Wave filtering and firing modes in a light-sensitive neural circuit*
Xiu-fang ZHANG, Jun MA,2
Wave filtering and firing modes in a light-sensitive neural circuit*
Xiu-fang ZHANG1, Jun MA†‡1,2
Department of Physics, Lanzhou University of Technology, Lanzhou 730050, China School of Science, Chongqing University of Posts and Telecommunications, Chongqing 430065, China †E-mail: hyperchaos@163.com
Inspired by the photoelectric effect, a phototube is incorporated into a simple neural circuit, and then the output voltage and dynamics become sensitive to external illumination within a specific frequency band. The firing modes are also dependent on the amplitude and frequency band in the illumination. In this paper, the signal outputs from a chaotic circuit are used as external optical signals, which are filtered and encoded by a phototube. Then, the functional neural circuit is excited to present a variety of firing modes and patterns. An exponential function of the filtering wave is proposed to discover the biophysical mechanism for frequency selection in the retina as most of wave bands of the external illumination are absorbed in the cathode material of the phototube while a specific band is effective in inducing a photocurrent for stimulating the visual neurons. Based on our light-sensitive neural circuit and model, external illumination is filtered and firing modes in the neuron are reproduced; furthermore, the mode transition induced by parameter shift is also investigated in detail. This result discovers the signal processing mechanism in the visual neurons and provides helpful guidance for designing artificial sensors for encoding optical signals and for repairing abnormalities in the retina of the visual system.
Light-sensitive neuron; Neural circuit; Phototube; Bifurcation; Information encoding
1 Introduction
Neurons from different functional regions in the brain can perceive and encode a variety of external stimuli and the nervous system is then excited to guide the most suitable gaits in the body. The auditory neurons (Machens et al., 2003; Heil, 2004; Koundakjian et al., 2007; Richter et al., 2008; Zhou et al., 2021a) can detect acoustic waves; the thermosensitive neurons (Nakayama, 1985; Zhang et al., 2004; Lee and Kenyon, 2009; Ruchty et al., 2010; Xu et al., 2020a) can discern changes of temperature; the visual neurons (Gabbiani et al., 2002; Peron and Gabbiani, 2009; Furtak et al., 2012; Wiederman and O’Carroll, 2013; Dipoppa et al., 2018) can perceive optical signals and light. Indeed, the activation and release of biophysical functions in nervous systems are associated with the neural activities of neurons and astrocytes (Li et al., 2016; Tang et al., 2017; Brazhe et al., 2018; Erkan et al., 2019; Du et al., 2020), while the collective behaviors and firing modes in neural networks are controlled by local kinetics and the links of nodes as well (Lv et al., 2019; Duan et al., 2020; Jiao et al., 2020; Yao, 2020; Zandi-Mehran et al., 2020). From a dynamical viewpoint, the local kinetics of the network can be described by many generic neurons, and even mathematical neurons. As a result, the coupling channels and external stimuli can be adjusted to trigger regular spatial patterns and ordered waves, and synchronization stability can also be controlled completely. In fact, neurons from different regions of the brain can develop specific functions and become sensitive to specific stimuli for generating appropriate firing modes even in the presence of noise. Some interneurons develop autapse connections (Seung et al., 2000; Song et al., 2019; Zhao et al., 2020; Li et al., 2021) and this self-adaption is enhanced by bridging an auxiliary loop when neurons are injured along the axon (Wang et al., 2017). When autapse connection is activated in a local area of the network, the switch between excitatory and inhibitory autapse will induce pacing stimulus and defects blocking in the wave propagation (Qin et al., 2014; Ma et al., 2015; Ge et al., 2019; Yao et al., 2019; Baysal et al., 2021), and so the collective behaviors of neural networks are regulated completely. On the other hand, the involvement of astrocytes (Achour and Pascual, 2012; MacVicar and Newman, 2015; Guo et al., 2017) connecting to neurons or the neural network will change the firing patterns in neural activities because calcium flow is changed to regulate the channel current and membrane potentials. Thus, the estimation and regulation of biological neurons and the neural network become a challenge when the biophysical effect and anatomic structure are considered (Ma et al., 2019).
The functional biophysical neuron (Wu et al., 2020) can be sensitive to specific stimuli, and similar dynamical properties can be reproduced in a large number of neural circuits by incorporating specific electric components into them. For the human auditory system, sound voice with the frequencies of 20 to 20 000 Hz can be heard effectively while bats are more sensitive in the ultrasonic. For the human visual system, light and illumination within the frequency band from 380 to 750 THz can be detected and, in particular, human eyes are most sensitive to green light with a wavelength of 500 to 560 nm. Therefore, specific electric components can be designed to enable the release and conversion of energy from external stimuli. For example, a memristor can be connected to a nonlinear circuit (Fitch et al., 2012; Vourkas and Sirakoulis, 2016; Babacan et al., 2017; Bao H et al., 2020; Bao BC et al., 2021), and then memristive synapse (Juzekaeva et al., 2019; Wu et al., 2019; Chen et al., 2020) is obtained and its dynamics relies on the initial value for the memory variable (magnetic flux). Connection of a thermistor to the nonlinear circuit (Lukić and Denić, 2015; Ibrahim et al., 2019; Xu et al., 2020b) makes its dynamics become sensitive to the temperature because its channel current is adjusted by the external temperature. The channel current across the Josephson junction (Saira et al., 2016; Pountougnigni et al., 2019; Koudafokê et al., 2021) can be regulated by an external magnetic field, and its involvement (Zhang et al., 2020, 2021) in the nonlinear circuit can enhance its ability to detect slight changes in the magnetic field. The coupling channel can also be controlled by an external field when a Josephson junction is used to connect neural circuits. A phototube can realize effective conversion from optical signals to electric signals, so the nonlinear circuit excited by a photocurrent (Liu et al., 2020a, 2020b) can be used to reproduce similar behaviors in eyes and visual system.
In this paper, a feasible light-sensitive neural circuit is proposed to discern how the external electromagnetic wave is filtered in the suggested frequency band for generating an effective photocurrent along the branch circuit. External illumination can be converted into an equivalent electric stimulus via the retina and then the visual neurons are excited for further information processing in the nervous system. Thus, the retina can be considered as an effective photoelectric converter and a phototube can be used to reproduce a similar biophysical function in light-sensitive neural circuits. The simple neural circuit is composed of a capacitor, an induction coil, a nonlinear resistor, and two linear resistors, and then a phototube is connected to enhance its dynamic dependence on external illumination. The phototube has two functional roles, wave filtering and photoelectric conversion, and then the equivalent neural circuit can be used to model the biophysical function and processing signal in visual neurons. The criterion for frequency selection in the electric component for wave filtering is suggested, and the filtered illumination can induce continuous electric stimulus in the neural circuit. The voltage outputs from a chaotic circuit are used as a realistic optical signal for activating the phototube; the photocurrent across the phototube is controlled under the suggested criterion for wave filtering. Furthermore, the functional neural circuit is excited to reproduce similar firing modes from biological visual neurons and the Hamilton energy is estimated when the firing modes are changed.
2 Model and scheme
As mentioned above, the eyes and visual neuron can perceive external illumination, and electromagnetic waves within specific frequency bands can be captured and converted to electric stimuli via the retina. The photoelectric conversion is realized via the phototube when external illumination is applied with high frequency beyond the threshold decided by the cathode material; the functional neural circuit coupled by a phototube is shown in Fig. 1.
