Relationship of proprioception,cutaneous sensitivity,and muscle strength with the balance control among older adults
Qipeng Song,Xinyn Zhng,Min Mo,Wei Sun,Cui Zhng,Yn Chen,Li Li
a College of Sports and Health,Shandong Sport University,Jinan 250102,China
b Department of Statistics and Analytical Sciences,Kennesaw State University,Kennesaw,GA 30144,USA
c Department of Allied Health,the University of North Carolina at Chapel Hill,Chapel Hill,NC 27599,USA
d Lab of Biomechanics,Shandong Institute of Sport Science,Jinan 250102,China
e Key Laboratory of Exercise and Health Sciences of Ministry of Education,Shanghai University of Sport,Shanghai 200438,China
f Department of Health Sciences and Kinesiology,Georgia Southern University,Statesboro,GA 30460,USA
Abstract Background: Balance impairment is one of the strongest risk factors for falls.Proprioception, cutaneous sensitivity, and muscle strength are 3 important contributors to balance control in older adults.The relationship that dynamic and static balance control has to proprioception,cutaneous sensitivity,and muscle strength is still unclear.This study was performed to investigate the relationship these contributors have to dynamic and static balance control.Methods:A total of 164 older adults(female=89,left dominant=15,age:73.5±7.8 years,height:161.6±7.1 cm,weight:63.7±8.9 kg,mean±SD)participated in this study.It tested the proprioception of their knee flexion/extension and ankle dorsi/plantarflexion,along with cutaneous sensitivity at the great toe,first and fifth metatarsals,arch,and heel,and the muscle strength of their ankle dorsi/plantarflexion and hip abduction.The Berg Balance Scale (BBS) and the root mean square (RMS) of the center of pressure (CoP) were collected as indications of dynamic and static balance control.A partial correlation was used to determine the relationship between the measured outcomes variables (BBS and CoPRMS)and the proprioception,cutaneous sensitivity,and muscle strength variables.Results: Proprioception of ankle plantarflexion (r=-0.306, p=0.002) and dorsiflexion (r=-0.217, p=0.030), and muscle strength of ankle plantarflexion(r=0.275,p=0.004),dorsiflexion(r=0.369,p<0.001),and hip abduction(r=0.342,p<0.001)were weakly to moderately correlated with BBS.Proprioception of ankle dorsiflexion(r=0.218,p=0.020)and cutaneous sensitivity at the great toe(r=0.231,p=0.041)and arch (r=0.285, p=0.002) were weakly correlated with CoP-RMS in the anteroposterior direction.Proprioception of ankle dorsiflexion(r=0.220, p=0.035), knee flexion (r=0.308, p=0.001) and extension (r=0.193, p=0.040), and cutaneous sensitivity at the arch (r=0.206,p=0.028)were weakly to moderately correlated with CoP-RMS in the mediolateral direction.Conclusion:There is a weak-to-moderate relationship between proprioception and dynamic and static balance control,a weak relationship between cutaneous sensitivity and static balance control,and a weak-to-moderate relationship between muscle strength and dynamic balance control.
Keywords: Body stability;Dynamic balance;Kinesthesia;Plantar sensation;Postural control
1.Introduction
Falls in older adults are a serious problem.They constitute the fifth leading cause of death among the age group,following common and severe illnesses such as cancer, heart disease,stroke,and respiratory disease.1Balance impairment is one of the strongest risk factors for falls among older adults.2-4In clinical practice and laboratory tests, performance-oriented functional mobility tests, such as the Berg Balance Scale(BBS) test and the center of pressure (CoP) test, are usually adopted to evaluate dynamic and static balance control,respectively.The BBS is widely used to determine the dynamic balance control of older adults through 14 balancerelated tasks, ranging from standing up from a sitting position to standing on 1 foot,5and is demonstrated to be successful in discriminating fallers from non-fallers.6A higher BBS score might indicate better balance control.7Likewise,the root mean square(RMS)of the CoP(CoP-RMS)while standing has been used in numerous studies as an indicator of static postural control.CoP-RMS is a time-domain “distance” measure, associated with either the displacement of the CoP from the central point of the stabilogram or the velocity of the CoP.8A decrease in the CoP-RMS might indicate improved postural control.9
Balance control involves integrating sensory input with information from the musculoskeletal systems.2Proprioception, cutaneous sensitivity, and muscle strength are 3 important contributors to balance control in older adults (Fig.1).Proprioception and cutaneous sensitivity, as primary components of the somatosensory system,10account for about 60%-70% of standing balance control, while visual and vestibular systems contribute the rest.11,12The ability of a person’s muscles to generate adequate force is also critical for maintaining balance control.13Together, proprioception,14cutaneous sensitivity,15and muscle strength16are the main elements that neural pathways utilize to control balance at the peripheral level.17,18When a disturbance occurs, muscle spindles are stretched while the muscle lengthens.This results in the generation of proprioceptive information on afferent fibers,which either form synapses with α motor neurons and interneurons or convey proprioceptive information to the sensorimotor cortex and the cerebellum.14Simultaneously,cutaneous mechanoreceptors convey pressure/vibration-related information to the central nervous system (CNS), aiding in spatial and temporal comparisons of the stimuli.15
