS=phosphate buffer saline;PCNA=proliferating cell nuclear antigen;rhFSTL1=recombinant human FSTL1.
3.4.FSTL1 promoted tube formation of HUVECs through Smad2/3 signaling
To confirm whether FSTL1 could promote angiogenesis even after the inhibition of TGFβR1, we conducted a tubule formation test to detect the angiogenesis ability of HUVECs.The results showed that, using the 20 h of non-intervention as a reference, the tubular-forming capacity was significantly reduced in the +LY2157299/-rhFSTL1 group (p< 0.001),and+LY2157299/+rhFSTL1 made the tubular-forming capacity increase (Fig.6A).But the HUVECs were loosely distributed and did not form tubular structures.When using the rhFSTL1 to treat HUVECs and not inhibiting TGFβR1(-LY2157299/+rhFSTL1 group),the number of tubular structures was increased by 66.67% compared with control group(p<0.001;Fig.6B),which was consistent with the change of VEGF-A protein level (Fig.5F).After treatment for 36 h and 48 h, the number of tubules in each group was increased.At 48 h, the number of tubules peaked and tended to be stable,which showed the characteristic of time dependence(Fig.6B).These results indicated that the inhibition of the TGFβ1-Smad2/3 signaling pathway affected the expression of VEGF-A, which is not conducive to angiogenesis.FSTL1 combined with its receptor, DIP2A, to regulate angiogenesis mainly through the Smad2/3 signal.Interestingly, FSTL1 could also directly activate Smad2/3 and was not affected by TGFβR1 activity.Both the expression of angiogenesis-related proteins and tubular formation of ECs could be promoted by FSTL1 through the Smad2/3 signaling mechanism.
4.Discussion
4.1.The endocrine of skeletal muscle stimulated by exercise has positive effects on cardioprotection
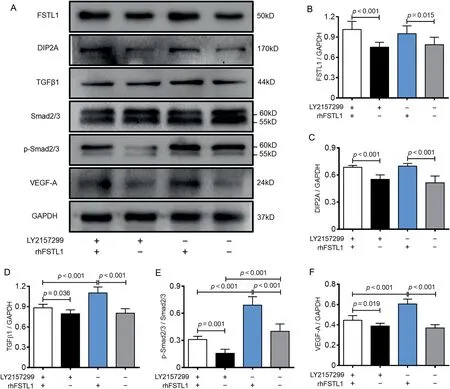
Fig.5.LY2157299 and rhFSTL1 regulated the expression of FSTL1,DIP2A,and VEGF-A and activity of TGFβ1-Smad2/3 signaling pathway in HUVECs.(A)The expression level of FSTL1,DIP2A,TGFβ1,Smad2/3,p-Smad2/3,and VEGF-A were measured by Western blotting after LY2157299 and/or rhFSTL1 treatment.The quantification of (B) FSTL1, (C) DIP2A, and (D) TGFβ1 expression.(E) The quantification of Smad2/3 phosphorylation.(F) The quantification of VEGF-A expression.Data presented are mean±SD(n=6).One-way ANOVA with post hoc LSD multiple comparison test was used(B-F).ANOVA=analysis of variance;DIP2A=disco interacting protein 2 homolog A;FSTL1=follistatin like-1;GAPDH=glyceraldehyde-3-phosphate dehydrogenase;HUVECs=human umbilical vein endothelial cells;LSD=least significant difference;p-Smad2/3=phosphorylated Smad2/3;rhFSTL1=recombinant human FSTL1;TGFβ1=transforming growth factor-β1;VEGF-A=vascular endothelial growth factor-A.