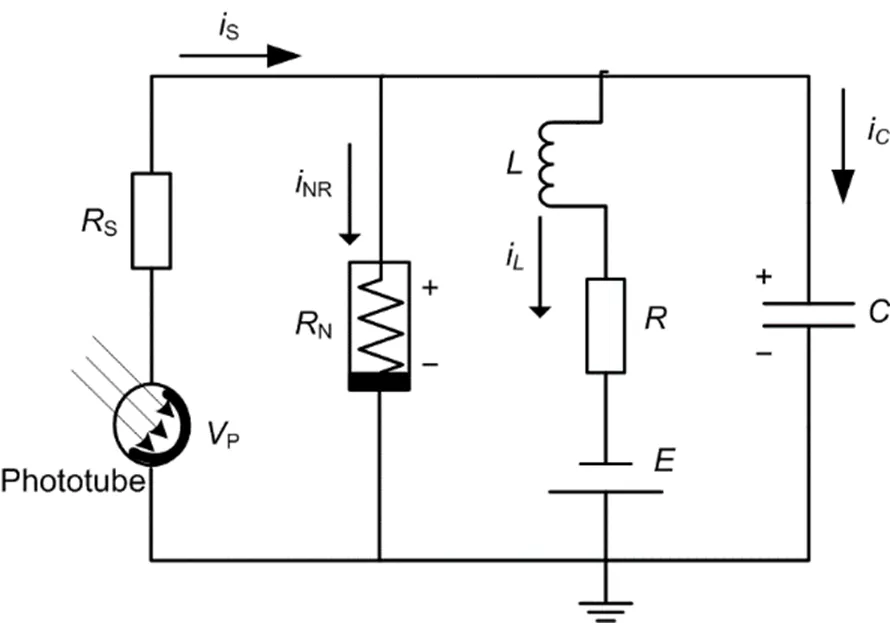
Fig. 1 Schematic diagram for a light-sensitive neural circuit controlled by the photocurrent across the phototube
P,,,,S,N, andrepresent the output voltage across the phototube, capacitor, inductor, linear resistors, nonlinear resistor, and constant voltage source, respectively.S,NR, i, and irepresent the channel currents along the linear resistors, nonlinear resistor, induction coil, and capacitor, respectively
Guided by Kirchhoff’s law, the circuit equations for Fig. 1 are obtained by
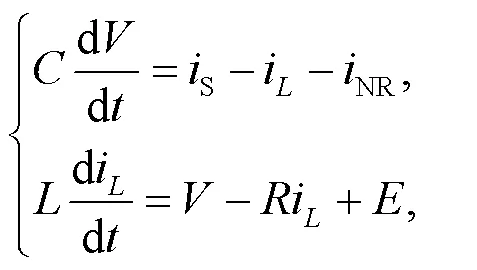
where the photocurrent across the phototube is described byS=(P−)/S.defines the voltage for the capacitor. The channel current (Kyprianidis et al., 2012) across the nonlinear resistorNis estimated by
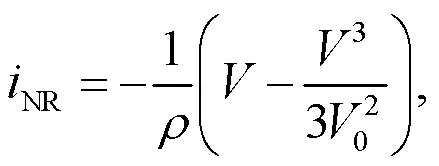
where the parametersand0represent the resistance in the linear region and the cut-off voltage for the nonlinear resistor, respectively. The phototube is used as a voltage sourcePwhen a large resistance is applied in the resistorS. In addition, the phototube can also be considered as a current sourceSwhen the linear resistorSis selected within a finite resistance, and then the photocurrent (Liu et al., 2020a) can be approached by
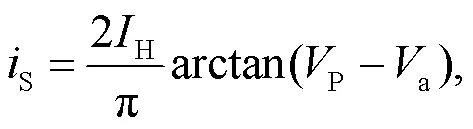
whereHandadenote the saturation current (maximal current value) and the reverse cut-off voltage, which can block the photocurrent across the phototube, respectively.
Firstly, we consider the case that the phototube is activated as the voltage source and a standard scale transformation (Wang et al., 2019) is applied for the physical variables and parameters in Eqs. (1)–(3) as follows:
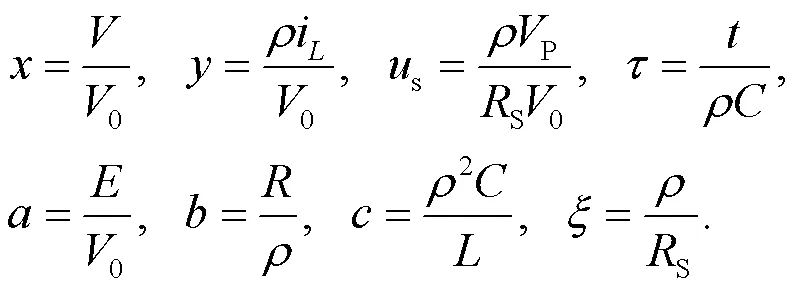
Thus, the dimensionless light-sensitive neuron can be described by
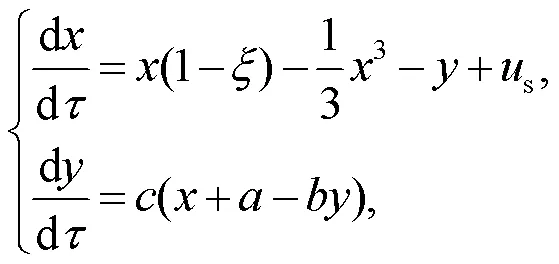
where the variables,, andsrepresent the membrane potential, recovery variable for slow current, and external stimulus from the phototube, respectively. Indeed, the phototube is sensitive to specific bands of external illumination and electromagnetic waves, and its amplitude is related to the frequency asfollows:
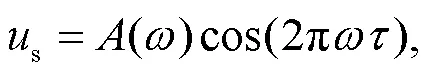
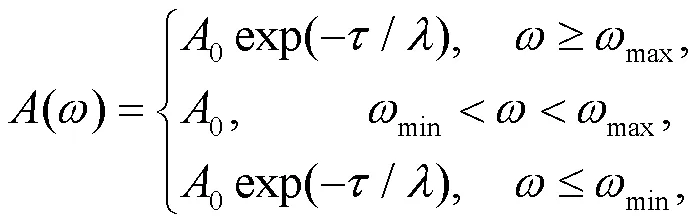
where the normalized parameteris considered as a decay factor, and is related to the properties of the cathode material of the phototube. The parametercan be controlled by using different coating materials while the parametersand0control the main frequency and amplitude in the filtered wave and voltage source, respectively. Some optical signals within specific bands of frequency will be absorbed and decayed via the phototube, and a lower value for the decay factormeans that the external illumination can be suppressed quickly. In addition,minandmaxdefine respectively the upper and lower limit thresholds for the frequency. As a result, the electromagnetic wave is filtered and its frequency remains active in the region [min,max], and the phototube is then activated effectively. In the experimental way, the equivalent voltage sourcesacross the phototube can be approached by using the Heaviside function(·) as follows:
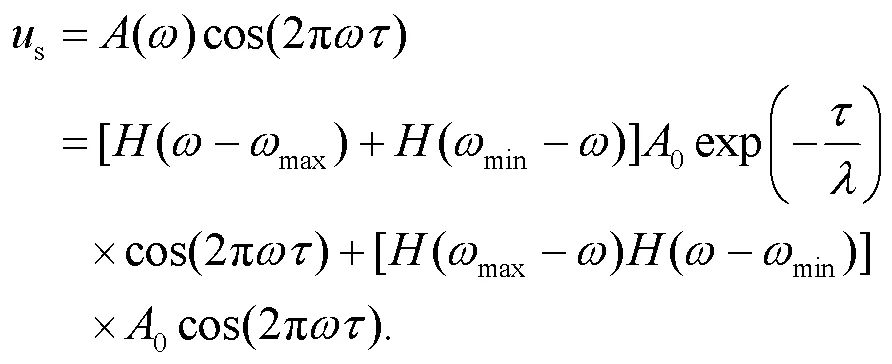
Indeed, the phototube can also be activated as a current source, and the photocurrent defined in Eq. (3) can be approached by using a simple dimensionless form as follows:
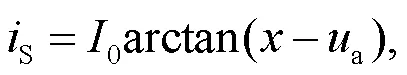
where0is the maximum value of the dimensionless photocurrent, and the normalized parameterais related to the material property of the phototube. In this way, the functional neuron driven by the photocurrent can be obtained by
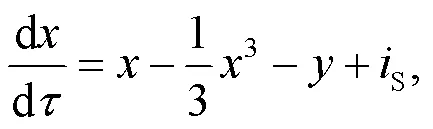
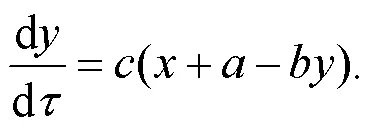
As is well known, the induction coil and capacitor of the neural circuit can pump and release field energy (Zhou et al., 2021b). The inner energy in the neural circuit is propagated and exchanged between electric components (capacitor and induction coil) when the output voltage is changed with the change of channel current across the induction coil as follows:
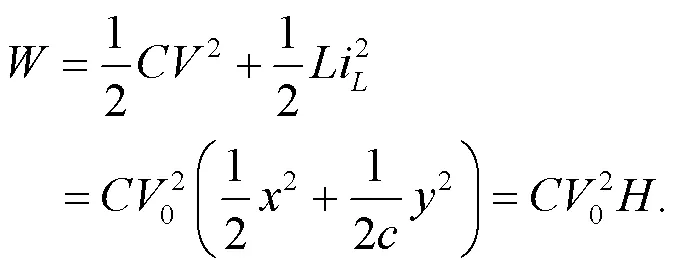
By applying similar scale transformation for the variables and parameters in the field energy shown in Eq. (10), the dimensionless Hamilton energyfor the functional neuron is updated as follows:
The realistic optical signal and electromagnetic wave show a wide band in frequency, and the output voltages from chaotic circuits meet this requirement. For simplicity but without losing generality, the output voltages from Pikovskii-Rabinovich (PR) (Pikovskii and Rabinovich, 1978) are used as a light signal, and their dynamics are described by
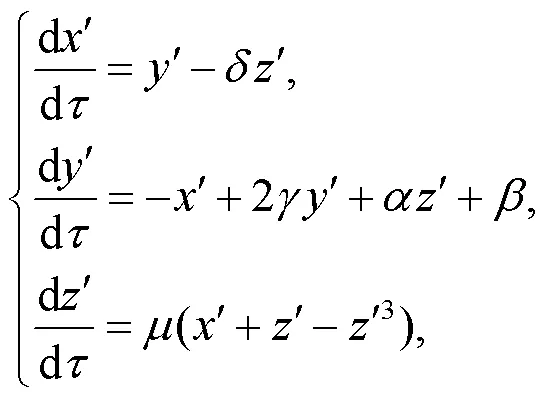
where the variables,, andcan be mapped from the channel currents across the induction coil and the voltages across the capacitors in the chaotic circuit; the normalized parameters are selected as=0.165,=0,=0.201,=0.66, and=1/0.047. In the next section, initial values are fixed at (0.1, 0.1, 0.1), and a chaotic series for variableis used as an external signal source which will be filtered and encoded by the phototube and an effective photocurrent is triggered to regulate the firing modes and patterns from the light-sensitive neuron.