Still, the relationship of proprioception, cutaneous sensitivity,and muscle strength to balance control remains unclear.Some studies showed that ankle proprioception was correlated with static balance control, as indicated by the single-leg stance time,19and CoP-RMS in both anteroposterior20and mediolateral21directions.On the contrary, other studies showed that ankle proprioception had no correlation with static balance control, as indicated by the CoP sway area and range.22One study demonstrated that cutaneous sensitivity in 9 plantar subareas had no relationship with dynamic balance control, as indicated by BBS,23while another study found that cutaneous sensitivity in the great toe has a relationship with dynamic balance control.24Most of the previous studies(e.g.,Spink et al.25)indicated that ankle plantarflexion muscle strength is correlated with static balance control,as indicated by body sway area.However, other studies indicated that static balance control,as measured by the CoP sway range,was not correlated with ankle plantar flexor and dorsiflexor muscle strength26or with leg muscle strength.27Previous studies mainly focused on single-joint proprioception and muscle strength when examining the relationship between these contributors and balance control.19,22,26Due to differences in participants and research protocols across studies,it has been difficult to determine the relative importance of these contributors to balance control.
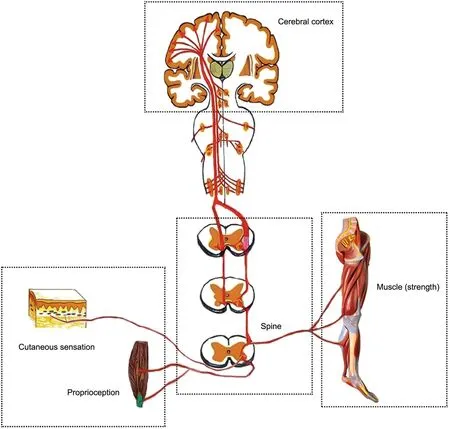
Fig.1.Schematic of neural pathways of postural stability.Proprioceptive and cutaneous mechanoreceptors detect physical deformation of the joints,muscles,or foot sole.Afferent information of nerve action potential is transmitted to spinal interneurons,whose facilitation level is modulated by the central nervous system,including the cerebral cortex,and then to α-motoneurons,which are connected to different muscle fibers.
Furthermore, most previous studies focused on static or dynamic balance separately.However,different sensory inputs or postural strategies may be adapted under static or dynamic balance control conditions.Studying the relationship these contributors have to static and dynamic balance control may reveal how their contributions manifest under specific circumstances.
Balance control involves a number of complicated neuromuscular activities,which allow individuals to make rapid and precise postural adjustments to maintain balance.28Therefore,investigating the relationship of proprioception,cutaneous sensitivity, and muscle strength to dynamic and static balance control might help identify individuals at risk of impaired balance control.Further understanding of these relationships can aid practitioners in choosing targeted physical exercise to improve balance control and to develop and implement personalized fall-prevention plans for older adults.This study aimed to investigate the relationship of proprioception,cutaneous sensitivity, and muscle strength to dynamic/static balance control in older adults.We hypothesized that proprioception,cutaneous sensitivity, and muscle strength are significantly related to dynamic/static balance control as measured by the BBS and the CoP-RMS.
2.Materials and methods
2.1.Participants
A total of 205 elderly participants were recruited by distributing flyers and providing presentations in local communities and nursing homes.The inclusion criteria were as follows: (1)age of 65 years or older and(2)independently ambulatory without the use of assistive devices.Individuals were excluded if they presented with (1) self-reported history or evidence of CNS dysfunction; (2) self-reported visual deficits; (3) selfreported dizziness,vertigo,or any other vestibular disorders;(4)self-reported psychological problems related to fall risks, such as fear of falling, anxiety, or depression; (5) evidence of foot sole ulcers; or (6) a score at or below the global cognitive impairment borderline (<24) as defined by Minithe-Mental State Examination.29Following the eligibility assessment, a total of 164 elderly participants (female=89, left dominant=15; age: 73.5 ± 7.8 years; height: 161.6 ± 7.1 cm;weight:63.7±8.9 kg,mean±SD)were enrolled and included in the final analysis.Human participation was approved by the Institutional Review Board of Shandong Sport University (No.19003)and was in accordance with the Declaration of Helsinki.