Skeletal muscle can be considered as the largest secretory organ,20producing and secreting more than 700 proteins in the body, mainly regulatory peptides, cytokines, adipokines,and growth factors,21so as to maintain the stability of the cardiovascular system.22,23Exercise is effective in stimulating skeletal muscle growth,metabolism,and endocrine function.24Similarly,FSTL1 can be also expressed and secreted by skeletal muscle after exercise intervention.20Other studies have shown that human myotubes express and secrete FSTL1 in a differentiation-dependent manner.8Moreover,in a study,after 60 min of cycle ergometer exercise,the level of serum FSTL1 increased significantly.8
In our previous study, we showed that exercise reverses skeletal muscle atrophy and increases FSTL1 expression in skeletal muscle and serum after MI.4Intermittent aerobic exercise induced more FSTL1+myocytes and FSTL1 protein than mechanical vibration training.4FSTL1 is systemic expressed cytokine, which is especially abundant in the heart, skeletal muscle, fat, kidneys, and lungs.In our FSTL1-related studies,we found that both aerobic4and resistance exercise stimulated the elevation of myocardial FSTL1(Fig.2C)in the absence of FSTL1 overexpression in the skeletal muscle of MI rats, indicating that, at least in part, endogenous myocardial FSTL1 may first respond to exercise and also play a role in cardioprotection.
In the present study, we emphasized the role of skeletal muscle on the basis of cardiac self-protection promoted by exercise.The tibialis anterior muscle was chosen for AAV-FSTL1 vector injection because it was easy to locate and take samples from, and was one of the muscles most biomechanically affected by resistance exercise.25We demonstrated that the endocrine of skeletal muscle-derived FSTL1 stimulated by exercise has positive effects on cardiac angiogenesis promotion,myocardial pathological remodeling reduction and cardiac function improvement.However, how much the heart and skeletal muscle contribute to cardioprotection remains to be further studied,as do the mechanisms leading to this contribution.
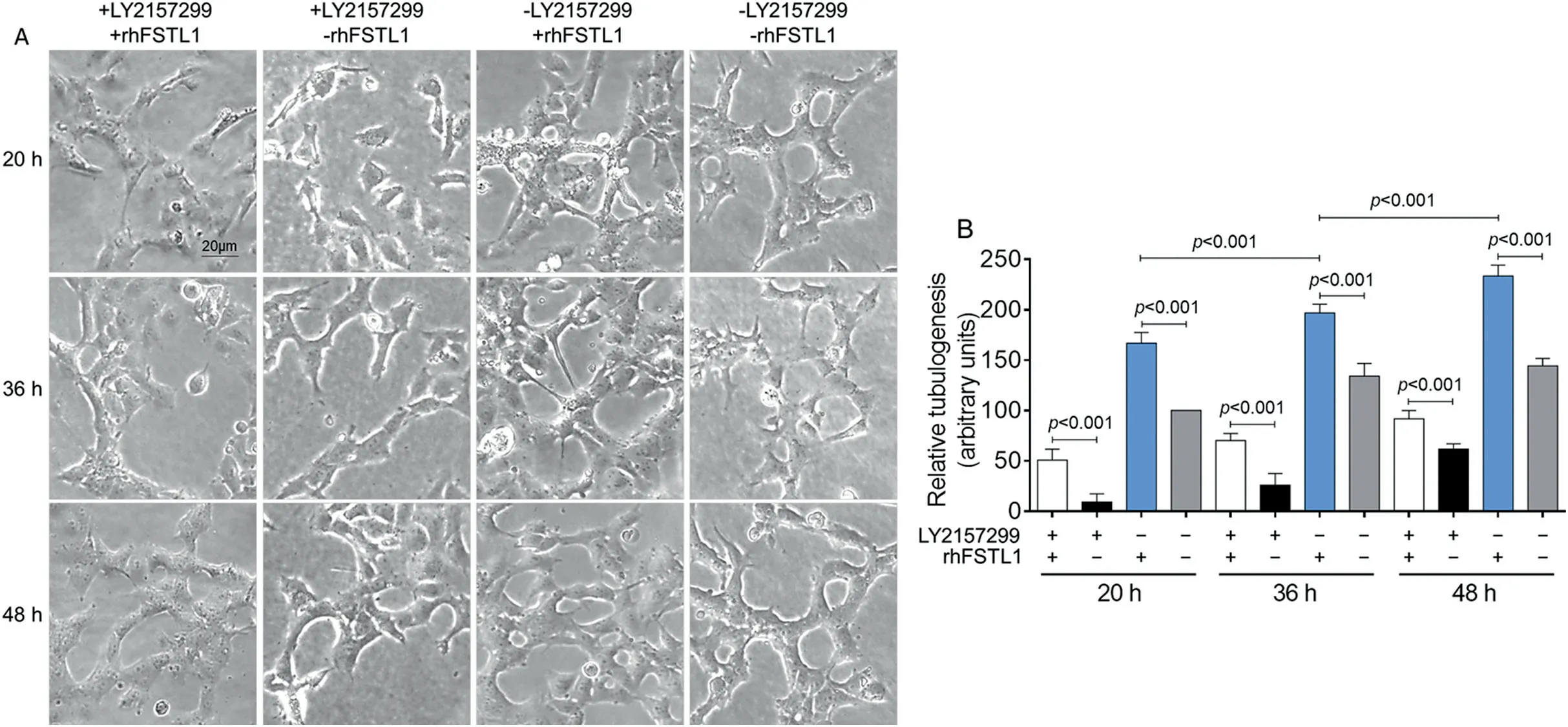
Fig.6.LY2157299 inhibited and rhFSTL1 promoted the small-tubes formation ability of HUVECs.(A)HUVECs were planted into Matrigel,and tubes formation was detected after LY2157299 and/or rhFSTL1 treatment for 20 h,36 h,and 48 h.(B)The quantification of tubes formation ability.Data presented are mean±SD(n=6).One-way ANOVA with post hoc LSD multiple comparison test was used(B).ANOVA=analysis of variance;HUVECs=human umbilical vein endothelial cells;LSD=least significant difference;rhFSTL1=recombinant human FSTL1.