3 Numerical results and discussion
The fourth-order Runge-Kutta algorithm is applied to find numerical solutions for the neuron model with time step=0.01, the initial value for the neuron model is selected as (0.2, 0.1), and the transient period is about 1000 time units. The chaotic series from the chaotic PR system are used as an unfiltered signal (electromagnetic wave) or a realistic illumination signal. In Fig. 2, chaotic seriesare presented and filtered.
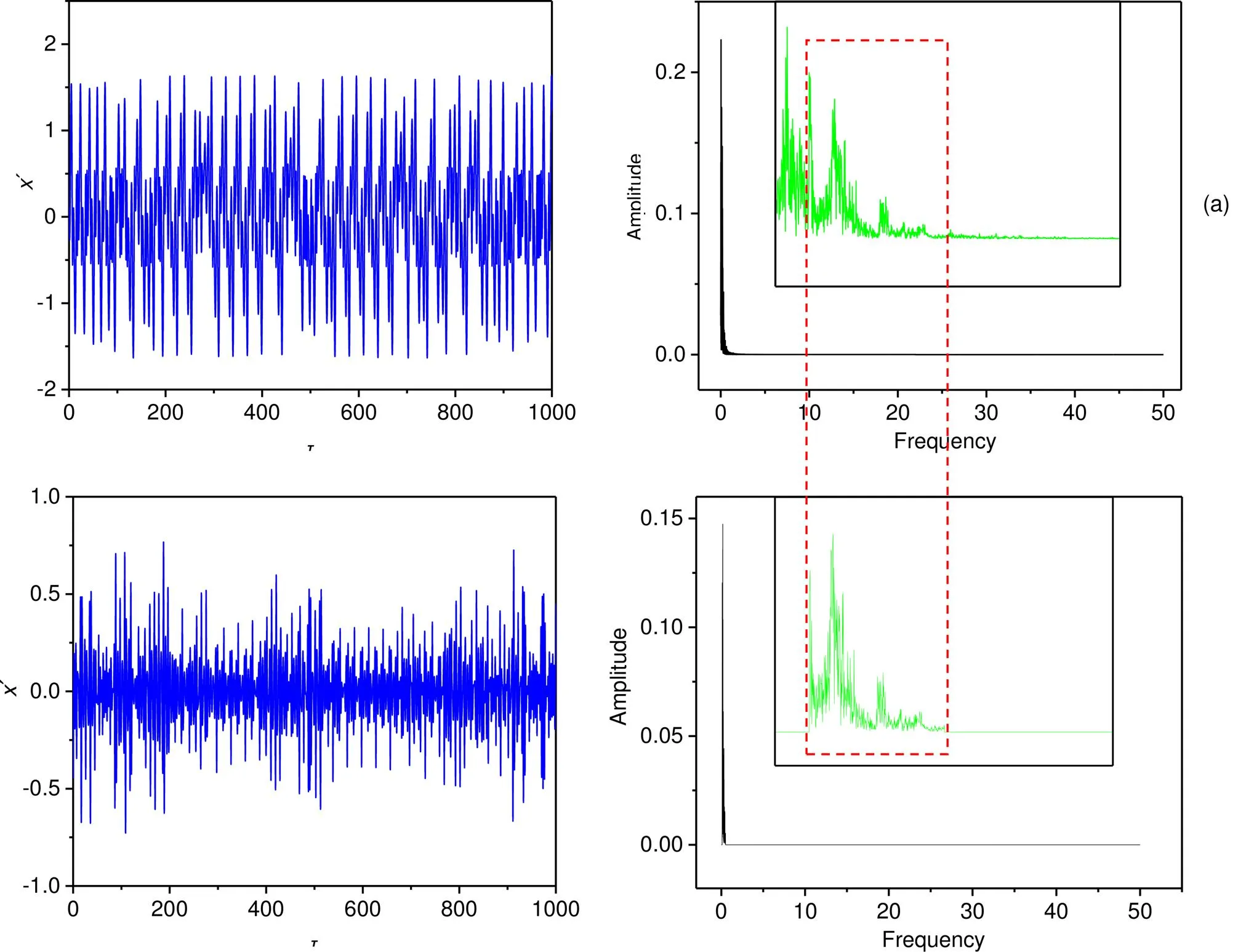
Fig. 2 Wave filtering and the power spectrum for the chaotic signals and filtered signals: (a) original chaotic series and power spectrum via fast Fourier transform (FFT); (b) filtered signal source and power spectrum. The thresholds for frequency selection and parameters are fixed at ωmin=0.1, ωmax=0.5, a=0.7, b=0.8, c=0.1, ξ=0.175, and λ=5. The inserted subfigure is an enlarged version and the frequency region is within [0.0, 1.0]
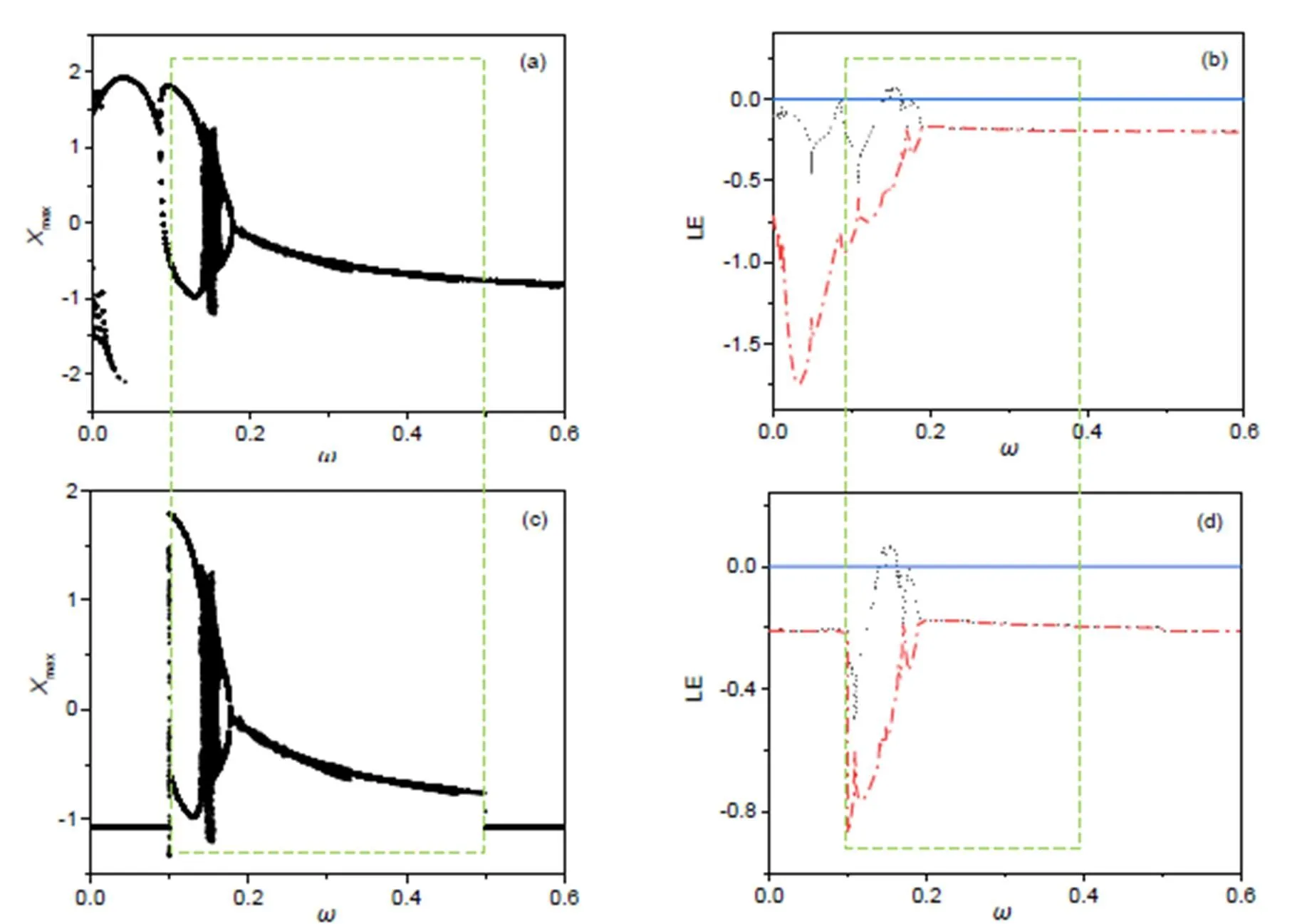
Fig. 3 Dependence of firing mode and Lyapunov exponent (LE) spectrum on the frequency in the voltage source us: bifurcation analysis for membrane potential (a) and LE spectra (b) for an isolated neuron driven by voltage source us=Acos(2πωτ) without wave filtering; bifurcation analysis for membrane potential (c) and LE spectra (d) for an isolated neuron driven by us=A(ω)cos(2πωτ) under wave filtering. The thresholds for frequency selection and parameters are fixed at ωmin=0.1, ωmax=0.5, a=0.7, b=0.8, c=0.1, ξ=0.175, λ=5, and A=0.9
From Fig. 2, the criterion for wave filtering plays an important role in frequency selection. As a result, the filtered wave shows a distinct difference in profile from the original signal source and the frequency band is kept in the region [min,max]; further decrease of the decay factorcan enhance the effect of wave filtering. Furthermore, the filtered wave is used to activate the functional neuron and the dynamics’ dependence on the main frequencyin the voltage source across the phototube. The results are plotted in Fig. 3.