2.2.Protocol
The participants came into the Biomechanics Laboratory and were given an overview of the research and an opportunity to ask questions.Participants signed an informed consent form after all of their questions were answered to their satisfaction and before any supplemental information was collected.The supplemental information included the Mini-Mental State Examination and participants’ medical history sheet and was used to determine whether they would be included or excluded from the study.Proprioception, cutaneous sensitivity, and muscle strength were measured for all of the eligible participants.The BBS and balance control tests were then conducted.The data for this study were collected in Jinan, Shandong Province,China,from July to September 2019.
2.3.Proprioception
The proprioception thresholds for the ankle and knee on each participant’s dominant side were assessed using a proprioception test device (Fig.2A), which showed good testretest reliability (intraclass correlation coefficient (ICC) =0.74-0.94).30The proprioception test device collected the minimum angular motion that the patient is able to detect during knee flexion/extension and ankle dorsal/plantarflexion.The device consists of a box and a platform that can rotate within the frontal and sagittal planes.The platform is driven by 2 electric motors at an angular velocity of 0.4°/s.The movement of the platform can be stopped at any time by a hand switch controlled by the participant.An electronic goniometer in the device recorded the angular displacement of the platform.Each participant was seated on a height-adjustable chair with their foot placed on the platform.During the ankle proprioception test,the knee and hip joints were flexed at 90°,and the leg was perpendicular to the surface of the platform when the platform was placed in a horizontal position.30During the knee proprioception test, the lateral axis of the instrumentation was parallel to the mediolateral axis of the knee joint.The hip and knee joints were each positioned at 90°,and the ankle joint was in a neutral position.Approximately 50% of the weight of the subject’s lower extremity was resting on the platform, and a thigh cuff suspension system was used to control unwanted sensory cues from contact between the platform and the plantar surface of the foot.31The participant sat with their eyes closed and wore headphones with music playing in order to eliminate potential environmental visual and auditory stimulation.The participant was instructed to concentrate on their foot and to press the hand switch to stop the movement of the platform when they could sense motion;they were then asked to identify the direction of rotation.The motor was equipped to rotate with a random time interval ranging from 2 s to 10 s after the indication to start a trial.At least 5 trials were performed for each direction in order to reduce random measurement errors.32

Fig.2.Test illustrations:(A)the proprioception test using a proprioception test device,(B)the cutaneous sensitivity test with a set of Semmes-Weinstein monofilaments,(C)the muscle strength test using the IsoMed 2000 muscle strength testing system,and(D)the center of pressure test on a KISTLER force plate.
2.4.Cutaneous sensitivity
The cutaneous sensitivity of a participant’s dominant foot was tested while they laid supine on the treatment table with a set of Semmes-Weinstein monofilaments (North Coast Medical, Inc.,Morgan Hill,CA,USA)(Fig.2B),which showed good test-retest reliability (ICC = 0.83-0.86).33Cutaneous sensitivity includes the monofilament gauge at the great toe, first and fifth metatarsals, arch, and heel.Monofilaments of 6 different sizes were used in this study: 2.83, 3.61, 4.31, 4.56, 5.07, and 6.65;each of them applies 0.07 g,0.40 g,2.00 g,4.00 g,10.00 g,and 300.00 g of force when pressed into a C-shape(bent 90°).The filament size was log10 (10×force in milligrams).The filaments were applied randomly to the skin on the bases of the great toe,first and fifth metatarsals, arch, and heel.These applications were performed for 1 s and with 2 repetitions.Randomized null-stimuli were added to ensure that the participants could not anticipate the application of the filaments.Plantar sensitivity was determined by initially applying the thin filaments and progressing to the thicker filaments until the participants were able to detect the touch.34When they perceived the stimulation, participants were asked to provide a verbal response about the localization of the area tested.The sensitivity threshold was determined by the minimum monofilament gauge detected correctly.23A lower sensitivity threshold indicates better plantar cutaneous sensitivity.