4.2.FSTL1 promotes angiogenesis and protects cardiac function in the MI heart
Angiogenesis plays an important role in cardiac rehabilitation after MI.In the early stages of MI,restoration of coronary circulating blood supply is a necessary condition for long-term LV physiological remodeling because it prevents heart failure in the late stages of MI.26,27The heart responds to myocardial ischemia with increased expression of FSTL1.28,29Mouse cardiomyocyte-specific knockout offstl1is more likely to cause cardiac dysfunction after transverse aortic constriction, and FSTL1 overexpressed mice are more tolerant to transverse aortic constriction-caused pathological reactions.30The research of Wei et al.31shows that FSTL1 from epicardium could promote the proliferation of immature cardiomyocytes and narrow the infarcted area after MI.In the present study, we did not evaluate the size of the infarction due to the post-operative changes of electrocardiogram, and hemodynamic parameters were significantly positively correlated with the quantitative result of triphenyltetrazolium chloride staining,32which could be a limitation of our study.In our study, we found that the endogenous myocardial FSTL1 was insufficient for long-term recovery from MI.Therefore, the MI heart needs a supply of FSTL1 from distant organs,such as skeletal muscle,for cardioprotection.
FSTL1 has been reported to promote angiogenesis in many studies.6,7FSTL1 from skeletal muscle could stimulate vascularization directly through the endothelial nitric oxide synthase pathway to inhibit apoptosis and improve differentiation and migration of ECs.6The addition of FSTL1 to cultured vascular smooth muscle cellsin vitroalso promoted the phosphorylation of adenosine monophosphate activated protein kinase and reduced the proliferation and migration of vascular smooth muscle cells.7The TGFβ1-Smad2/3 signaling pathway is one of the main mechanisms regulating angiogenesis,33and the activity of its upstream member,Activin, is directly regulated by FSTL1, which affects the activity of the TGFβ receptor complex and downstream Smad2/3 phosphorylation, thereby regulating angiogenesis(Kyoto Encyclopedia of Genes and Genomes database).It is noteworthy that overexpression of FSTL1 by adenovirus accelerates angiogenesis in ischemic muscle tissue but not in muscles with normal oxygen content.This evidence indicates that the function of FSTL1 in responding to the tissue ischemia and promoting angiogenesis may be related to the niche conditions of ECs.
