Exercise attenuates bone mineral density loss during diet-induced weight loss in adults with overweight and obesity:A systematic review and meta-analysis
Jku Mesinovic,Pul Jnsons,Ayse Zengin,Bror de Courten,Alexnder J.Rodriguez,c,Roin M.Dly,Peter R.Eeling,Dvid Scott,d
a Department of Medicine,School of Clinical Sciences at Monash Health,Monash University,Clayton,VIA 3168,Australia
b Institute for Physical Activity and Nutrition(IPAN),School of Exercise and Nutrition Sciences,Deakin University,Burwood,VIA 2134,Australia
c School of Medical and Health Sciences,Edith Cowan University,Perth,WA 6027,Australia
d Department of Medicine and Australian Institute of Musculoskeletal Science,Melbourne Medical School-Western Campus,University of Melbourne,St Albans,VIA 3021,Australia
Abstract Background:Weight-loss-induced fat loss improves cardiometabolic health in individuals with overweight and obesity;however,weight loss can also result in bone loss and increased fracture risk.Weight-loss-induced bone loss may be attenuated with exercise.Our aim was to compare changes in bone mineral density(BMD)in adults with overweight and obesity who undertook diet-induced weight loss alone or in combination with exercise.Methods:We included randomized controlled trials(RCTs)in adults with overweight or obesity(aged ≥18 years;body mass index ≥25 kg/m2)that prescribed diet-induced weight loss alone or in combination with supervised exercise,and measured any bone structural parameters.Risk of bias was assessed using the Cochrane Risk of Bias tool.Random-effects meta-analyses determined mean changes and net mean differences(95%confidence intervals(95%CIs))in the percentage of areal BMD(aBMD)change between groups.Results: We included 9 RCTs.Diet-induced weight loss led to significant losses in femoral neck aBMD (mean change: -1.73% (95%CI:-2.39%to-1.07%),p<0.001)and total hip aBMD(-2.19%(95%CI:-3.84%to-0.54%),p=0.009).Femoral neck aBMD losses were significantly greater in the diet-induced weight loss group compared to the exercise plus diet-induced weight loss group (net difference: -0.88%(95%CI: -1.73% to -0.03%)); however, there were no differences in aBMD changes at any other skeletal site: total hip (-1.96% (95%CI:-4.59%to 0.68%))and lumbar spine (-0.48% (95%CI:-1.81% to 0.86%)).aBMD changes did not differ significantly according to exercise modality(resistance exercise,aerobic exercise,or a combination of the two)during diet-induced weight loss.Conclusion:Diet-induced weight loss led to greater femoral neck bone loss compared to diet-induced weight loss plus exercise.Bone loss at the total hip and lumbar spine was not attenuated by exercise during diet-induced weight loss.The lack of consistent skeletal benefits may be due to the insufficient duration and/or training intensities of most exercise interventions.Additional RCTs with appropriate,targeted exercise interventions should be conducted.
Keywords: Bone mass;Exercise;Obesity;Overweight;Weight loss
1.Introduction
The global prevalence of obesity has almost doubled since 1980,which has led to an increase in cardiometabolic diseases and premature mortality.1,2Caloric restriction supports weight loss and improves cardiometabolic health in individuals with overweight and obesity,3but it can also result in bone and muscle loss and increased fracture risk.4,5
In the study of osteoporotic fractures, older women with overweight and obesity who intentionally and unintentionally lost body weight (≥5%)over 6 years had a 2.5-fold increased risk for hip fracture compared with those who either maintained(<5%)or gained(>5%)body weight.5Similar associations between weight loss and increased fracture risk have also been observed in older men.6Randomized controlled trials(RCTs) have demonstrated that weight loss with and without subsequent weight regain can result in sustained bone loss.7-9Meta-analyses of exercise interventions have shown that programs incorporating moderate- to high-intensity resistance and impact exercise can either increase or maintain areal bone mineral density (aBMD).10,11Therefore, exercise is often promoted as a strategy to attenuate bone loss during weight loss.
RCTs investigating whether exercise can attenuate bone loss during diet-induced weight loss have reported inconsistent findings.Ostensibly, the first RCT that addressed this research question showed that postmenopausal women completing combined resistance and aerobic exercise during diet-induced weight loss experienced significant lumbar spine aBMD losses (-2.4%), while those randomized to diet-induced weight loss alone had no significant change(-1.6%).12It should be noted that between-group differences in aBMD changes were not reported, so it is unclear whether differences between groups undertaking dietinduced weight loss alone, and in combination with exercise, were statistically significant.12Whole-body and radial aBMD did not change with either intervention.12Other RCTs have reported no changes in femoral neck or total hip aBMD,13attenuated aBMD loss at the total hip,14and increased lumbar spine and total hip aBMD15in response to resistance exercise, aerobic exercise, or the combination,during diet-induced weight loss.A recent meta-analysis examining the effects of any type of exercise plus dietinduced weight loss compared to diet-induced weight loss alone reported that exercise during diet-induced weight loss improved total hip (mean difference: 0.03 g/cm2(95%confidence interval (95%CI): 0.01-0.04)) and femoral neck aBMD (mean difference: 0.03 g/cm2(95%CI:0.01-0.05)).16However, this review was not prospectively registered in the international prospective register of systematic reviews (PROSPERO), which is important for transparency and minimizing bias.17Furthermore, this meta-analysis had several limitations, such as the inclusion of nonrandomized studies, despite stating that only RCTs were included, omission of a relevant study,18combining of percentage and absolute aBMD changes in the same analysis, and the inclusion of multiple reports from the same study.In light of these methodological flaws, we have comprehensively re-evaluated the literature concerning this important topic.