2.5.Muscle strength
The muscle strength test was performed on each participant’s dominant lower extremity using the IsoMed 2000 muscle strength testing system (D.& R.Ferstl GmbH, Hemau,Germany) (Fig.2C), which showed good testretest reliability with respect to measuring lower extremity muscle strength (ICC = 0.77-0.98).35In this study, muscle strength consists of maximum joint torque (normalized by body mass)during ankle dorsal/plantarflexion and hip abduction.Ankle and hip muscle strength were tested because when a person steps in response to an external perturbation,the first attempt to return their body to its initial position comes from exerting their ankle or hip torque.36During the ankle muscle strength test,the participant was asked to lay in a supine position on the dynamometer bed with hips and knees in full extension.37The waist and thigh of the test leg were constrained with straps.The participant performed the ankle isokinetic muscle strength test with maximal exertion at an angular velocity of 30°/s, which has been used previously in related studies among older adults,38,39and had a good test-retest reliability(ICC=0.86-0.97).39Ankle plantarflexion started at 5° of dorsiflexion and stopped at 30° of plantarflexion,while dorsiflexion started at 30°of plantarflexion and stopped at 5° of dorsiflexion.Ankle plantarflexion and dorsiflexion torques were recorded as functions of time for each trial.During the hip muscle strength test,the participant was asked to lay on their side with the hip fully extended.They were then stabilized with belts strapped around the pelvis.The test leg was stabilized by straps with the knee fully extended, and the other leg was stabilized on the bed with the knee slightly flexed.The participant was instructed to abduct the tested leg with maximal exertion at an angular speed of 30°/s.Hip abduction motion started from 0°hip abduction and stopped at 30°hip abduction.Hip abduction torque was recorded as a function of time for each trial.The mean value of 3 successful trials from each direction was used for data analysis.The participant had at least a 2-min break between 2 consecutive trials.
2.6.BBS
The BBS test was adopted to evaluate dynamic balance control because it showed excellent test-retest reliability among older adults (ICC = 0.97).40A stopwatch (HS-70w;Casio, Tokyo, Japan), a 15-inch-long ruler, two 16-inch-high chairs (one with armrests, one without armrests), and an 8-inch-high stool were used to perform the evaluation.The balance assessment consisted of 14 subtests performed in the following order: sit to stand, stand unsupported, sit unsupported,stand to sit, transfers, stand with eyes closed, stand with feet together, reach forward with an outstretched arm, retrieve object from the floor, turn to look behind, turn 360°, place alternate foot on a stool, stand with 1 foot in front, and stand on 1 foot.Each task was scored on a 5-point scale (0-4)according to the quality of the performance or the time taken to complete the task, as determined by the test developers.A total score was recorded after all tests were completed.The maximum score for this assessment was 56.
2.7.CoP test
The participant was instructed to stand upright with eyes closed and feet together(no space between the feet)on a force plate (9287BA; KISTLER, Bern, Switzerland) for 120 s(Fig.2D).The Kistler force plate has moderate to good test-retest reliability when measuring CoP data(ICC=0.62-0.89).41To make the support base consistent for each participant, they were asked to stand with a narrow base26and to close their eyes in order to exclude the influence of the visual system.In order to reduce the volume of data, the study used the vertical ground reaction force data from between 60 s and 90 s of the standing trials, allowing participants to stabilize at the beginning of the trial,after stepping on the force plate,and eliminating subtle perturbations closer to the end of the trial.42The participant was instructed to look straight ahead with arms down beside their trunk in a comfortable position and to stand still during the entire testing session.Ground reaction force data were collected at a sample rate of 1000 Hz.Each participant had 3 successful trials(defined as a trial in which the participant holds the standing position as instructed without assistance and the data are collected appropriately).Each participant had at least a 1-min break between 2 consecutive trials.The output data were the position of CoP in the anteroposterior and mediolateral directions.The CoP data were filtered by a fourth-order low-pass Butterworth filter with a cut-off frequency of 12 Hz.The filtered data were used to calculate the RMS of the CoP displacement (in millimeters)for each participant by using the following equations:
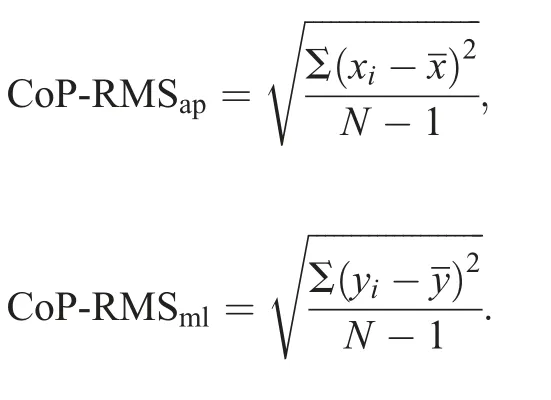
In these equations,andare the mean positions of CoP in the anteroposterior(ap)and mediolateral(ml)directions.
2.8.Data analysis
The statistical analysis involved descriptive analysis and partial correlation.Mean and SD were summarized for all measured variables in balance control,proprioception,cutaneous sensitivity,and muscle strength.Then,a partial correlation was used to determine the relationship of the measured outcomes variables from the category of balance control (BBS,CoP-RMSap, and CoP-RMSml) with each of the proprioception, cutaneous sensitivity, and muscle strength variables,while controlling for the covariates, age, height, and weight.Ninety-five percent confidence intervals (95%CIs) of the correlation coefficients were calculated.The thresholds for the correlation coefficient (r) were as follows: trivial: 0-0.1;weak: >0.1-0.3; moderate: >0.3-0.5; strong: >0.5.43All analyses were conducted in SPSS Version 24.0 (IBM Corp.;Armonk, NY, USA) and SAS Version 9.4 (SAS; Cary, NC,USA).Significant level was set at α=0.05.
