Structural,magnetic,and dielectric properties of Ni–Zn ferrite and Bi2O3 nanocomposites prepared by the sol-gel method∗
Jinmiao Han(韩晋苗),Li Sun(孙礼),†,Ensi Cao(曹恩思),Wentao Hao(郝文涛),Yongjia Zhang(张雍家),and Lin Ju(鞠林)
1College of Physics and Optoelectronics,Taiyuan University of Technology,Taiyuan 030024,China
2College of Physics and Electrical Engineering,Anyang Normal University,Anyang 455000,China
Keywords:Ni–Zn ferrite,Bi2O3,magnetic properties,nanocomposites
1.Introduction
Ferrite is a kind of material with great research value,which possesses a few good magnetic properties,namely,high electric resistivity,low coercivity,and negligible eddy current loss for high frequency electromagnetic wave propagation.[1–6]Therefore,a ferrite is suitable for making high-frequency weak current electromagnetic devices,such as filters,loud speakers,and so on.[1]
The chemical formula of a spinel ferrite is MFe2O4(such as NiFe2O4),which adopts the face-centered cubic crystal and each crystal cell contains eight molecular formulas.[3,7]In the spinel structure,oxygen ions are arranged approximately in a cubic close packing.Meanwhile,besides containing 32 oxygen ions,there are 32 octahedral positions and 64 tetrahedral positions as well in the crystal cell,among which 16 octahedral positions(B sites)and 8 tetrahedral positions(A sites)are filled by specific cations.[2,8,9]Depending on the potential applications,ferrite materials could have tailored values of magnetic parameters including coercive field(Hc),threshold field and ferromagnetic resonance band.[3]
As we know,the magnetic properties and microstructures of Ni–Zn ferrites are highly sensitive to some factors involved in the fabrication process(e.g.,sintering conditions,preparation method,and nature of the metal oxides including dopants and/or impurities).[2]Until now,in order to improve the physical properties of a nickel–zinc ferrite,researchers have been devoted to the study of nickel–zinc ferrites,among which the construction of the composite system or doping of nickel–zinc ferrites is the most extensive.[7,10–12]
Zhang et al.reported the dielectric,magnetic and magnetoelectric properties of Ni0.5Zn0.5Fe2O4–Pb(Zr0.48Ti0.52)O3composite ceramics prepared by the solid-state reaction method.XRD and SEM results showed that high dense composite ceramics without any foreign phases were obtained.The properties of the samples sintered at 1120°C and 1140°C showed similar behaviors,including phases,microstructure and dielectric,magnetic and magnetoelectric properties.The ceramics showed excellent dielectric and magnetic properties,which were stable in a large frequency range.[10]Lin et al.prepared laminated Ca(Zn1/3Nb2/3)O3(CZN)–Ni0.8Zn0.2Fe2O4(NZO)composites using the conventional solid-state sintering method.With increasing the NZO concentration,the diffraction peaks of NZO phase become strengthened gradually.At a lower frequency,the permittivity and dielectric loss of the CZN–NZO composites increase with the increasing NZO ratio.All the samples showed excellent magneto-dielectric properties in a wide frequency range from 10 MHz to 1 GHz.[11]Hu et al.investigated the composition,structural and magnetic properties of Co-doped Ni–Zn ferrite,which was prepared by the solid-state method.Moreover,the saturation magnetization(Ms)increased with increasing Co substitution and reaches the maximum value 135.5 emu/g at x=0.05.[12]Li et al.showed that structure and static magnetic properties of Tisubstituted Ni–Zn–Co ferrite thin films synthesized by the solgel process.The average grain size of Ti-substituted Ni–Zn–Co ferrite thin films increased with Ti substitution.The saturation magnetization gradually increased with the increase of Ti substitution when x≤0.06,and decreased when x>0.06.Besides,the coercivity initially increased with the increase of Ti substitution when x≤0.03,and decreased when x>0.03.[7]