The aim of this systematic review and meta-analysis was to compare changes in aBMD and other bone structural parameters among adults with overweight and obesity undertaking diet-induced weight loss alone and in combination with exercise.We hypothesized that exercise would attenuate bone loss at weight-bearing sites.
2.Methods
This study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analysis(PRISMA) statement19(PRISMA checklist can be found in Supplementary Appendix 1)and met A MeaSurement Tool to Assess systematic Reviews 2(AMSTAR2)specifications for a high-quality systematic review.20The study protocol was registered with PROSPERO(registration number CRD42019127460).
2.1.Search strategy and study selection
A systematic search for articles comparing the effects of diet-induced weight loss alone and diet-induced weight loss in combination with exercise on bone structural parameters in adults with overweight and obesity was conducted from April 2020 until June 2020 using Embase (1980 to present),PubMed, Web of Science, SPORTDiscus, CINAHL Plus, and Scopus databases.We also searched Cochrane Central Register of Controlled Trials and ClinicalTrials.gov records for relevant articles, as well as bibliographies of all eligible articles and our personal reference libraries.Titles,abstracts,and keywords were searched using the terms found in Supplementary Appendix 1.Screening was performed by 2 independent reviewers (JM and PJ) using Covidence software (Veritas Health Innovation, Melbourne, Australia), and a third investigator(DS)adjudicated for consensus.
We included parallel-group RCTs with populations aged≥18 years with overweight or obesity determined by body mass index (≥25 kg/m2) that were prescribed either diet-induced weight loss alone or diet-induced weight loss in combination with supervised exercise.We only included studies with supervised exercise to ensure that both adherence and intervention fidelity were high.Eligible RCTs also had control groups that underwent diet-induced weight loss without exercise and presented any bone structural parameters.Studies were excluded if participants had diseases known to affect bone-mineral metabolism (e.g., chronic kidney disease) or exercise performance(e.g., chronic obstructive pulmonary disease).Review articles,book chapters, conference proceedings, case reports,and letters to the editor were also excluded.Studies that were not conducted in humans, did not have a control group, were nonrandomized,had a crossover design, involved surgical or pharmacological weight loss interventions,or were not published in English were also excluded.
2.2.Data extraction and quality assessment
Data were independently extracted by 2 reviewers(JM and PJ).We extracted sample size, age, country, anthropometry,health status, diet composition, exercise protocol, and dual-energy X-ray absorptiometry (DXA)-determined wholebody,total hip,femoral neck,radius,and lumbar spine aBMD data from eligible studies.We also extracted total hip,femoral neck,and lumbar spine volumetric BMD,as well as estimates of bone strength measured via computed tomography (CT).If required, we contacted corresponding authors from eligible and potentially eligible studies and requested additional data.We were unable to obtain mean and standard deviation (SD)percentage change data for 3 eligible studies,15,18,21so test and summary statistics were used to calculate these values.Specifically, absolute mean changes were converted to percentage mean changes by dividing absolute mean changes by baseline values and multiplying by 100.Algebraic recalculation of missing change SDs were calculated from actualpvalues and/ortstatistics using methods described by Higgins et al.22All calculations were performed using Review Manager (RevMan) software (Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration,Copenhagen,Denmark).Quality assessment (risk of bias) of included studies was performed by 2 reviewers (JM and PJ) using the Cochrane Risk of Bias tool.23Studies were classified as low,unclear,or high risk of bias based on the following 5 bias domains: (1)selection, (2) performance, (3) detection, (4) attrition, and (5)reporting.Publication bias was not assessed due to the low number of included studies.Discrepancies between the 2 reviewers (JM and PJ) during data extraction or study quality assessment were adjudicated by a third reviewer(DS).
2.3.Training regimens
We also compared all training regimens in the included studies to the Exercise and Sports Science Australia position statement on recommendations for exercise prescription for the prevention and management of osteoporosis.24Given that studies included in this meta-analysis had populations with overweight or obesity, we applied an assumption that most individuals had either normal or high aBMD and low risk for osteoporosis.25Therefore, we compared training regimens in the included studies against “low-risk” exercise guidelines.Exercise prescriptions for the prevention of osteoporosis in low-risk individuals included high-impact activities(e.g.,hopping, jumping,etc.) that produced ground reaction forces 4-fold greater than what is experienced when in a standing position (i.e., >4 body weights) completed >4 days/week,with 10-20 repetitions and 3-5 sets; progressive resistance training at high to very high intensity(80%-85%1 repetition maximum (1RM)) completed 2 days/week, with 8 repetitions and 2-3 sets; and balance exercise with challenging intensity as often as possible.