3.Results
The descriptive characteristics are shown for all the variables in Table 1.Mean, SD, minimum, and maximum are reported for balance control, proprioception, cutaneous sensitivity,and muscle strength.
The results for partial correlation outcomes of the BBS,CoP-RMSap,and CoP-RMSmlwith each of the proprioception,cutaneous sensitivity,and muscle strength variables are shown in Table 2.Both ankle plantarflexion (moderate,r=-0.306,95%CI:-0.484 to-0.092,p=0.002)and dorsiflexion(weak,r=-0.217,95%CI:-0.438 to-0.015,p=0.030)were correlated with BBS.However, ankle dorsiflexion was weakly correlated with CoP-RMSml(r=0.220, 95%CI: 0.021-0.482,p=0.035).Neither knee flexion nor extension proprioception were correlated with BBS, though both correlated with CoPRMSml—moderately for flexion (r=0.308, 95%CI: 0.073-0.509,p=0.001)and weakly for extension(r=0.193,95%CI:0.003-0.429,p=0.040).Proprioception of knee flexion was also weakly correlated with CoP-RMSap(r=0.218, 95%CI:0.023-0.416,p=0.020).
None of the cutaneous test results were correlated with BBS.The cutaneous sensitivity at the great toe was weakly correlated with CoP-RMSap(r=0.231, 95%CI: 0.017-0.441,p=0.041).The cutaneous sensitivity at arch was weakly correlated with CoP-RMSap(r=0.285, 95%CI: 0.095-0.442,p=0.002) and CoP-RMSml(r=0.206, 95%CI: 0.024-0.423,p=0.028).No other correlation was detected between the cutaneous sensitivity and COP movements(Table 2).
All 3 muscle strength test results were correlated with BBS but not with the COP movements.Partial correlations between BBS and ankle plantarflexion,dorsiflexion,and hip abduction,respectively, were weak (r=0.275, 95%CI: 0.119-0.419,p=0.004),moderate(r=0.369,95%CI:0.208-0.509,p<0.001),and moderate(r=0.342,95%CI:0.149-0.482,p<0.001)(Table 2).
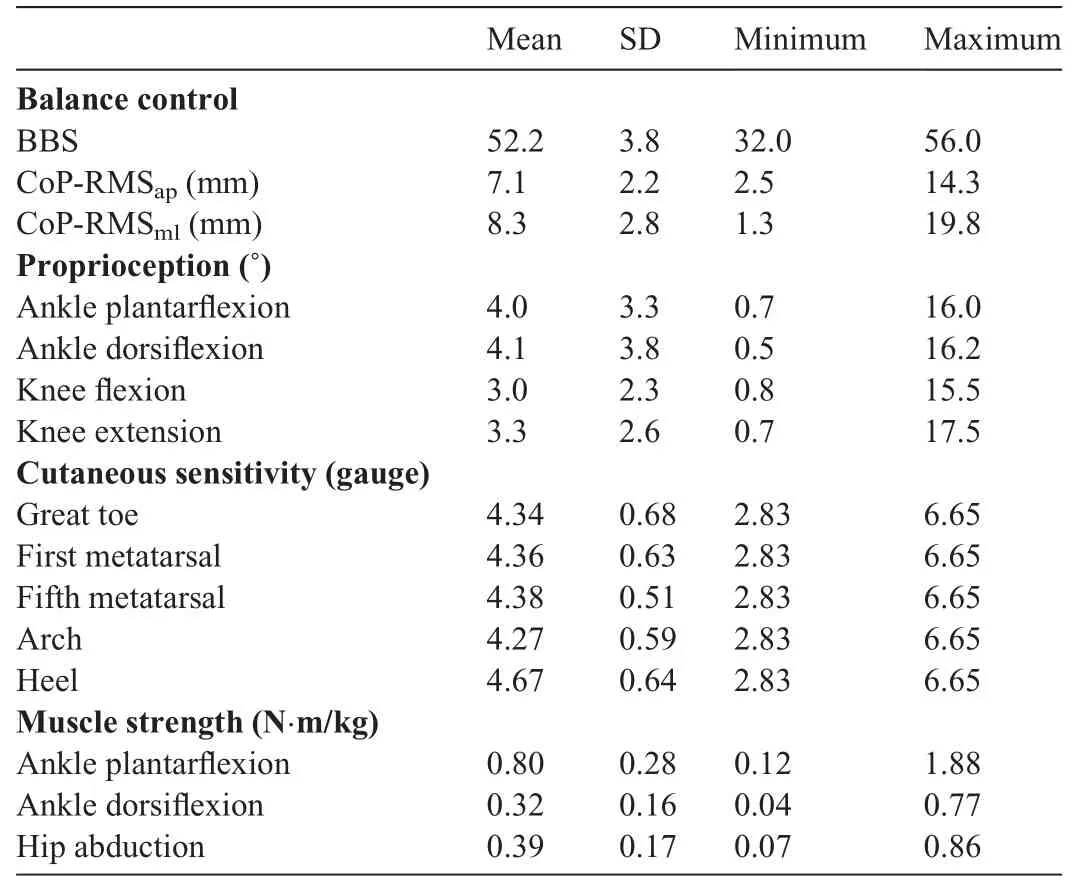
Table 1Descriptive characteristics of the outcome variables.