In previous studies,Bi2O3was used to improve the low-temperature densification of Ni–Cu–Zn ferrites,[13]since Bi2O3could promote grain growth,enhance the density of the sample and reduce the porosity via liquid phase sintering.And Bi2O3could segregate at the grain boundaries as a liquid phase layer.Moreover,the addition of moderate content of Bi2O3can enhance the intensity of x-ray diffraction peaks of Bi-doped ferrites.[14]Gan et al.reported that ion substitution can adjust magnetic properties of M-type barium ferrite,while Bi2O3aids can lower the sintering temperature and improve ferrite densification.[15]It is noteworthy that influences of Bi2O3on microstructure and magnetic properties of MnZn ferrite have been studied by Yu et al.The results indicate that Bi2O3promotes a solid-state reaction and grain growth,reduces porosity and enhances density.In the MnZn ferrite doped with 0.06% Bi2O3,the grains are most homogeneous,the pores are the least,the initial permeability and saturation magnetic induction are the maximal,but the coercivity is the minimal.[16]Gan et al.synthesized spinel Mg0.6Cd0.4Co0.05Fe1.95O4ferrite with Bi2O3dopant by using the LTCC technique.The results indicated that densification sintering results in ultra-low dielectric loss tanδεand magnetic loss tanδµ(tanδε≈0.003,tanδµ≈0.035).In addition,the samples presented enhanced magnetic properties,such as high saturation magnetization(approximately 37.94 emu/g)and appropriate coercivity(approximately 60.5 Oe)at 940°C.[17]Xu et al.synthesized Ni–Cu–Zn ferrites with Bi2O3–Nb2O5composite additives.Ni–Cu–Zn ferrites with low coercivity(Hc=94.75 A/m)was obtained when 0.25 wt% Nb2O5–0.50 wt% Bi2O3was added.It is proved that the improvement of compactness can enhance magnetic properties even though grain growth was suppressed.[18]Therefore,we speculated that the combination of Bi2O3and Ni–Zn ferrite materials can enhance the density and improve the magnetic and dielectric properties of a Ni–Zn ferrite.
In our current work,Ni0.5Zn0.5Fe2O4–x%Bi2O3(x=0,2.5,5)nanocomposites with different Bi2O3molar ratios were prepared by the sol-gel method.The microstructure,magnetic and dielectric properties were investigated.And the dielectric and magnetic properties of Ni–Zn ferrite were found to be improved by the addition of Bi2O3.These results indicated that Ni0.5Zn0.5Fe2O4–x% Bi2O3(x=0,2.5,5)ferrites might be a potential candidate for high-density recording media applications.[19]
2.Experimental details
2.1.Fabrication
The mixing of Bi2O3and Ni0.5Zn0.5Fe2O4composites were prepared by the sol-gel auto-combustion method. The starting materials used were nickel nitrate hexahydrate[Ni(NO3)2·6H2O,99%],zinc nitrate hexahydrate[Zn(NO3)2·6H2O,99%],bismuth nitrate pentahydrate[Bi(NO3)3·5H2O,99%],ferrite nitrate nonahydrate[Fe(NO3)3·9H2O,99%],polyethylene glycol[PEG 2000]and citric acid(C6H8O7,>99.5%).The metal nitrates weighted in precision and citric acid were dissolved in a beaker using minimum amounts of deionized water,keeping the citric acid to the metal ion ratio of 1:1.2 to form an aqueous solution.Then,the PEG and the ammonia were added into the mixed solution until the pH value was adjusted to 7.The mixture was stirred constantly at 80°C using a water bath to form a dry gel.To remove the organic matter,the resulting gel is heated to spontaneous combustion and ground to form a powder.Next,the removal of organics and nitrate components can be further achieved by calcining ferrite powder at 600°C for 2 hours at a heating rate of 10°C/min in a muffle furnace and subsequently cooled to room temperature at the same rate in the furnace.The calcined powder is thoroughly ground and then pressed at a pressure of 40 MPa to form pellets with a diameter of 10 mm and a thickness of 1.5 mm.These pellets were sintered at 1000°C for 5 hours and then cooled to room temperature to get the final ferrite ceramics.The temperature ramps for sintering are 5°C/min for heating and 10°C/min for cooling to 200°C,then cooling naturally to room temperature.In order to test the electrical properties,some samples were silvered.