2.4.Statistical analysis
CT-derived bone parameters were not compared among groups because only 1 study21reported these outcomes.Mean changes were calculated as aBMD at follow-up minus aBMD at baseline in the control and treatment groups.Net mean differences were calculated as the mean change in the control group minus the mean change in the intervention group, or as mean final aBMD value in the control group minus final aBMD value in the intervention group.We extracted aBMD data from timepoints where exercise interventions were supervised,and data were pooled using random-effects meta-analyses.If the standard error (SE) was reported, the SD was computed by.If the 95%CI was reported, we multipliednby the difference between the upper and lower limits and then divided this value by 3.92.τ2,Qstatistic,andI2values were used to measure heterogeneity between studies.I2values of 25%,50%,and 75%were considered to be indicative of low,moderate,and high heterogeneity,respectively.26We investigated potential sources of heterogeneity using the following predefined subgroup analyses: age (<60 yearsvs.≥60 years), trial duration (<6 monthsvs.≥6 months), and exercise modality (resistance exercisevs.aerobic exercisevs.combined resistance and aerobic exercise).One study had multiple treatment groups21(diet-induced weight loss plus resistance exercise and diet-induced weight loss plus aerobic exercise),which were combined using the calculator function in RevMan(The Nordic Cochrane Centre)in all analyses except the subanalyses comparing exercise modes.We performed a leave-one-out sensitivity analysis to determine how each study influenced our overall estimates at each skeletal site.We also performed meta-regression to determine the impact of several moderator variables (change in total weight loss, fat mass, and lean mass)on the net mean differences in aBMD changes at all skeletal sites.Meta-analyses were performed using RevMan (The Nordic Cochrane Centre), and meta-regression was performed using R software (Foundation for Statistical Computing, Vienna, Austria).27p<0.05 was considered statistically significant.
3.Results
The search of databases identified 721 unique citations.The full texts of 29 articles were screened, and 9 were included in the qualitative and quantitative synthesis(Fig.1).Studies were excluded because their exercise prescriptions were not supervised throughout a portion of28or throughout29the entire trial period, because they did not report bone outcomes,30or because they were nonrandomized.31
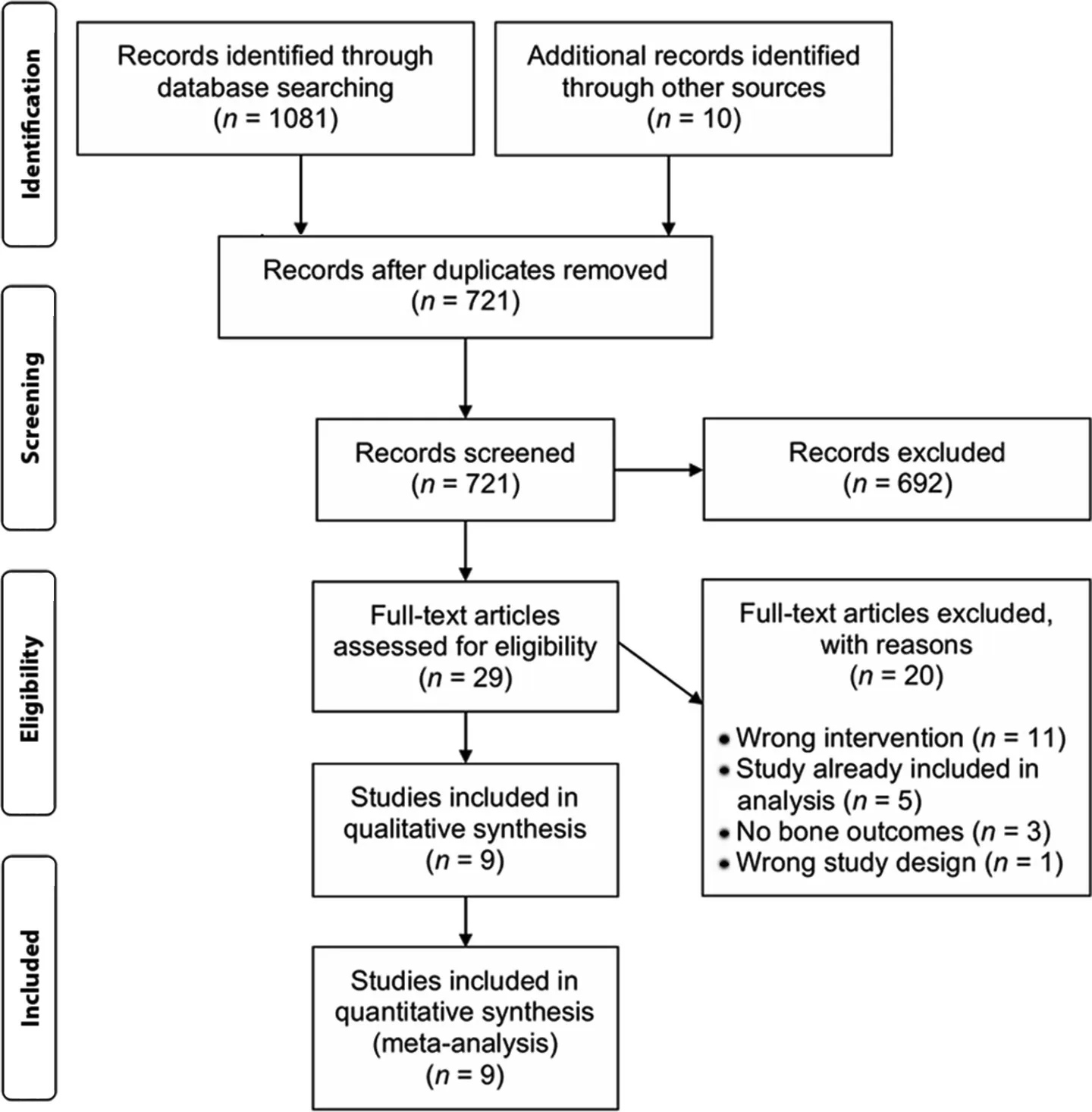
Fig.1.Preferred reporting items for systematic reviews and meta-analyses diagram of information flow in this systematic review and meta-analysis.