4.3.FSTL1-DIP2A and its downstream members can be regulatory mechanisms
In vivoandin vitroresearch has demonstrated that DIP2A is the only receptor of FSTL1.34,35DIP2A is a kind of membrane receptor protein with an intracellular domain,18and its expression in adults is concentrated in neurons, mesenchymal cells,ECs, smooth muscle cells, and cardiomyocytes.36After interfering with the expression of DIP2A in cultured ECs, the effects of FSTL1-induced cell survival, migration, and differentiation into tubular structures is weakened,34which indicates that intracellular molecular cascades induced by FSTL1-DIP2A is a key mechanism in regulating angiogenesis.In the present study, we found that the expression of DIP2A in cultured HUVECs was highly consistent with the proliferation of HUVECs after treatment of rhFSTL1 (Fig.4).The phosphorylation of Smad2/3 and the expression of PCNA and VEGF-A were also closely related to DIP2A.Other studies have suggested that the deletion of thefstl1gene in vascular endothelium leads to the activation of the Smad1/5/8 signal and increases the expression of bone morphogenetic protein (BMP)/Smad-regulated genes such asjagged1,endoglin, andgata2, and that the output of the right ventricle also decreases, which is related to the damage of pulmonary microvascular remodeling.37TGFβ1 can rapidly activate the Smad, mitogen-activated protein kinase and protein kinase B (Akt) signaling pathways, but only the inhibition of Smad2/3 can eliminate the TGFβ1-induced expression of FSTL1.38These results suggest that Smad2/3 may be regulated by DIP2A and can act as a downstream molecule of the FSTL1-DIP2A signal to regulate EC activity,and that Smad2/3 also responds to and has feedback regulation in the expression of FSTL1 (Fig.7).
After the knockout ofdip2ain cultured cardiomyocytes,the protective effect of FSTL1 on anoxia- and reoxygenation-induced apoptosis is reduced, and the level of FSTL1-induced Akt phosphorylation decreaseds.34Anoxia-reoxygenation intervention in cardiomyocytes simulates the pathophysiological changes of MIin vivo.After being activated by FSTL1, DIP2A enhances the activation of the downstream Akt,35which is of great significance for cardiomyocytes protection, apoptosis inhibition, and improvement of myocardial fibrosis (Fig.7).