2.2.Characterization
The x-ray diffraction(XRD)patterns of Ni–Zn ferrite and Bi2O3composites were acquired using Cu Kαradiation on an Ultima IV diffractometer.The fractured cross-sectional microstructures were investigated with a JSM-7100F field emission scanning electronic microscope(FE-SEM).Magnetic behavior of samples was determined using a vibrating sample magnetometer(VSM,BKT-4600 with a 1.5 T electromagnet).The dielectric properties of the Ni–Zn ferrite and Bi2O3composite samples deposited with silver were measured by an LCR meter Agilent E4980A with an oscillation voltage of 0.5 V over the frequency range from 20 Hz to 2 MHz.
3.Results and discussion
3.1.Structural properties
The crystal structure was determined by the XRD technique.Figure 1 shows the XRD patterns of composites samples of Ni0.5Zn0.5Fe2O4–x% Bi2O3(x=0,2.5,5)with different Bi2O3molar ratio.It can be clearly seen that the sample has good crystallinity.All the diffraction peaks of Ni0.5Zn0.5Fe2O4were well matched with the standard diffraction peaks of powder(JCPDS:08-0234),and no other impurity peaks appeared,indicating that the prepared samples had good spinel structure.When the Ni0.5Zn0.5Fe2O4is combined with Bi2O3,the two substances can coexist well without other impurities,and the newly formed peaks still had a typical spinel structure.Also,all the crystal plane indexes of corresponding diffraction peaks were assigned in Fig.1.
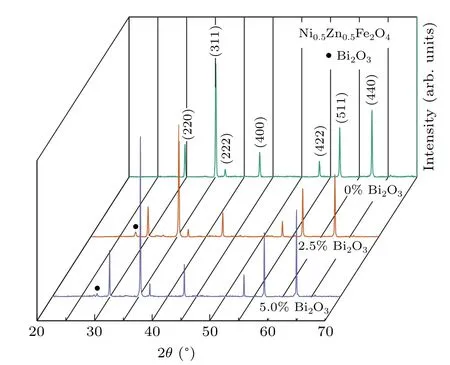
Fig.1.XRD patterns of Ni0.5Zn0.5Fe2O4–x%Bi2O3(x=0,2.5,5)ceramic samples sintered at 1000 °C.