Descriptive characteristics of the included studies are presented in Supplementary Table 1.Two studies were conducted in populations with poor cardiometabolic health,13,21and others included overweight and obese but otherwise healthy,adults.12,14,15,32-34Most studies were conducted in the USA,14,18,21,32,34and the other 4 studies were conducted in Australia,13Egypt,15Japan,33or Denmark.12Six studies used DXA machines manufactured by GE Healthcare,13,15,18,21,32,33and 3 studies used DXA machines manufactured by Hologic.12,14,34Of the included RCTs,3 RCTs were conducted in older adults(aged ≥60 years),13,14,215 RCTs only included women,12,15,18,32,33and 4 RCTs had combined cohorts of women and men.13,14,21,34Only 1 study exclusively recruited postmenopausal women.12Most studies recruited populations with overweight and obesity,12,13,18,212 studies recruited individuals with only overweight,33,34and 3 studies recruited individuals with obesity.14,15,32The average weight loss in the diet-induced weight loss group was 8.7 kg (range:3.0-19.4 kg),and the average weight loss in the exercise plus diet-induced weight loss group was 9.7 kg (range:2.4-16.6 kg).Supervised exercise frequency 3—5 days/week.Four studies included resistance exercise,13,21,32,334 studies included aerobic exercise,15,18,21,34and 2 studies included combined resistance and aerobic exercise12,14during diet-induced weight loss.All aerobic exercises included walking, most included cycling,12,14,18,34and some also included running.12,34All resistance exercise interventions were progressive; 2 studies performed progressive resistance exercise at an intensity of ≤65%1RM.12,33Beavers et al.21progressed to an intensity of 75%1RM,Villareal et al.14progressed to an intensity of 80%1RM,and Daly et al.13progressed to an intensity of 75%-85% 1RM.Andersen et al.32did not specify the intensity at which resistance training was performed, but the authors stated that participants were able to complete 8 repetitions but no more than 12 repetitions, and that resistance was increased whenever subjects were able to perform more than 12 repetitions for 2 consecutive workouts.Exercise session durations ranged 30-90 min/day.Of the 9 studies,six reported mean/median exercise session adherence, which ranged between 83%-97%.12-14,21,32,33Six studies had interventions that were ≥6 months in duration,13,14,18,21,32,34and 3 studies had interventions that were <6 months in duration.12,15,33When compared with the Exercise and Sports Science Australia position statement on exercise recommendations for bone health,24we observed that no studies included in our meta-analysis prescribed adequate impact-loading exercise.Four studies13,14,21,32likely prescribed resistance exercise at the recommended intensity,but 2 studies32,33prescribed resistance exercise at an insufficient intensity to manage or prevent osteoporosis.Only 1 study incorporated balance training.14
Study quality assessments performed using the Cochrane Risk of Bias tool are presented in Supplementary Fig.1.Most studies had unclear selection bias;3 studies provided information pertinent to random sequence generation and had a low risk of bias,14,18,21and no studies provided information pertinent to allocation concealment.All participants completed supervised exercise; therefore, all studies had a high risk of performance bias because it was not possible for investigators and participants to blind themselves to the intervention arm allocation.Two studies had unclear detection bias13,15because they did not specify whether outcome assessors were blinded to the treatment allocations;all other studies had a low risk of bias.All studies had a low attrition and reporting bias.Two studies had an unclear risk of other bias,15,18which was due to insufficient information about the accuracy of aBMD measures(no short-term DXA coefficient of variation data).
Diet-induced weight loss led to significant bone loss at the femoral neck (mean change: -1.73% (95%CI: -2.39% to-1.07%),p<0.001) and total hip(-2.19% (95%CI:-3.84%to -0.54%),p=0.009), but not at the lumbar spine (-0.61%(95%CI:-1.68%to 0.46%),p=0.26)(Supplementary Fig.2).There were no significant aBMD changes at any skeletal site in response to diet-induced weight loss plus exercise (Supplementary Fig.3).Effects of all exercise interventions plus diet-induced weight lossvs.diet-induced weight loss alone on percentage change in total hip, femoral neck, lumbar spine,and radial aBMD are presented in Fig.2.The diet-induced weight loss group had greater femoral neck aBMD losses compared with the exercise plus diet-induced weight loss group(net difference: -0.88% (95%CI: -1.73% to -0.03%)).The net differences for the change in aBMD were not significantly different between the exercise and diet-induced weight loss group and the diet-induced weight loss alone group at any other skeletal site: total hip (-1.96% (95%CI: -4.59% to 0.68%)),lumbar spine(-0.48%(95%CI:-1.81%to 0.86%)),whole-body(-0.20%(95%CI:-0.57%to 0.17%),k=7),and radius (0.11% (95%CI: -0.70% to 0.93%)).Heterogeneity was low at the femoral neck (τ2=0.00;I2=0% (95%CI:0%-70%); andQstatistic=2.63) and radius (τ2=0.00;I2=0% (95%CI: 0%-90%); andQstatistic=0.65), but high at the total hip (τ2=5.95;I2=89% (95%CI: 75%-95%); andQstatistic=27.53), and lumbar spine (τ2=2.14;I2=76%(95%CI:49%-89%);andQstatistic=24.79).In a sensitivity analysis that excluded Hosny et al.,15heterogeneity at the total hip was moderate (τ2=0.89;I2=63%; andQ-statistic=5.40) and heterogeneity at the lumbar spine was low(τ2=0.00;I2=0%; andQstatistic=4.76).Studies included in our meta-analyses had similar risk of bias, which prevented us from performing subgroup analyses in studies with low risk of bias.We also performed a leave-one-out sensitivity analysis comparing the effect of exercise plus diet-induced weight lossvs.diet-induced weight loss alone on aBMD changes at different skeletal sites (Supplementary Table 2).The diet-induced weight loss alone group still experienced significantly greater femoral neck aBMD losses compared with the exercise plus diet-induced weight loss group after excluding Andersen et al.32and Redman et al.34but not after excluding any other studies.13,14,21There were no significant differences between groups at any other skeletal site.