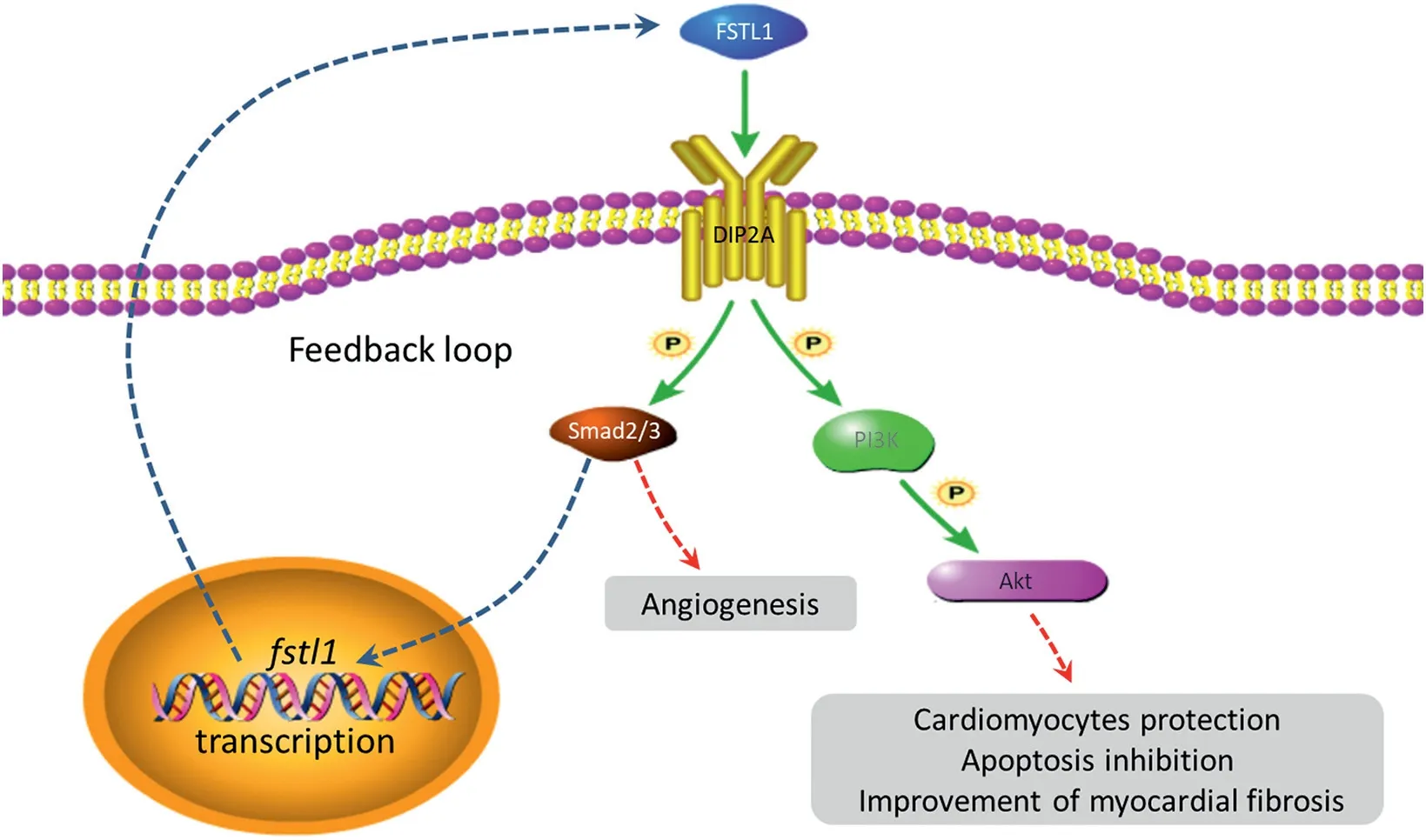
Fig.7.The predicted mechanisms of FSTL1-DIP2A functioning.Akt=protein kinase B; DIP2A=disco interacting protein 2 homolog A; FSTL1=follistatin like-1;P=phosphorylation;PI3K=phosphoinositol 3 kinase.
5.Conclusion
Dynamic resistance exercise stimulates the expression of skeletal muscle-derived FSTL1, which could supplement the insufficiency of cardiac FSTL1 and promote cardiac rehabilitation through the DIP2A-Smad2/3 signaling pathway in MI rats.Further exploration of the interactive modes and mechanisms between the heart and distant organs will provide new ideas for exercise rehabilitation research on the MI heart.
Acknowledgment
The following grants supported this research: The National Natural Science Foundation of China (No.31671240 to ZT and No.31900828 to YX).
Authors’contributions
YX performed the experiments and drafted the manuscript;MH and QL contributed to perform the experiments and prepared the figures; ZT and YL participated in design of the experiments; DWG contributed to write the manuscript.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
All authors declare that they have no competing interests.
 Journal of Sport and Health Science2021年5期
Journal of Sport and Health Science2021年5期
- Journal of Sport and Health Science的其它文章
- A systematic review of running-related musculoskeletal injuries in runners
- Most ankle sprain research is either false or clinically unimportant:A 30-year audit of randomized controlled trials
- Effects of plyometric vs.resistance training on skeletal muscle hypertrophy:A review
- Pacing profiles and tactical behaviors of elite runners
- Exercise attenuates bone mineral density loss during diet-induced weight loss in adults with overweight and obesity:A systematic review and meta-analysis
- Adverse associations of sedentary behavior with cancer incidence and all-cause mortality:A prospective cohort study