Figures 2(a)–2(c)shows the FE-SEM diagram of the cross section of the above samples and the mathematical statistical distribution of grain size obtained from the FE-SEM diagram(Figs.2(d)–2(f)).It can be seen that the grain size distribution of all the samples is relatively uniform and the microstructure is relatively dense.The average grain size are respectively 0.26µm,0.28µm and 0.50µm with the increase of Bi2O3content.It is obvious that the Bi2O3is effective in the densification and enhancement of grain growth.When the molar ratio reaches 5%,the average grain size increases greatly,indicating that Bi2O3and Ni0.5Zn0.5Fe2O4combined well in this proportion.Bi2O3is a low melting point substance with a melting point of 820°C.In the sintering process of Ni–Zn ferrites and Bi2O3composites,liquid phase will be formed,thus reducing the reflected activation energy and further promoting the growth of grains.[14]
Moreover,some researchers have shown that the ferrite ceramic samples containing Bi2O3complex may form eutectic and deposit at grain boundary during sintering.[16]At the same time,the liquid phase formed by Bi2O3will accelerate the diffusion and separation of ions in the reaction process.Grains grow larger and grain boundaries become more obvious,the porosity is easy to escape from the particles along the grain boundary,thus reducing the porosity.[16]The formation of Bi2O3in the liquid phase can promote the growth of the grain size of the Ni–Zn ferrite and increase the density.[14,16]
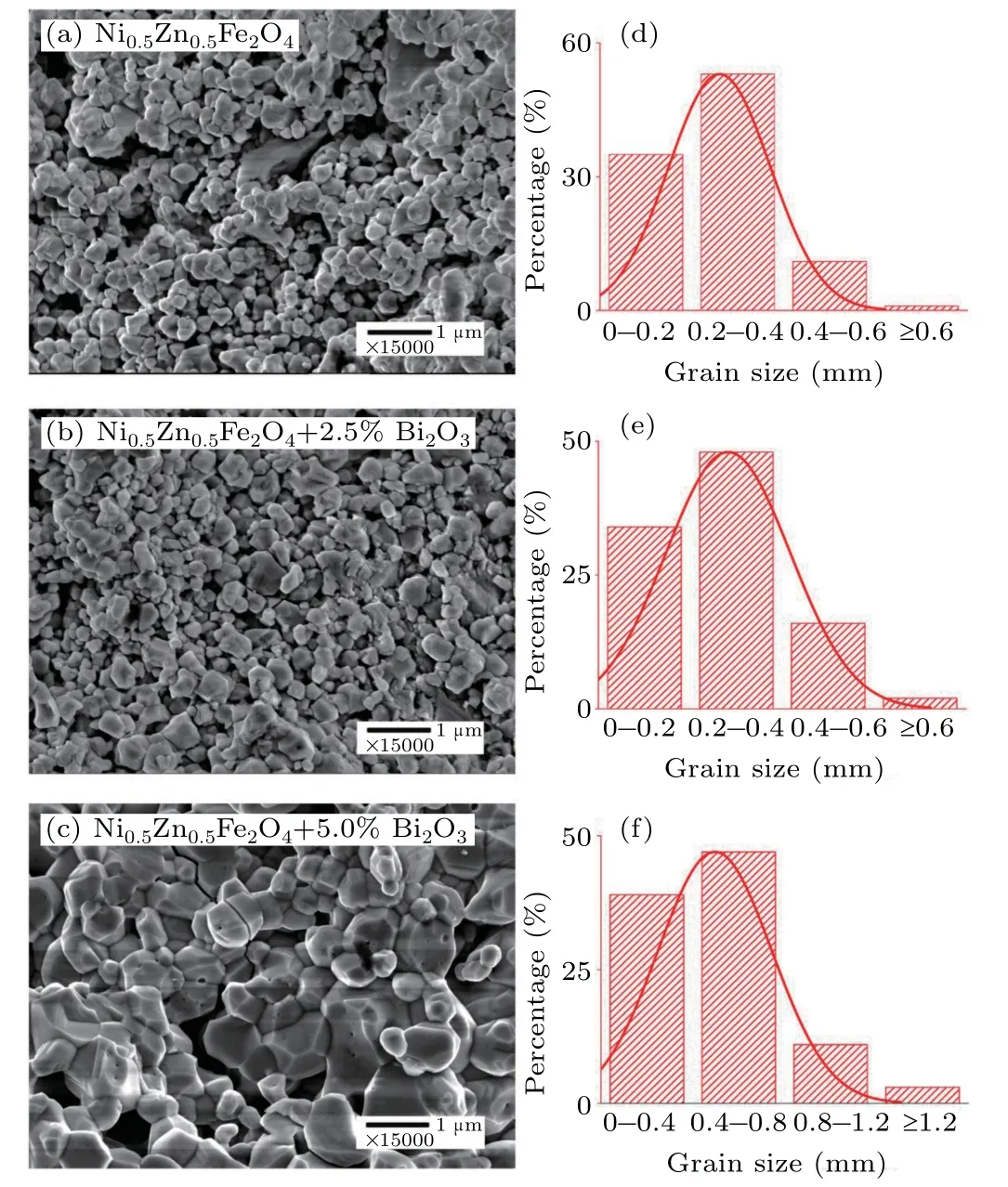
Fig.2.FE-SEM micrographs of Ni0.5Zn0.5Fe2O4–x% Bi2O3(x=0(a),x=2.5(b),x=5(c)).The graphs of(d)–(f)are histograms for grain size of Ni0.5Zn0.5Fe2O4–x%Bi2O3 samples(x=0(d),x=2.5(e),x=5(f)).