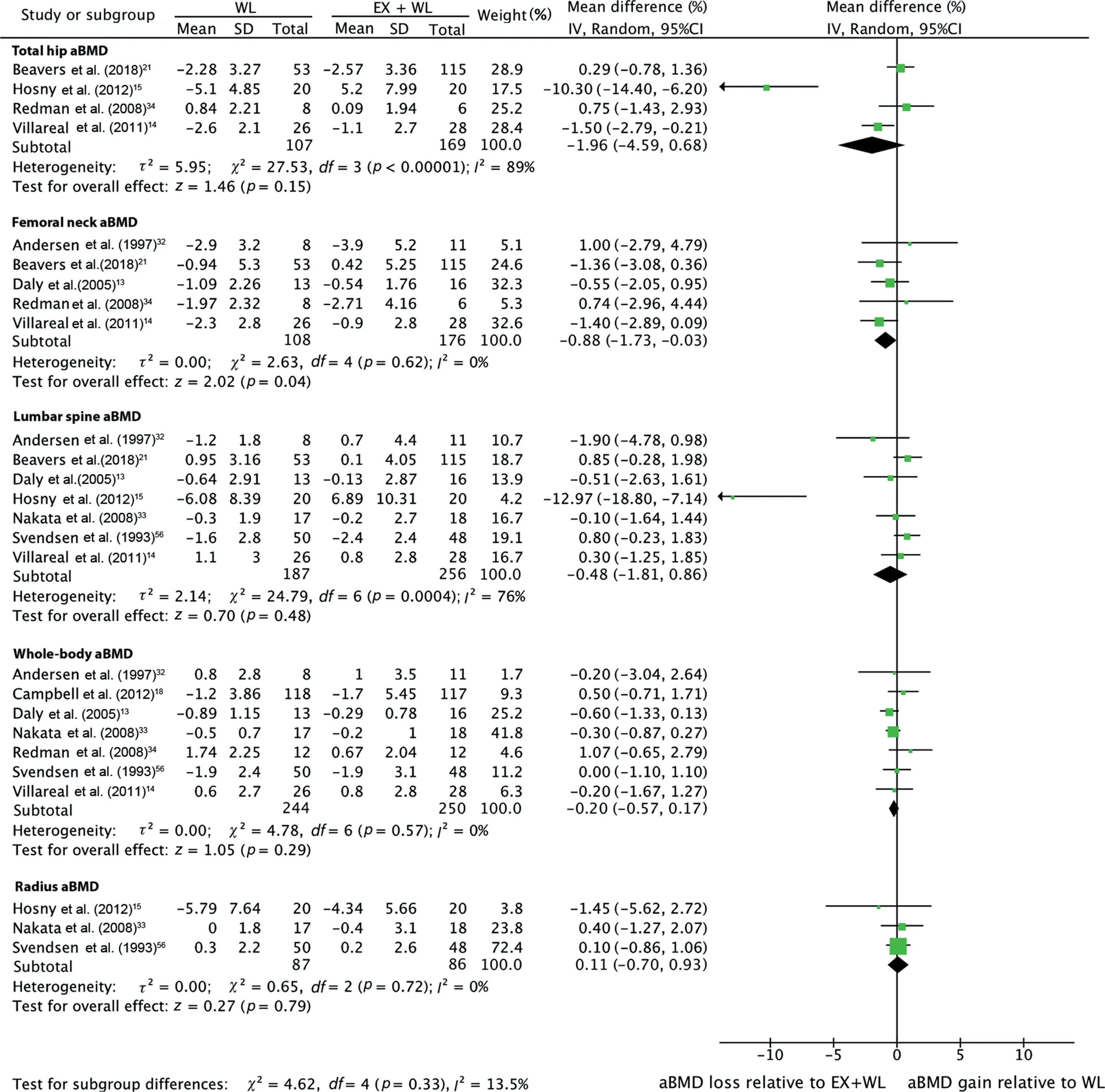
Fig.2.Effect of exercise plus diet-induced weight loss vs.diet-induced weight loss alone on percentage change in areal bone mineral density at various skeletal sites.Total refers to the number of included participants at the end of the study.95%CI=95%confidence interval;aBMD=areal bone mineral density;EX=exercise;IV=inverse variance;WL=weight loss.