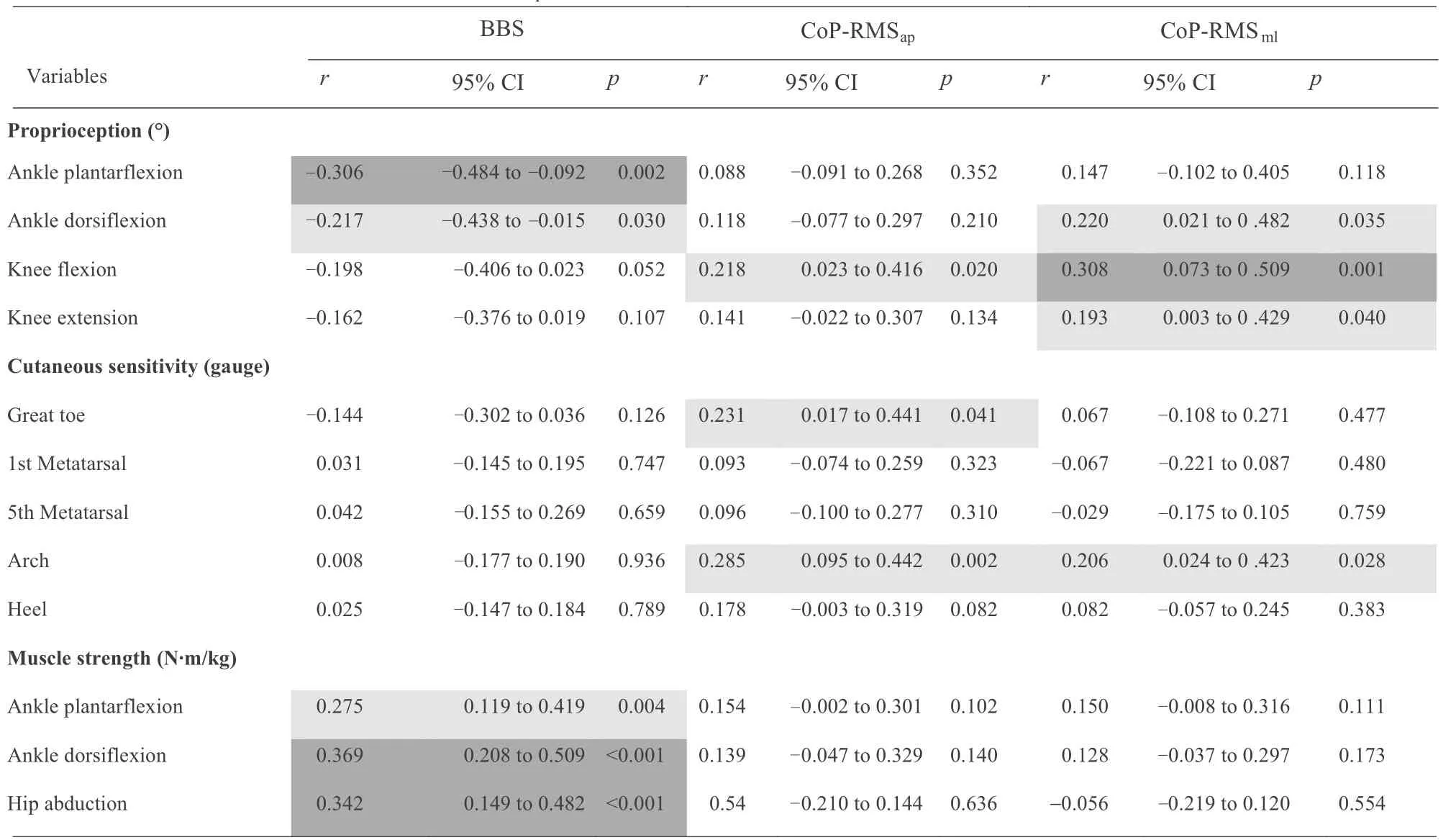
Table 2Partial correlation outcomes of the BBS,CoP-RMSap,and CoP-RMSml with proprioception,cutaneous sensitivity,and muscle strength variables.