3.2.Magnetic properties
The room temperature magnetic hysteresis loops for different Ni0.5Zn0.5Fe2O4–x% Bi2O3(x=0,2.5,5)samples sintered at 1000°C are displayed in Fig.3.And Fig.4 showed variation of Msand Hcof composites samples of Ni0.5Zn0.5Fe2O4–x% Bi2O3(x=0,x=2.5,x=5)in different molar proportions sintered at 1000°C.All the samples showed good magnetic properties of the ferrite.With the increase of x,the Msand Hcof the sample decrease gradually.As seen,the addition of Bi2O3has a noticeable effect on the magnetic properties of samples Msdropped from 99.15 emu/g to 82.43 emu/g.And Hcdescend from 82.08 Oe to 63.83 Oe.As discussed previously,with the increase of Bi2O3content,the liquid phase reaction would be intensified,and the average grain size of the sample would increase gradually.It also means that Bi2O3mainly segregates and concentrates in the grain boundary regions of Ni–Zn ferrites,and Bi2O3would block the motion of the domain wall,which would cause the decrease of Ms.[16]In addition,the increase of non-magnetic Bi2O3also weakens the magnetization,leading to the decrease of Ms.[20]
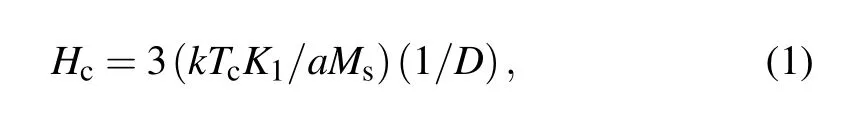
Similarly,the samples not combined with Bi2O3show that the maximum value of Hcat 1000°C.It could be explained by the relation as follows:[21]where K1is the first magneto crystalline anisotropy constant,Tcis Curie temperature,Msis saturation magnetization,a is lattice constant and D is grain size.In this study,the rate of change of Msis small,so it can be seen from the above equation that the average grain size is the main factor affecting the Hcvalue.Hcis inversely proportional to the grain size and a decrease in grain size increases Hc.A smaller grain size results in a high Hcdue to pinning of domain walls by grain boundaries that requires a higher energy for switching.[22]Therefore,the addition of Bi2O3will increase the Hcof the Ni–Zn ferrite.
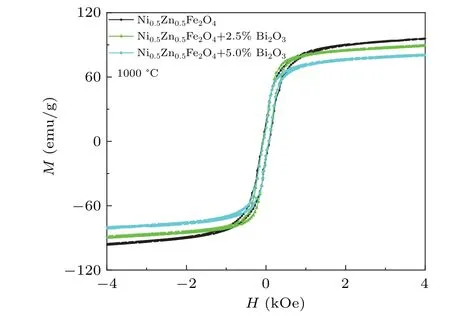
Fig.3. The room temperature magnetic hysteresis loops for Ni0.5Zn0.5Fe2O4–x% Bi2O3(x=0,2.5,5)ceramic samples sintered at 1000 °C.
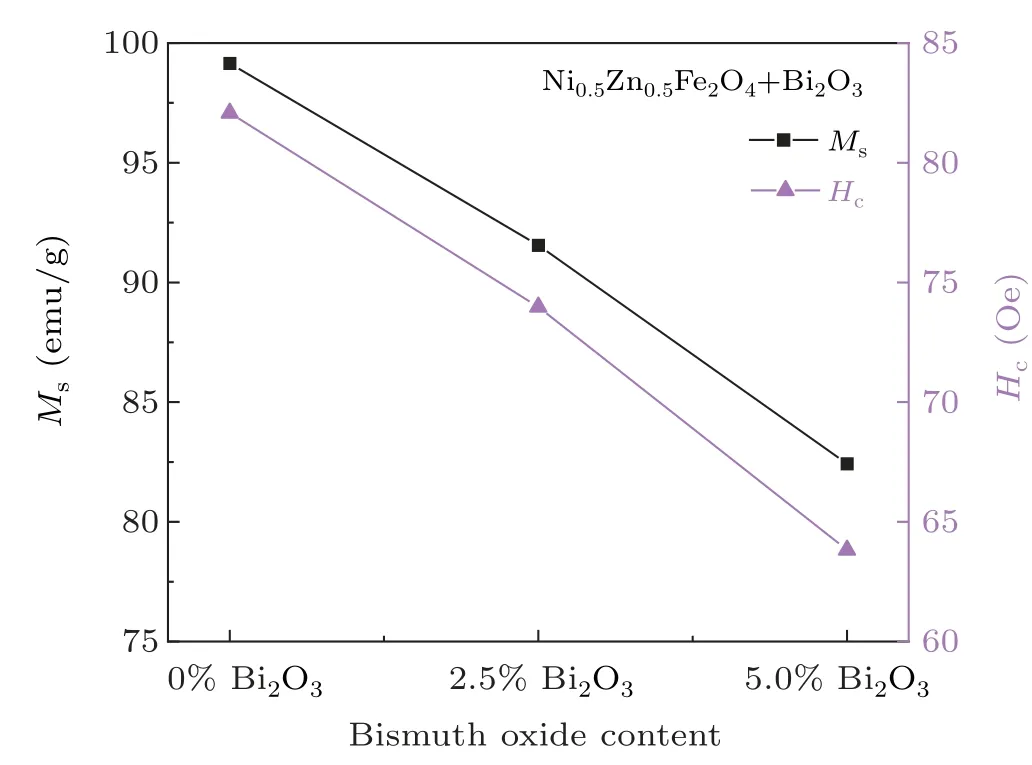
Fig.4.Variation of Ms and Hc of Ni0.5Zn0.5Fe2O4–x%Bi2O3(x=0,2.5,5)ceramic samples sintered at 1000 °C.