The effects of combined exercise plus diet-induced weight lossvs.diet-induced weight loss alone on aBMD changes in several subgroups are presented in Table 1.The diet-induced weight loss alone group had greater femoral neck aBMD losses compared with the exercise plus diet-induced weight loss group in adults aged ≥60 years (-1.08%(95%CI:-1.98%to-0.18%)) and in trials that were ≥6 months in duration(-0.88% (95%CI: -1.73% to -0.03%)).Heterogeneity was low in all subgroup analyses at the femoral neck.There were no significant differences in aBMD changes at the total hip,lumbar spine, or whole-body in studies that used resistance exercise,aerobic exercise,or combined aerobic and resistance exercise during diet-induced weight loss.There were also no significant differences in aBMD changes at sites other than the femoral neck in studies that were <6 months and ≥6 months in duration, or between studies that included individuals aged<60 yearsvs.≥60 years.In our subgroup analyses, heterogeneity was moderate or high at the total hip, low at the lumbar spine (where Hosny et al.15was excluded) and low at the whole body.We also performed subgroup analyses in studies that only included women; there were no net differences in aBMD changes at the whole-body(-0.16%(95%CI:-0.66%to 0.35%),k=3) or lumbar spine (-4.14% (95%CI: -9.32%to 1.05%),k=3).Subgroup analyses were not performed at the radial site because there were an insufficient number of studies.We also performed meta-regression to determine the impact of several moderator variables on effect sizes;total losses in weight,fat mass,and lean mass did not significantly alter effect estimates at any skeletal site(Supplementary Table 3).
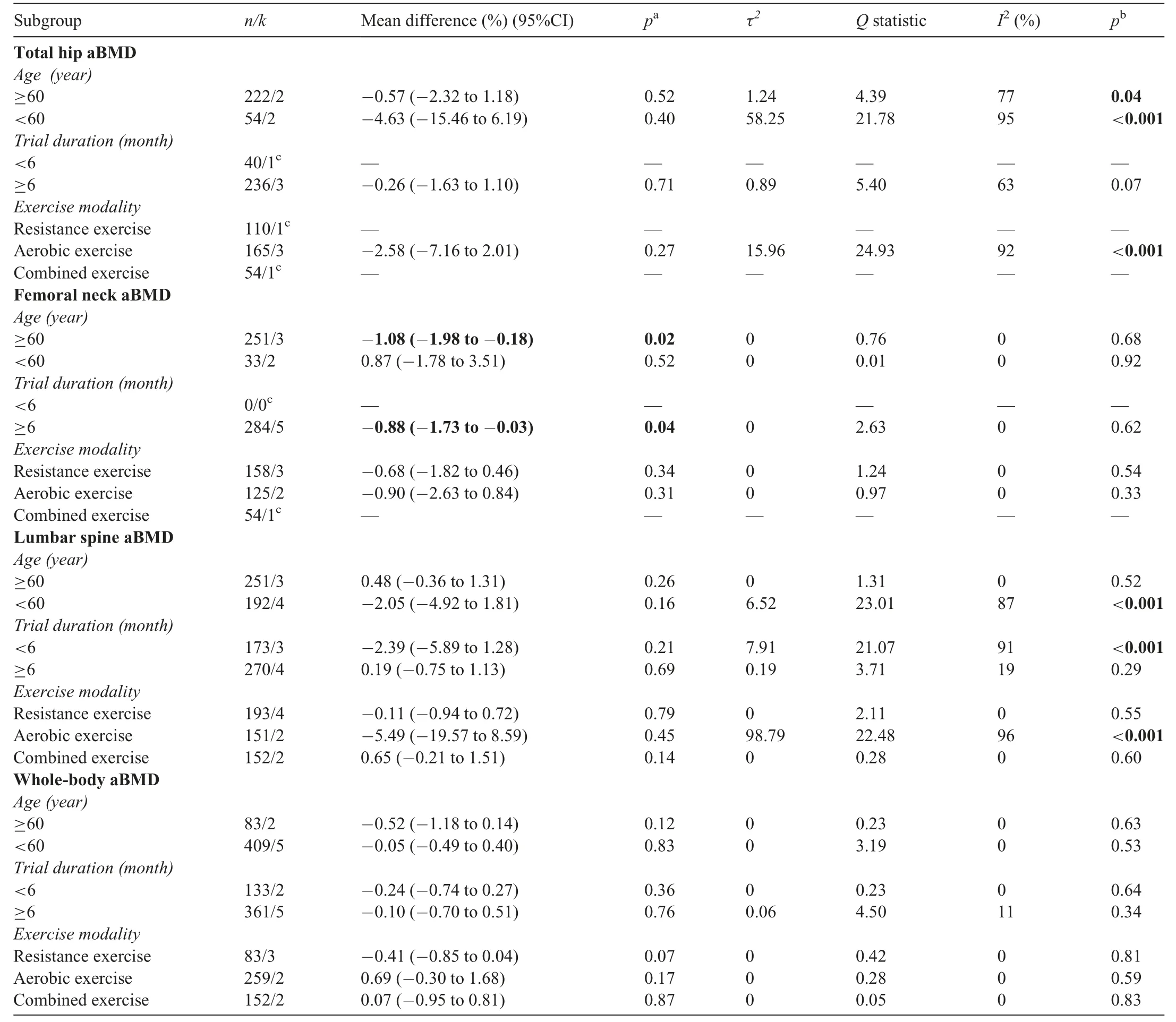
Table 1Meta-analyses comparing the effects of combined exercise plus diet-induced weight loss vs.diet-induced weight loss alone on percentage change in aBMD in several subgroups.
4.Discussion
Our meta-analysis demonstrated that diet-induced weight loss leads to greater femoral neck bone loss compared to dietinduced weight loss plus exercise.Bone loss at the total hip,lumbar spine,and radius was not attenuated by exercise during diet-induced weight loss.However, because bone loss at the femoral neck was attenuated by only ~1%, the clinical relevance of this finding remains uncertain.It is likely that thelack of any marked benefits of the exercise interventions on bone outcomes was related to insufficient durations and/or inadequate training intensities to maintain or improve aBMD during diet-induced weight loss.Additional RCTs with appropriate,targeted exercise interventions should be conducted.