4.Discussion
This study used partial correlation to investigate the relationship of proprioception, cutaneous sensitivity, and muscle strength with dynamic/static balance control,as represented by BBS and CoP-RMS.The outcomes partially supported our hypothesis that proprioception is correlated with dynamic and static balance control, that cutaneous sensitivity is correlated with static balance control, and that muscle strength is correlated with dynamic balance control.The mean and range of outcomes we collected in this study were similar to and comparable with previous studies that investigated proprioception,32,44cutaneous sensitivity,45and muscle strength46among healthy older adults.
The results showed that proprioception is related to both dynamic and static balance control,which was consistent with the majority of previous studies.19,21While our results did disagree with those of one previous study,22the conflict may be explained by the age difference among participants (Amin’s study recruited young athletes who averaged 24.67 years of age).Proprioception is important for smooth and coordinated movements, maintenance, and regulating balance control.47A previous study measured the postural sway in 74 healthy subjects from different age groups and found that all of the groups were more dependent on proprioception than on vision tomaintain balance control.48That is because the mechanoreceptors (i.e., muscle spindles and Golgi tendon organs) in and around joints provide important spatial and temporal afferent information regarding the positions and movements of body segments as well as information about the space between body segments.49,50Mechanoreceptor afferent information is transmitted to the CNS, and these receptors, in turn, are organized and managed in various high-order areas; motor control commands are sent to relevant muscles around the joint to ensure effective coordinated movement.50
It needs to be emphasized that people with impaired static balance control, when compared to people whose static balance control is not impaired, may rely more heavily on proprioception for their dynamic postural control.51This means that proprioception may be even more important for individuals with static postural impairment, which may become a problem for older adults as proprioception sensitivity has been shown to decrease with age.For example, the ankle joint proprioception threshold is 47.5% higher among older adults than younger adults.52Given that this study confirmed the relationship of proprioception to both dynamic and static balance control,exercises that are typically suggested to improve proprioception, such as Tai Chi,32backward walking,53or exergaming,54could be used to improve proprioception functions among older adults.
Our outcomes indicated that muscle strength was correlated with dynamic balance control but not with static balance control.Our observations agreed with reports from most previous studies26,27with the exception of one that had shown no relationship between muscle strength and dynamic balance control.25The differences between that study and the present one could be explained in the following ways: First, their study collected data on muscle strength during isometric contraction instead of on muscle strength during isokinetic contraction as our study did.It can be inferred that dynamic balance control is better reflected by isokinetic contraction because both processes involve dynamic muscle contraction;isometric contraction, on the other hand, involves static muscle contraction.Second,the participants they recruited(65-95 years)are older than the participants we recruited(65-82 years),which is relevant due to the fact that sensory impairment increases with aging.55Muscle would not be recruited if sensory inputs were impaired,thereby failing to provide the necessary information for the body to engage process of balance control.56
The aging process results in a reduced ability to develop maximal and explosive force.57In general,a 40%reduction in lower body muscle strength has been reported among older adults than young healthy adults.58Unfortunately, it is believed that a decline in muscle strength is one of the leading causes of balance control impairment and, thus, increases the risk of falling.In this study,we observed that muscle strength was related to BBS, which is often used to evaluate balance control and mobility impairment in older adults.59The results of a previous study contradicted our findings by showing that muscle strength does not affect falling or risk of falling in older women, particularly those who can function independently.60This conflict may be due to the fact that their study measured knee flexor and extensor muscle strength, while our study measured ankle dorsiflexor and plantar flexor along with hip abductor muscle strength.Ankle and hip functions are vital in returning the body to equilibrium after destabilization.36Furthermore, only women were included in their study, while our study included both men and women.
In contrast to proprioception, no relation was detected between cutaneous sensitivity and dynamic balance control.Our outcomes agreed with one study, which indicated that cutaneous sensitivity was not correlated with BBS,23but disagreed with another, which argued that cutaneous sensitivity in the great toe has some relationship to dynamic balance control.24Unlike ours, however, the latter study used only one monofilament (5.07 g, 10 g) to get a cutaneous tactile score.Peripheral sensory signals transmit along with different types of sensory neurons, for example, large Type Ia and II sensory neurons responsible for proprioceptive sensitivities,and smalldiameter Type III sensory neurons for cutaneous sensitivities.10Type III sensory neurons are slower and weaker than type Ia and II sensory neurons,10so it makes sense that dynamic balance control relies more on proprioceptive signals than cutaneous signals.