3.3.Dielectric properties
Figure 5 shows the variation of a real dielectric constant(ε′)and dissipation factor(tanδ)with frequency and temperature.It is obvious thatε′tends to decrease with increasing frequency between 20 Hz and 2 MHz.This is because in the hopping process of electrons,the accumulation of electrons in the grain boundary will lead to the increase of the grain boundary resistance,so theε′value is small at high frequencies.[23]The value of dielectric constant increases with the increase of temperature.The results show that the dielectric properties of the ferrite can be improved effectively when the Bi2O3is combined with nickel–zinc ferrite.Because the charge hopping is thermally activated process,dielectric polarization increases with increasing temperature resulting into an increase in dielectric constant.[24]The dielectric constant is usually affected by dipolar,electronic,ionic and interfacial polarizations.[25]And at high frequencies,electron polarization plays a major role,electronic contribution is weakly temperature dependent and is confirmed in Fig.5.
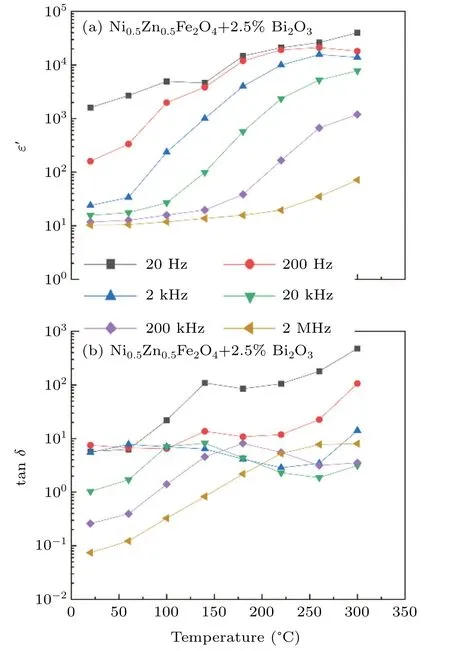
Fig.5.Different temperature frequency dependence ofε′and tanδof the prepared Ni0.5Zn0.5Fe2O4–2.5%Bi2O3 ceramic samples.
Besides,it can be seen from the Fig.5 that the tanδof different frequencies increases first and then decreases with the increase of temperature.As the frequency increases,tanδ decreases gradually because at low frequencies,the application field frequency is equal to the hopping frequency of the current carrier.The hopping frequency is inconsistent with the applied frequency in the high frequency,which results the low tanδ.[26]And another reason for the lower tanδat high frequencies may be the suppression of domain wall motion.[27]
Figure 6 shows the variation of real dielectric constant(ε′)and dissipation factor(tanδ)with frequency of Ni0.5Zn0.5Fe2O4–x% Bi2O3(x=0,2.5,5)ceramic sample.It is obvious that the relative dielectric constant of the sample increases with the increase of Bi2O3content.Since Bi2O3is a kind of material with low melting temperature.It is easy to transform into liquid during the sintering process,and then flows to the grain boundary to help and support the grain growth,which makes the sample denser and lower porosity.As a result,the dielectric constant increases.[17]
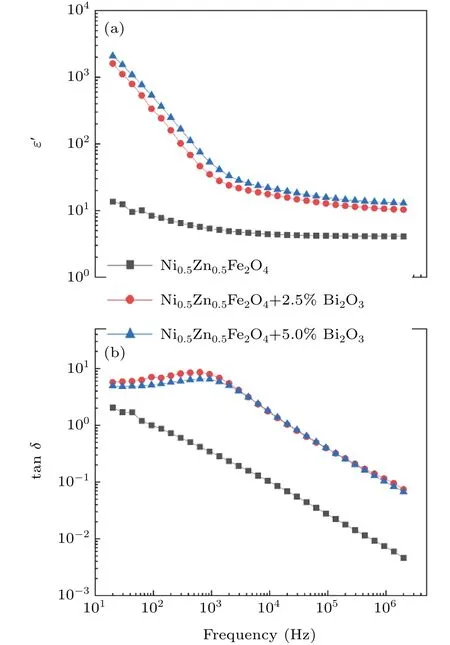
Fig.6.Room temperature frequency dependence ofε′and tanδof the prepared Ni0.5Zn0.5Fe2O4–x%Bi2O3(x=0,2.5,5)ceramic samples.