Our findings are inconsistent with a previous systematic review and meta-analysis,16which reported that both femoral neck and total hip aBMD increased in response to combined exercise and diet-induced weight loss compared with diet-induced weight loss alone.We find the above-mentioned results16unlikely due to the reduction in mechanical loading that occurs at these sites during weight loss and also due to the inadequate exercise prescriptions (e.g., resistance exercise intensity is too low to prevent or manage osteoporosis24) in several of the studies included in the previous meta-analysis.16Instead,the discrepancies between our study and that previous meta-analysis16are probably attributable to methodological differences described earlier.Interestingly, although femoral neck aBMD losses were attenuated by exercise in our analyses,there were no benefits to hip aBMD.Although this may be related to different studies’ being included in the analyses at both skeletal sites,it could also be related to the training principle of specificity.Several studies have demonstrated that lower-limb exercises can improve bone mass in some hip regions but not in others.A study by Welsh and Rutherford35reported that 12 months of stepping and jumping exercises led to significant increases in greater trochanteric aBMD(+2.21%) but nonsignificant increases in femoral neck aBMD(+1.57%)in postmenopausal women and men aged>50 years.Similarly, Kerr et al.36reported that 12 months of high-load and low-repetition strength exercises (e.g., leg presses, hamstring curls,and hip abductions and adductions)increased trochanteric (+1.7%) and intertrochanteric aBMD (+1.5%) but not femoral neck aBMD (0.0%) in postmenopausal women.The inconsistent effects of exercise on bone mass in differing hip regions can probably be attributed to the differing load/force distributions that occur during various exercises.Martelli et al.37demonstrated that lower-limb exercises, such as chair stands,step-ups, knee extensions, and hip abductions, led to changes in peak strain energy at the femoral neck that were unlikely to induce bone formation.However, other lower-limb exercises,such as walking, hip extensions and flexions,and vertical jumping,led to changes in peak strain energy that were likely to induce bone formation at this skeletal site.37Similar to our findings,previous exercise interventions in older adults have also reported gains in femoral neck,but not total hip,aBMD.38,39Thus,the prescribed exercises in the studies included in our analyses probably applied different loads across the femoral neck and other hip regions.37,40Our subanalyses at the femoral neck also demonstrated that bone loss was attenuated by exercise in adults aged≥60 years but not in those aged<60 years.Exercise may,therefore, be particularly important for older adults with overweight and obesity when undergoing diet-induced weight loss, and this is supported by the evidence that weight loss is associated with increased likelihood of fractures in older populations.5,6
Exercise intervention duration is also important for detecting true physiological changes in aBMD.A normal bone remodeling cycle is approximately 120-200 days in cortical and cancellous bone,respectively.41,42Although primary mineralization,which accounts for ~60%-70%of total mineralization, occurs during the first 3 weeks following the initial deposition of collagen, secondary mineralization can continue for over a year.43,44Therefore, it is possible that some RCTs included in our analyses were too brief to observe true physiological changes in aBMD using DXA,and longer-term studies should be conducted in the future.One study that found the most substantial differences between groups at all skeletal sites reported that only 12 weeks of treadmill walking exercise performed at 70% maximum heart rate during diet-induced weight loss significantly increased lumbar spine (+6.9%) and total hip aBMD (+5.2%), but decreased radial aBMD(-4.3%).15In contrast,the diet-induced weight-loss group that did not exercise experienced significant aBMD losses at all skeletal sites.15It should be noted that this study did not report any short-term precision data (coefficient of variation) for DXA measurements, which calls into question the validity of these findings.Furthermore, these aBMD improvements are greater than those that occur in response to current osteoporosis pharmacotherapies such as zoledronic acid infusions,which have been reported to increase lumbar spine and femoral neck aBMD by approximately 4%compared with placebo after 12 months in postmenopausal women with low aBMD.45Nevertheless, our leave-one-out sensitivity analysis demonstrated that the results of Hosny et al.15did not affect our overall findings.
The net difference in femoral neck aBMD changes between the exercise plus diet-induced weight loss group and the diet-induced weight loss alone group was ~1%, which may not be clinically meaningful in terms of fracture risk reduction.A recent study by Black et al.46reported that a 2.13%net difference in total hip aBMD change between treatment and placebo groups would significantly reduce the risk of having a nonvertebral fracture.It is possible that net differences in aBMD changes between those undergoing diet-induced weight loss with and without exercise would continue to increase over time; however, this would likely depend on the magnitude of weight loss as well as the osteogenic potential of prescribed exercises.A well-known training principle that should be considered when designing osteogenic exercise regimens is the principle of progressive overload.47Progressive overload is important because loads or strains imparted on bone via muscle or gravitational forces must exceed typical loading patterns experienced during everyday activities for osteogenesis to occur; and as bone adapts, loading stimulus should be increased progressively.48,49Other important overload training characteristics to consider in addition to load magnitude when prescribing osteogenic exercise include load pattern (distribution), rate, and frequency.50-52In the studies included in our review, all resistance exercise prescriptions during diet-induced weight loss were progressive; however, most did not include moderate- to high-impact exercises (e.g., hopping and jumping)performed at sufficient intensities to elicit significant skeletal adaptations,which might explain why there were no marked differences in aBMD changes between groups.