The isolated neuron can present periodic firing at<0.14, and chaotic firing patterns are induced at 0.14<<0.17. When the wave filtering and frequency selection are switched on, the signal series with low frequency and high frequency are filtered from the chaotic signal source. The cone cells are of three kinds and they can perceive the electromagnetic waves as long wavelength, moderate wavelength, and short wavelength. For biological neurons, the excitability can be controlled by external stimuli and then appropriate firing modes are stabilized in the neural activities. Therefore, we will discuss three cases in which the optical signal and illumination are presented with low frequency, moderate frequency, and high frequency, respectively. First, we discuss the case for low frequency and the thresholds are selected asmin=0.005,max=0.16. In Fig. 4, the spectra for the signal source before and after wave filtering, and the phase portraits are plotted.
When the voltage source after wave filtering is kept in the low frequency band, the combined electric stimuli can induce firing patterns within multiple modes. That is different from the case where the neuron is excited by a sole periodic forcing current with low frequency. Thus, the filter wave criterion and mechanism make the functional neuron present rich firing modes and patterns under excitation from the filtered wave with combined signals.
In the case of moderate frequency, the thresholds for frequency selection are fixed atmin=0.16,max=0.3, and the results are presented in Fig. 5.
With moderate frequency in the source signal across the phototube, the photocurrent can enhance chaotic firing patterns effectively, and chaotic attractors are formed in the phase space.
The case of high frequency, with the thresholdmin=0.3,max=0.5, is calculated in Fig. 6.
Similar to the results in Fig. 5, the firing patterns in the electric activities show a chaotic series, and attractors are formed completely when a filtered wave with high frequency is used to activate a similar voltage source across the phototube with high frequency. As mentioned above, the Hamilton energy is dependent on the membrane potential and channel current, and any changes in the firing modes will change the Hamilton energy. In Fig. 7, the evolution of Hamilton energy is calculated to discern the effect of wave filtering in the electromagnetic wave imposed on the phototube.