Compared with pure ferrite,theε′of the ferrite complex containing Bi2O3increased significantly at low frequency,but decrease rapidly as frequency increases.In the high frequency region,the contribution of the orientation mechanism of the inherent electric moment to polarization decreases to zero due to the high molecular mass and high inertia.[28]With low mass and low inertia,the electron is able to keep up with the changes of high-frequency electric field.Thus,at high frequencies,only electron polarization is left,leading to a sharp drop in the dielectric constant.This phenomenon shows dielectric dispersion at lower frequencies which might be due to the interfacial polarization.[19]According to the double-layer dielectric model,polycrystalline spinel ferrite and composites have a two-layer structure called low resistivity grain boundary and high resistivity grain boundary.[29]In composites,the low conductivity grain is surrounded by the high resistivity grain boundary,so the composites will form a non-uniform structure.[29]Because the electrical conductivity of Bi2O3and Ni0.5Zn0.5Fe2O4is different,the two materials form an uneven double layer when they are combined.Due to the polyphase structure and non-uniform internal conduction of the composite material,the relative dielectric constant is relatively large in the low frequency region.[29]As can be seen from Fig.6,the dielectric constant of the complex is larger at low frequencies.As the frequency gradually increases,ferrite ions will gradually aggregate at the grain boundary.Meanwhile,grain boundaries become more active and provide a rapid response at lower frequencies to the applied electric field,leading to high polarization and high resistivity grain boundary.[29]Therefore,a large number of ferrite ions accumulate at the grain boundary interface,resulting in a higher dielectric constant.[29]The dielectric constant of Ni0.5Zn0.5Fe2O4–Bi2O3composites are relatively stable at the frequency of 105Hz,which might be due to the inconsistency of electric dipole with the fast variation of electric field.[29]The tanδof the sample is shown in Fig.6.In the low frequency region,the tanδof the sample is high,and with the increase of frequency,the tanδdecreases gradually.In addition,ferrite composites containing Bi2O3exhibit higher tanδ.This is because the space charge polarization in the low frequency band leads to high tanδ.[29]When the frequency increases,the frequency of the alternating electric field becomes faster,the charge accumulation decreases,and the tanδdecreases.[29]A dielectric relaxation peak appears in tanδ–T curve(see Fig.5(b)),which is due to the conduction in ferrite grain and grain boundaries.[29]
4.Conclusion
In this study,different Ni0.5Zn0.5Fe2O4–x%Bi2O3(x=0,2.5,5)samples were prepared by the sol-gel auto-combustion method.A small addition of Bi2O3is a benefit for the grain growth but does not affect the spinel structure of the Ni–Zn ferrite.The dielectric properties of the composite materials are greatly improved in the low frequency region.From 200 Hz to 20 kHz,the temperature has a significant effect on the dielectric properties of the composite.The magnetic properties are also improved with the increase of Bi2O3content.
- Chinese Physics B的其它文章
- Multiple solutions and hysteresis in the flows driven by surface with antisymmetric velocity profile∗
- Magnetization relaxation of uniaxial anisotropic ferromagnetic particles with linear reaction dynamics driven by DC/AC magnetic field∗
- Influences of spin–orbit interaction on quantum speed limit and entanglement of spin qubits in coupled quantum dots
- Quantum multicast schemes of different quantum states via non-maximally entangled channels with multiparty involvement∗
- Magnetic and electronic properties of two-dimensional metal-organic frameworks TM3(C2NH)12*
- Preparation of a two-state mixture of ultracold fermionic atoms with balanced population subject to the unstable magnetic field∗