Furthermore, alternative sensory information sources can be used to compensate for individuals who have impairments with particular sensory input.The CNS uses one type of sensory stimulus that is coupled with the control of balance control to compensate for another weakened sensory input.10Cutaneous sensitivity is not a primary function for balance control because it can be compensated for by proprioceptive61or visual information.62Therefore, it could be inferred that older adults depend more on proprioception than on cutaneous sensitivity to control dynamic balance.Other studies that supported our observations report that cutaneous sensitivity is not related to dynamic balance control or falls and point out that the loss of sensitivity may be compensated by other sensorial systems.23,61
Our outcome agrees with those of previous studies63that found cutaneous sensitivity but not muscle strength to be related to static balance control.Less muscle strength is required to maintain balance when an individual is standing still than during functional mobility.In this circumstance, the function of muscle strength is limited.Even when a person is standing still, the position of CoP changes according to the subtle variation of ground reaction forces; the perception of forces under the feet during the stance could be used to generate an internal estimate of the body center of mass location.64Cutaneous sensitivity afferents could provide valuable feedback to the CNS regarding ankle torque production, weight transfer, and limb loading.64From this viewpoint, cutaneous sensitivity affects static balance control.Given that most of the falls occur when the body is moving rather than standing still,65our outcomes showed that the effects of cutaneous sensitivity are limited within static balance control.
Our outcome is supported by a previous study which found that the arch is the most sensitive region of the foot sole56and indicated that it was related to CoP-RMS in both anteroposterior and mediolateral directions.Another study pointed out that the arch was the thinnest and softest region, followed by the great toe, the fifth metatarsal, and the heel.66Considering that is the same order of foot sole cutaneous sensitivity found in our study,our results support the idea that cutaneous sensitivity is partially influenced by mechanical properties of the skin.66Furthermore, our outcomes indicated that foot sole regions with better sensitivity, like the arch and great toe,had a closer relationship with static balance control.
This study has several limitations.First, this study only examined the relationship of proprioception,cutaneous sensitivity, and muscle strength with balance control.Other contributors, such as CNS, visual, vestibular, or cognitive functions,could also influence balance control.Second, an intact sensory system is thought to provide accurate information to assist balance control.Still, it has been established that alternative sensory information sources can be used to compensate for those that are damaged/impaired.In this regard,although we revealed no relationship between specific variables and balance control(e.g., cutaneous sensitivity and dynamic balance control), the relationship may change when other balance control subsystems/contributors are impaired/damaged.Third, all the participants in this study were recruited from the same city,indicating that they had similar backgrounds; thus, subgroup analyses are recommended to determine the effectiveness of interventions among people with different characteristics.Fourth,older adults have different capabilities for the range of motion at the ankle.A fixed degree of range of motion may limit the ability to produce force at the ankle and hip joints due to the relationship between muscle length and force.
5.Conclusion
There are weak-to-moderate relationships between proprioception and dynamic and static balance control, a weak relationship between cutaneous sensitivity and static balance control, and a weak-to-moderate relationship between muscle strength and dynamic balance control.
Acknowledgments
This project was funded by Shandong Province Youth Innovative Talent Induction Program (grant number 2019-183),and the National Key R&D Program of China(2018YFC2000600).The authors would like to thank Huifen Zheng, Hengshuo Zhang, Xiao Gao, Xiu Hu, Fang Liu, and Teng Zhang, graduate students at Shandong Sport University,for participating in the experiment or data acquisition for this work.
Authors’contributions
QS participated in the design of the study, contributed to data collection and data reduction/analysis; XZ participates in the design of the study, data reduction/analysis; MM and WS participated in the design of the study,contributed to data collection and data reduction/analysis;CZ contributed to data collection and interpretation of results of the study; YC participated the design and interpretation of results of the study; LL participated in the design of the study, contributed to data analysis and interpretation of results.All authors contributed to the manuscript writing.All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
 Journal of Sport and Health Science2021年5期
Journal of Sport and Health Science2021年5期
- Journal of Sport and Health Science的其它文章
- Association between physical activity and cardiovascular risk factors:Dose and sex matter
- Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2A-Smad2/3 in rats following myocardial infarction
- Associations of accelerometer-determined physical activity and sedentary behavior with sarcopenia and incident falls over 12 months in community-dwelling Swedish older adults
- Physical activity and its relationship with COVID-19 cases and deaths:Analysis of U.S.counties
- Adverse associations of sedentary behavior with cancer incidence and all-cause mortality:A prospective cohort study
- Exercise attenuates bone mineral density loss during diet-induced weight loss in adults with overweight and obesity:A systematic review and meta-analysis