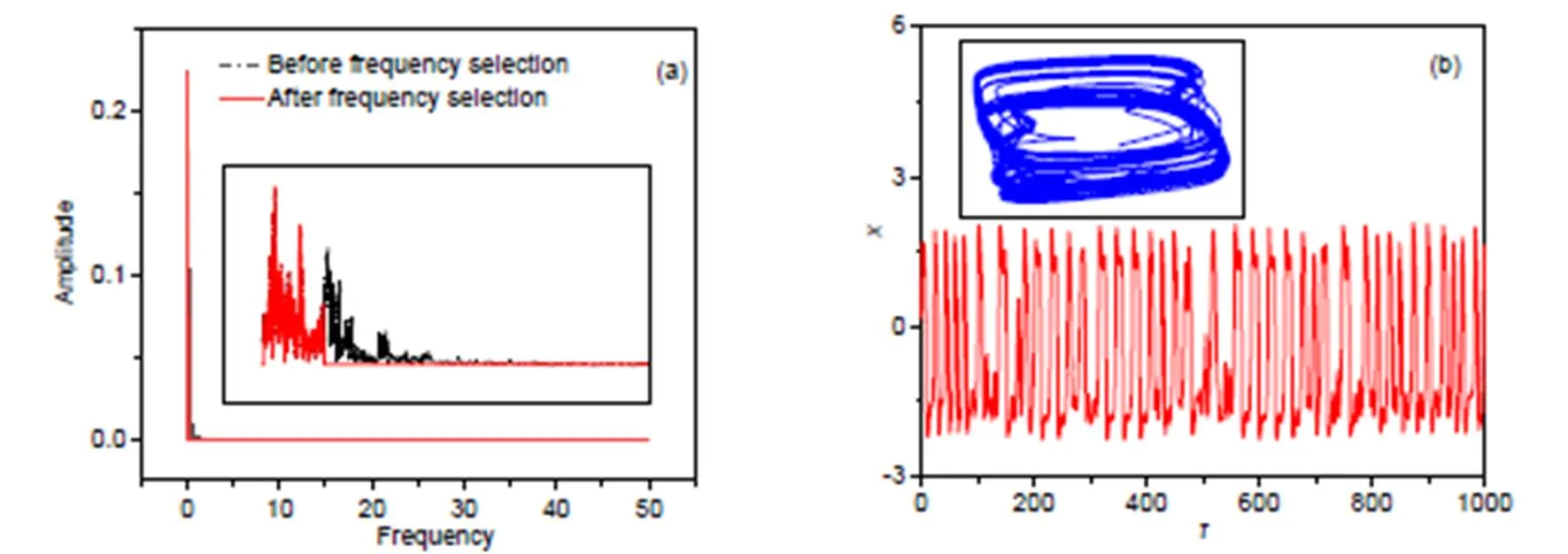
Fig. 4 Frequency domain for exciting signals and phase portraits for the neuron: (a) frequency domain for voltage source with and without wave filtering; (b) phase portraits and firing patterns with wave filtering at ωmin=0.005, ωmax=0.16. The parameters are fixed at a=0.7, b=0.8, c=0.1, ξ=0.175, and λ=5
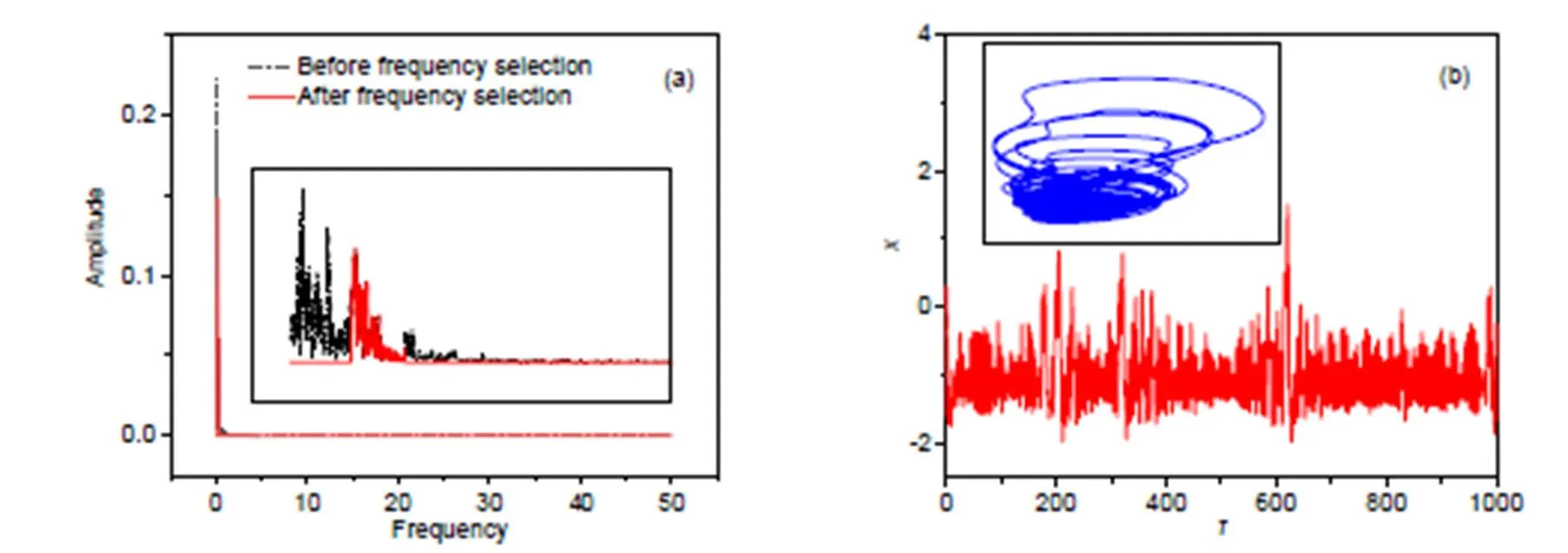
Fig. 5 Frequency domain for exciting signals and phase portraits for the neuron: (a) frequency domain for voltage source with and without wave filtering; (b) phase portraits and firing patterns with wave filtering at ωmin=0.16, ωmax=0.3. The parameters are fixed at a=0.7, b=0.8, c=0.1, ξ=0.175, and λ=5
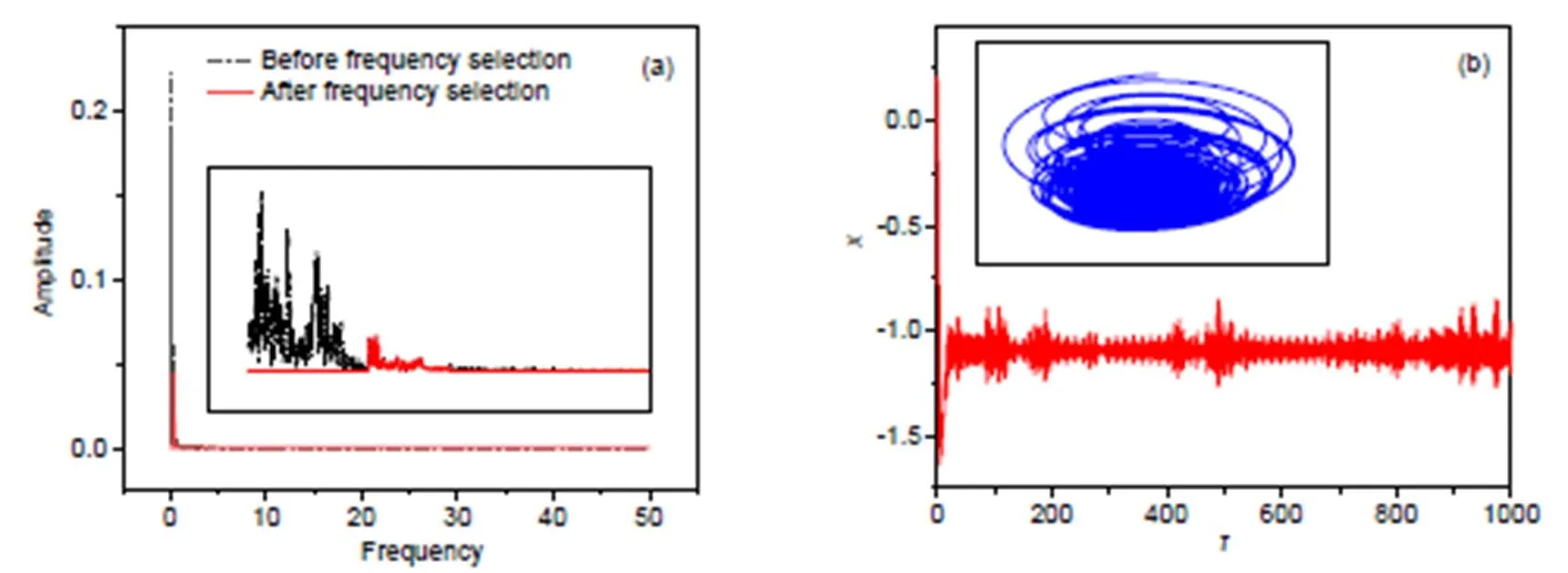
Fig. 6 Frequency domain for exciting signals and phase portraits for the neuron: (a) frequency domain for voltage source with and without wave filtering; (b) phase portraits and firing patterns with wave filtering at ωmin=0.3, ωmax=0.5. The parameters are fixed at a=0.7, b=0.8, c=0.1, ξ=0.175, and λ=5. The inserted subfigure in Fig. 6a is an enlarged one in the region of frequency [0.0, 1.0]
As shown by the dashed line in Fig. 7a, the neural circuit is excited by a chaotic series ass=′(), and the neuron energy shows multiple modes and its evolution is changed greatly when wave filtering is switched on. In addition, the fluctuation range of Hamilton energy is decreased when the voltage source with moderate or high frequency is applied to trigger chaotic firing patterns in the electric activities. That is, chaotic firing modes make neurons show lower Hamilton energy. On the other hand, as shown in Eq. (11), any changes in the intrinsic parameterwill change the energy directly, and any changes in the intrinsic parameters (,,,) will change the firing mode, and the energy in the neural circuit is regulated subsequently. In Fig. 8, the sole periodic electric stimulus from the phototube ass=cos() is applied to detect the mode dependence of membrane potentialon the intrinsic parameters (,).
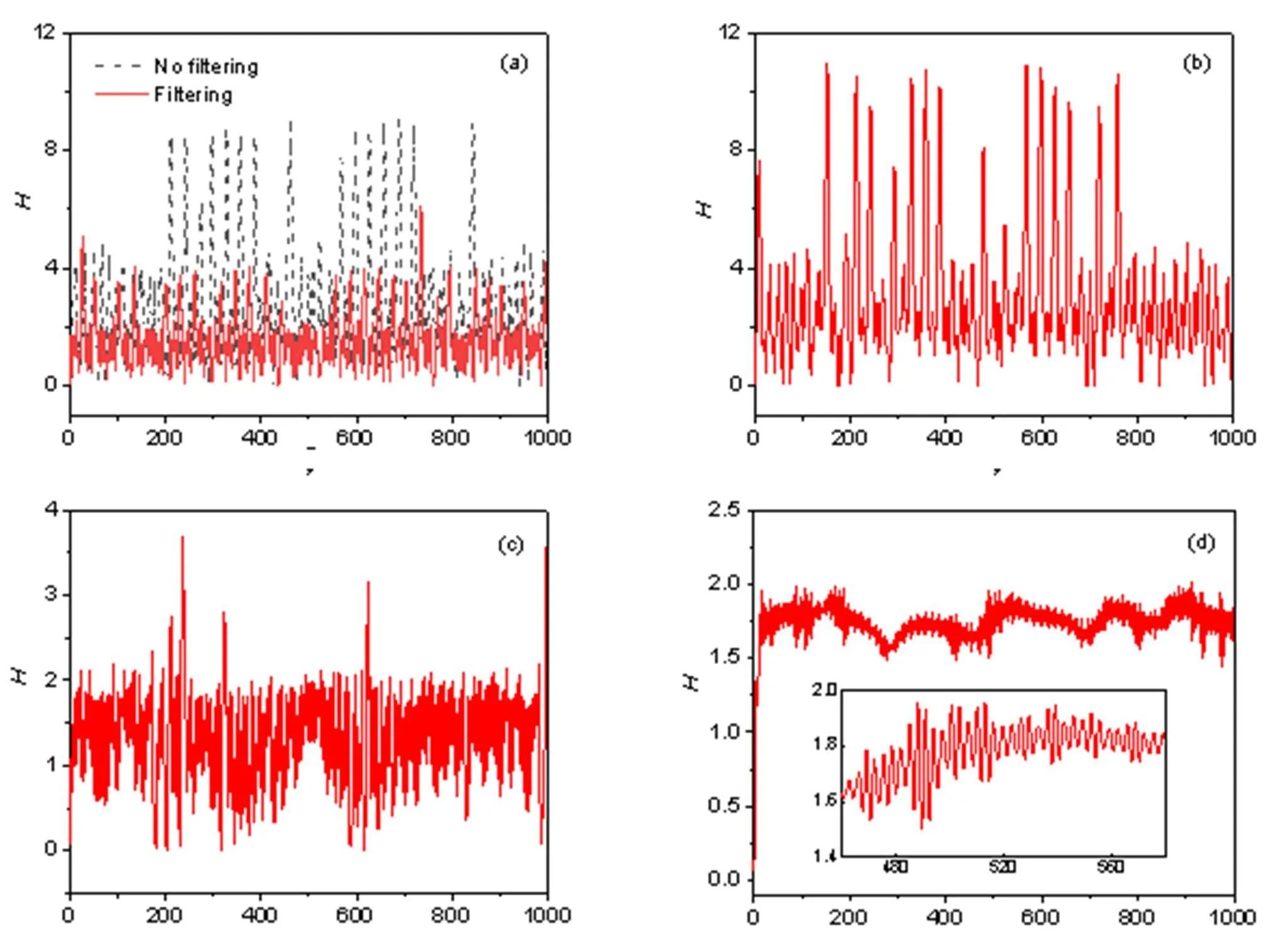