Our finding that there were no net differences in changes in lumbar spine aBMD between the groups in our study could be attributed to several factors.First,the diet-induced weight loss group did not experience significant bone loss at the lumbar spine,nor did the exercise plus diet-induced weight loss group.Given that there was no significant lumbar spine bone loss,it is not surprising that there were no net differences between groups at this site.Second, lumbar spine aBMD might not have improved in response to exercise due to the lack of training specificity; for instance, all aerobic exercise interventions in our meta-analysis involved walking, which has been reported in other meta-analyses to have a small beneficial effect on femoral neck aBMD, but not on lumbar spine aBMD.53,54Interestingly,our subgroup analyses indicated that resistance exercise also had no effect on lumbar spine aBMD,which could be due to the type, or frequency, of resistance exercises included in some training regimens.Thus, targeted exercises might have led to greater improvements in aBMD at this site and significant net differences between groups.Finally, it is possible that lumbar spine aBMD estimates were influenced to some degree by the imaging modality used.Previous studies have demonstrated that factors such as tissue depth or fat layering can decrease the precision and reproducibility of DXA-derived lumbar spine aBMD estimates.55-57Three-dimensional imaging technology such as CT has similar limitations but to a lesser degree57and may be particularly useful in weight-loss studies involving obese populations.
There are several ways that body composition changes can affect aBMD and fracture risk.There is a biomechanical and physiological link between bone and muscle; skeletal muscle applies forces directly to the skeleton during locomotion, and both bone and muscle engage in auto- and paracrineregulation.58,59Lean mass losses increase fracture risk more than fat mass losses.A recent study by Leslie et al.60showed that in 9622 middle-aged and older adults, prior loss of lean mass,but not fat mass,independently predicted major osteoporotic and hip fractures.The Concord Health and Aging in Men Project also demonstrated that increases in appendicular lean mass relative to fat mass change residuals over 2 years decreases the hazard for hip fracture (hazard ratio=0.68(95%CI:0.47-0.99)per 1 SD increase)from 2.0-8.8 years in older men.61Despite these findings, losing fat mass can adversely affect aBMD through subsequent decreases in mechanical loading and also by altering hormonal profiles,and it might also increase fracture risk via the loss of soft tissue padding.28,62-64In our meta-regression analyses, changes in body weight or fat mass or lean mass did not influence effect size estimates at any skeletal site.These findings need to be interpreted with caution, given the small number of studies that were included in our analyses;however,the nonsignificant results in our study may also be attributed to several other factors.First, DXA does not directly measure muscle mass;instead,it derives lean mass measurements that include organs and fibrotic tissues,a process that can lead to imprecise muscle mass estimates.65Second,significant changes in body composition have been shown to affect aBMD estimates,55-57which could explain why accounting for fat mass losses also did not influence effect size estimates.Finally,given the small number of studies included in our analyses, we could not perform meta-regression in trials that were more than 6 or 12 months in duration, which might also explain the small effect sizes we observed.Future studies should be of appropriate duration(ideally ≥12 months)and should utilize more precise measures of muscle mass and body composition in order to better understand how body composition changes during exercise and weight loss affect aBMD.
Our study has a number of limitations.First, we did not analyse individual patient data for any study, and because of the small number of included articles,we were unable to assess publication bias.There were only 9 articles that met the inclusion criteria for our systematic review,which limited our ability to determine how factors such as osteoporosis and menopause status and diet and exercise adherence influenced aBMD changes at the various skeletal sites.Also,some of our subgroup analyses might not have had sufficient power to detect significant net differences in aBMD changes between groups at certain skeletal sites.Two studies included populations with poor metabolic health,13,21which may have influenced aBMD changes in response to exercise and diet-induced weight loss.All but 1 study21were limited to aBMD assessments, and it is possible that 3-dimensional imaging may have detected earlier changes in bone microarchitecture.
5.Conclusion
Our findings suggest that diet-induced weight loss leads to greater femoral neck bone loss compared to diet-induced weight loss plus exercise.Bone loss at the total hip, lumbar spine, and radius was not attenuated by exercise during diet-induced weight loss.However,due to heterogeneity in the design of the exercise interventions and the failure to adhere to exercise recommendations specific to the prevention and management of osteoporosis, additional long-term RCTs using osteogenic exercise interventions during diet-induced weight loss,particularly in populations most at risk for falls and fractures, are warranted.Future studies should also use 3-dimensional imaging to further understand how the combination of exercise and weight loss interventions affects bone microstructure and geometry and estimates of whole bone strength.
Acknowledgments
JM is supported by a Research Training Program Scholarship; DS is supported by an Australian National Health and Medical Research Council (NHMRC) RD Wright Biomedical Career Development Fellowship (GNT1123014) and an NHMRC Investigator Grant(GNT1174886).
Authors’contributions
JM drafted the manuscript, performed data analysis, and was responsible for manuscript revision and preparation; PJ and AJR performed data analysis and were responsible for manuscript revision and preparation; AZ, RMD, BdC, PRE,and DS were responsible for manuscript revision and preparation of this review.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jshs.2021.05.001.
 Journal of Sport and Health Science2021年5期
Journal of Sport and Health Science2021年5期
- Journal of Sport and Health Science的其它文章
- A systematic review of running-related musculoskeletal injuries in runners
- Most ankle sprain research is either false or clinically unimportant:A 30-year audit of randomized controlled trials
- Effects of plyometric vs.resistance training on skeletal muscle hypertrophy:A review
- Pacing profiles and tactical behaviors of elite runners
- Adverse associations of sedentary behavior with cancer incidence and all-cause mortality:A prospective cohort study
- Physical activity and its relationship with COVID-19 cases and deaths:Analysis of U.S.counties