Fig. 7 Energy pumping and release in the functional neuron with and without wave filtering under different thresholds in frequency: (a) ωmin=0.1, ωmax=0.5; (b) ωmin=0.005, ωmax=0.16; (c) ωmin=0.16, ωmax=0.3; (d) ωmin=0.3, ωmax=0.5. The parameters are fixed at a=0.7, b=0.8, c=0.1, ξ=0.175, and λ=5. The dashed line describes the evolution of Hamilton energy in the neuron driven by chaotic signals without wave filtering, and the solid lines represent the energy series under wave filtering with different thresholds
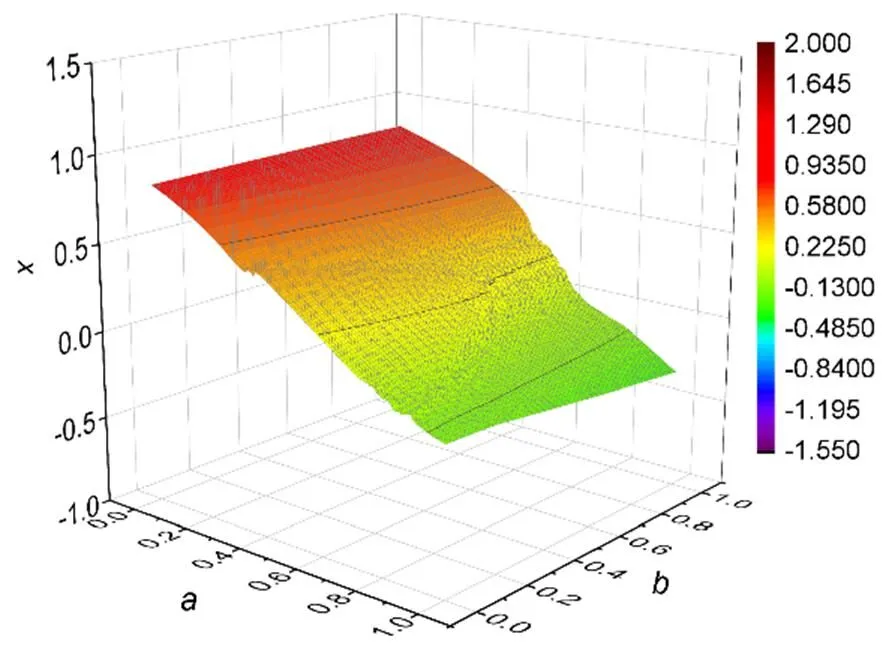
Fig. 8 Evolution of membrane potentials in the two-parameter space (membrane potentials for variable x in the neuron driven by periodic stimulus us=Acos(ωτ), with the parameters fixed at A=0.9, ω=0.16, c=0.1, and ξ=0.175)
For greater clarity, the bifurcation analysis is calculated in Fig. 9 by changing one of the intrinsic parameters in the neural circuit driven by periodic stimulus. When the parameteris fixed, in case of a periodic stimulus, the neuron shows a distinct transition from periodic firing to chaotic firing at=0.46 and then the chaos is suppressed at=0.8 for retrieving periodic firing modes. When the parameteris fixed, the neuron driven by periodic forcing goes through periodic firing to chaotic firing modes from=0.54, and further increase of the parameterwill wake periodic firing completely. In Fig. 10, the evolution of membrane potential is plotted when one intrinsic parameter is jumped with a constant step.
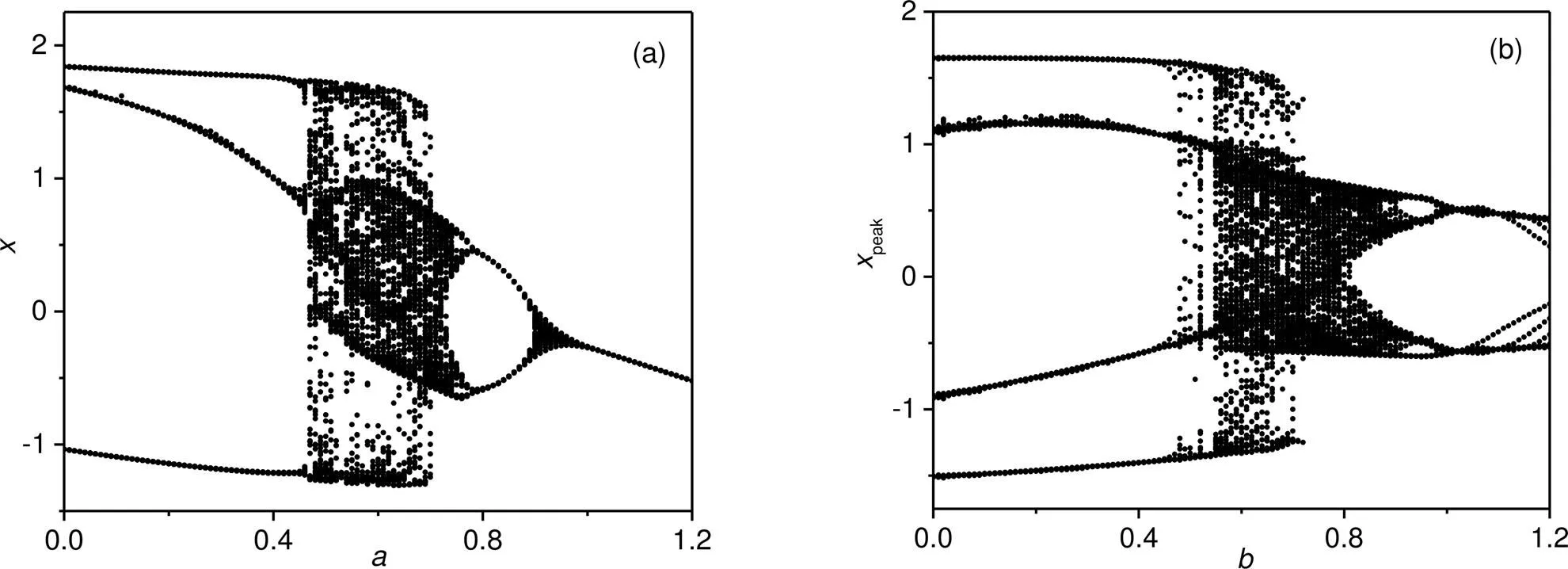
Fig. 9 Bifurcation diagrams for membrane potential x by changing one intrinsic parameter: (a) b=0.7; (b) a=0.7. The parameters are fixed at A=0.9, ω=0.16, c=0.1, and ξ=0.175
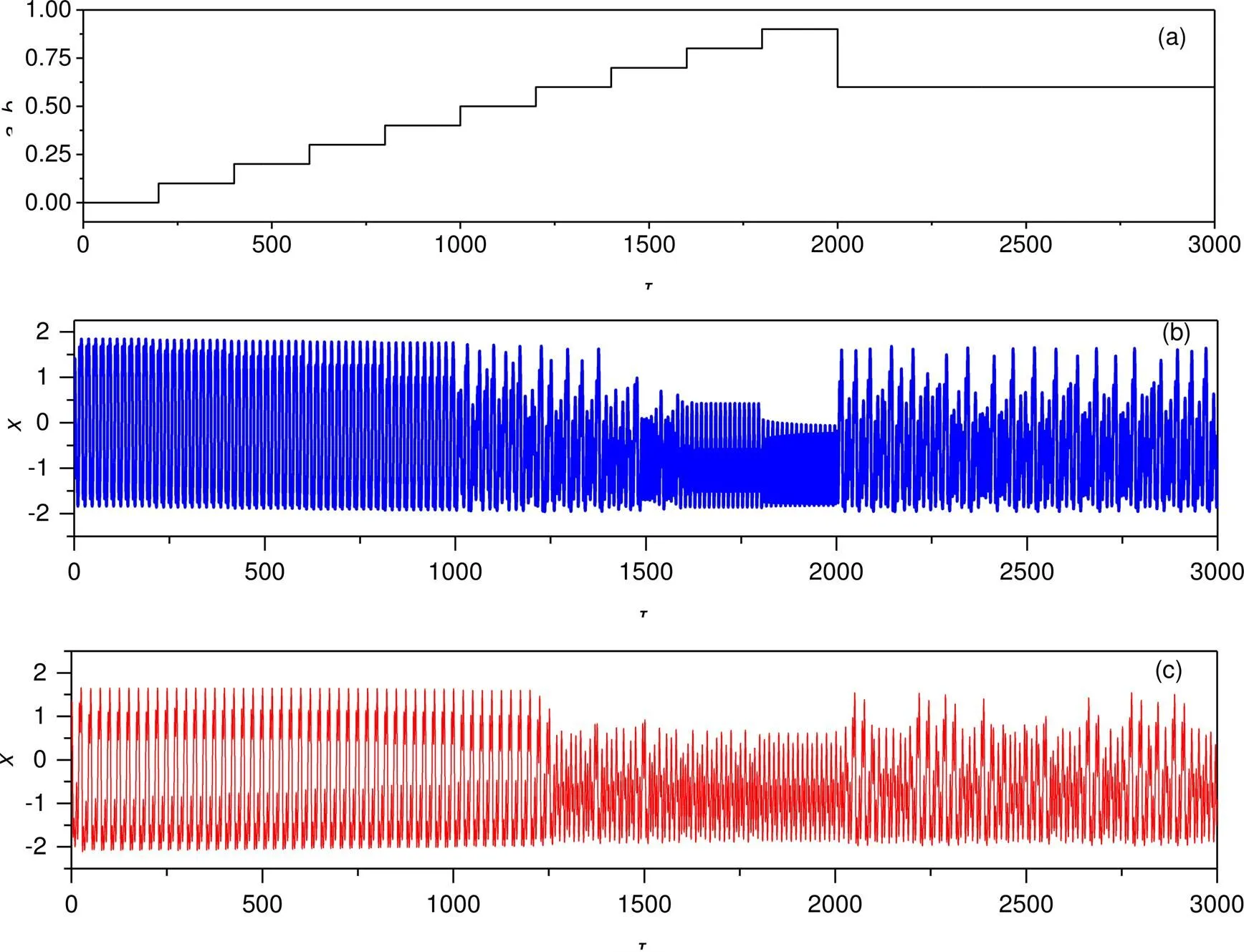
Fig. 10 Firing patterns in electric activities when two intrinsic parameters are jumped: (a) parameters jumping in constant steps with time; (b) jumped parameter a with fixed b=0.7; (c) jumped parameter b with fixed a=0.7. The other parameters are fixed at A=0.9, ω=0.16, c=0.1, and ξ=0.175
Indeed, any changes in one of the intrinsic parameters will induce distinct mode transition in the neural activities when periodic stimulus from the phototube is applied. Furthermore, we consider the case when the phototube is activated and it is used as current source; the firing mode’s dependence on intrinsic parameters is plotted in Fig. 11.
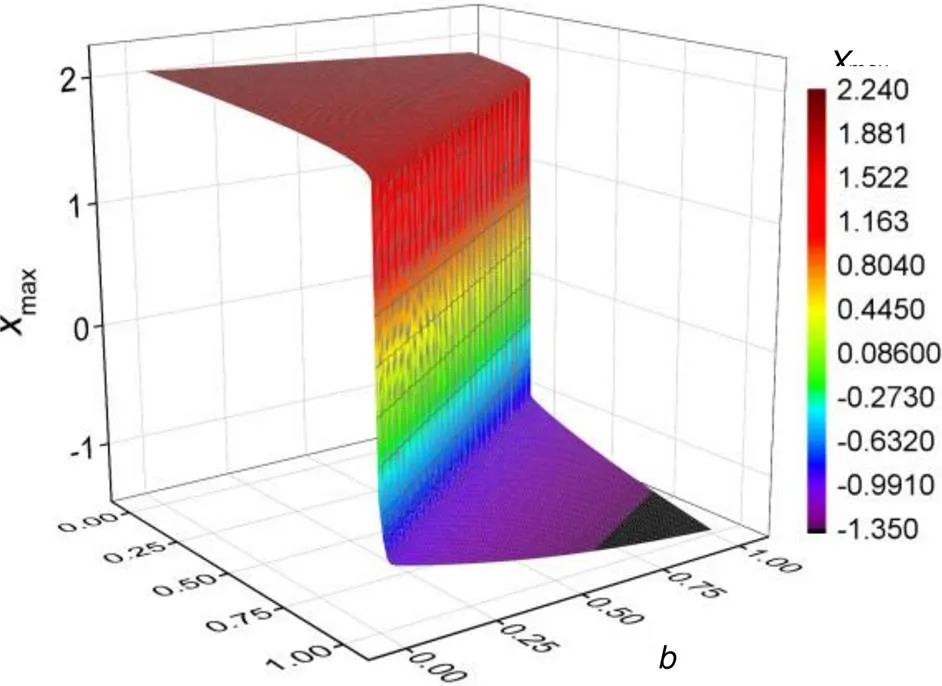
Fig. 11 Evolution of membrane potentials in the two-parameter space (membrane potentials for variable x in the neuron driven by photocurrent iS=I0arctan(x−ua)). The parameters are fixed at I0=0.05, ua=0.1, c=0.1, and ξ= 0.175
When the phototube is handled as a current source, the neuron tends to present periodic firing modes and bifurcation analysis as shown in Fig. 12.
In fact, the neuron used to present periodic firing modes when the stimulus from the phototube connected to the neural circuit can be regarded as a continuous current source, and the firing modes become regular but with their parameters shifted greatly. In the same way, one parameter is shifted to detect the transition in the membrane potentials with the results shown in Fig. 13.
As presented in Fig. 13, appropriate switch and jump in any of the two intrinsic parameters (,) will induce transition from periodic to quiescent states. For example, periodic firing is generated at<0.8,<0.4 while the neuron becomes quiescent at>0.8,>0.4. That is, the physical property of the phototube and its outputs can control the excitability and firing modes of the functional neuron completely. Extensive numerical calculation is carried out, and it indicates that the Hamilton energy prefers higher values in the case of periodic firing modes.
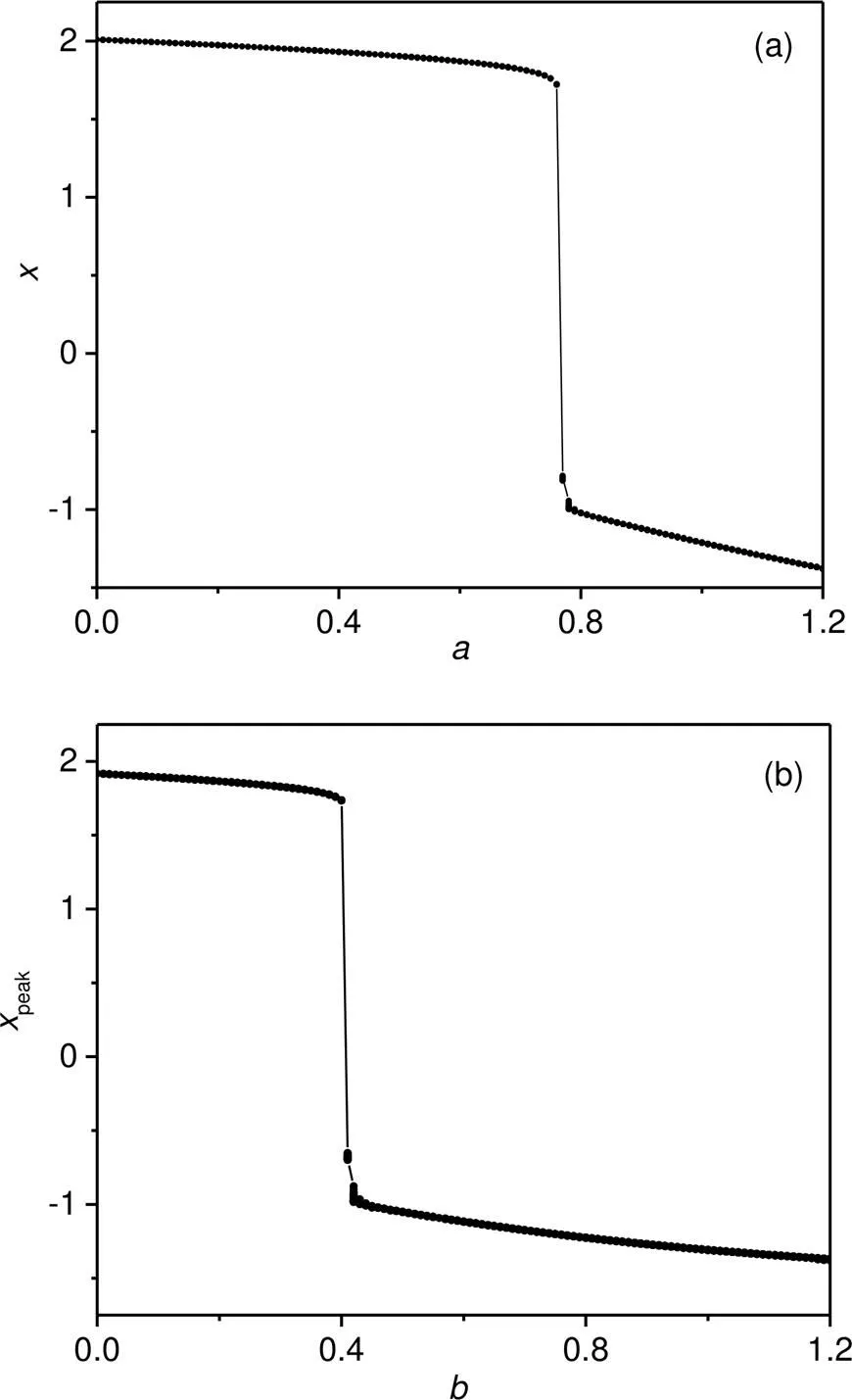
Fig. 12 Bifurcation diagrams for membrane potential x by changing one intrinsic parameter: (a) b=0.32; (b) a= 0.7. The parameters are fixed at I0=0.05, ua=0.1, c=0.1, ξ=0.175, and the photocurrent is estimated at iS= I0arctan(x−ua)
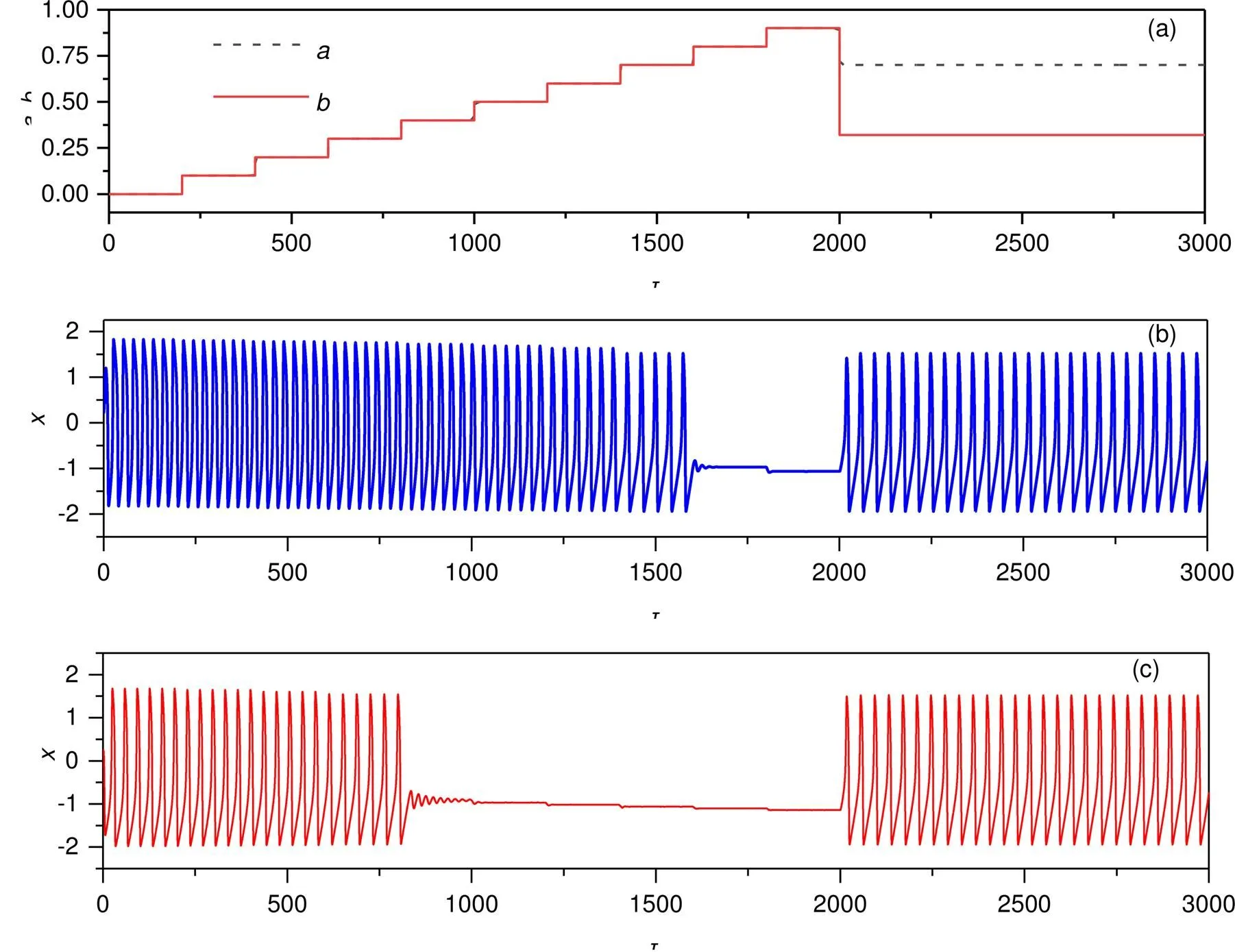
Fig. 13 Firing patterns in electric activities when two intrinsic parameters are jumped: (a) parameters jumping in constant steps with time; (b) jumped parameter a with fixed b=0.32; (c) jumped parameter b with fixed a=0.7. The other parameters are fixed at I0=0.05, ua=0.1, c=0.1, and ξ=0.175
In summary, most realistic optical signals often have wide bands, e.g. the chaotic signals are also generated with wide bands. The illumination and electromagnetic wave within certain frequency bands can be detected in the visual system by generating equivalent and effective electric stimuli in the neurons and neural circuit loop. Therefore, wave filtering becomes critical and the thresholds for frequency selection in the electromagnetic wave are relative to the physical property of the phototube (and of the retina in the visual system). In a practice, a variety of coating films can be coated and adhered to the photoelectric conversion device to select and/or filter illuminations within specific frequency bands. For the light-sensitive neural circuit/neuron, the equivalent photocurrent generated from the phototube can change the excitability and energy pumping effectively when the external illumination is filtered effectively. That is to say that the criterion for wave filtering and frequency selection well explains the potential biophysical mechanism for photoelectric conversion and coding in the visual system, and provides possible guidance for enhancing photoelectric conversion in artificial eyes and signal encoding in intelligent devices. Also, this proposal is effective in exploring wave filtering in auditory systems (Guo et al., 2021) because the ears can be sensitive to acoustic waves within specific frequency bands. In fact, electromagnetic radiation has an important impact on the wake-sleep cycle (Jin et al., 2019), and appropriate devices for filtering electromagnetic waves can be designed for ensuring healthy sleep. In experiments and extensive applications, the light-sensitive neural circuit can be excited by a silicon photocell, and a color absorber (filter) can be preplaced to select an optical wave with a suitable frequency band. Therefore, these light-sensitive neural circuits can be connected to build a network, which is capable of detecting optical signals and can control electric devices by using different illuminations. As reported in (Perc, 2007a, 2007b), the collective behaviors in the neural networks are controlled by the local excitability and connection topology, and adaptive adjusting in the coupling channels; links and local kinetics will be sensitive for detecting external stimuli and then appropriate wave propagation and energy flow can be controlled completely. Based on our proposal and criterion for wave filtering, more wave sources can be applied for detecting pattern formation, wave propagation, and energy flow in a network driven by filtered waves with spatiotemporal diversity.
4 Conclusions
Visual neurons are sensitive to electromagnetic waves and external illumination within specific frequency bands, and the potential mechanism is that the retina can absorb certain bands of electromagnetic wave. In this paper, a phototube is incorporated into the neural circuit for improving its sensitivity to illumination and light. A criterion for wave filtering is suggested to select specific bands from a chaotic series, which is used to model a realistic signal source, and the filtered wave is effective in realizing photoelectric conversion via a phototube. As a result, the photocurrent across the phototube is dependent on the external illumination and filtered wave, and it is effective in triggering a variety of firing modes; the Hamilton energy is also adjusted effectively. This result can clarify the potential biophysical mechanism for signal processing and frequency selection in visual neurons, and the proposed criterion for wave filtering is also helpful for further designing artificial light-dependent and piezoelectric sensors and networks. For further investigation and application, appropriate coating films can be plated on the phototube and piezoelectric devices for wave filtering and selection of frequency, and then artificial functional sensors can be designed to perceive different physical signals.
Contributors
Xiu-fang ZHANG designed the research, processed the corresponding data, and wrote the first draft of the manuscript. Jun MA helped to organize the manuscript, and revised and edited the final version.
Conflict of interest
Xiu-fang ZHANG and Jun MA declare that they have no conflict of interest.
Achour SB, Pascual O, 2012. Astrocyte–neuron communication: functional consequences., 37(11):2464-2473. https://doi.org/10.1007/s11064-012-0807-0
Babacan Y, Yesil A, Kacar F, 2017. Memristor emulator with tunable characteristic and its experimental results., 81:99-104. https://doi.org/10.1016/j.aeue.2017.07.012
Bao BC, Zhu YX, Ma J, et al., 2021. Memristive neuron model with an adapting synapse and its hardware experiments., 64(5): 1107-1117.
https://doi.org/10.1007/s11431-020-1730-0
Bao H, Chen M, Wu HG, et al., 2020. Memristor initial-boosted coexisting plane bifurcations and its extreme multi-stability reconstitution in two-memristor-based dynamical system., 63(4):603-613. https://doi.org/10.1007/s11431-019-1450-6
Baysal V, Erkan E, Yilmaz E, 2021. Impacts of autapse on chaotic resonance in single neurons and small-world neuronal networks., 379(2198):20200237. https://doi.org/10.1098/rsta.2020.0237
Brazhe AR, Postnov DE, Sosnovtseva O, 2018. Astrocyte calcium signaling: interplay between structural and dynamical patterns., 28(10):106320. https://doi.org/10.1063/1.5037153
Chen M, Qi JW, Wu HG, et al., 2020. Bifurcation analyses and hardware experiments for bursting dynamics in non-autonomous memristive FitzHugh-Nagumo circuit., 63(6):1035-1044. https://doi.org/10.1007/s11431-019-1458-5
Dipoppa M, Ranson A, Krumin M, et al., 2018. Vision and locomotion shape the interactions between neuron types in mouse visual cortex., 98(3):602-615. https://doi.org/10.1016/j.neuron.2018.03.037
Du MM, Li JJ, Yuan ZX, et al., 2020. Astrocyte and ions metabolism during epileptogenesis: a review for modeling studies., 29(3):038701. https://doi.org/10.1088/1674-1056/ab6961
Duan KR, Fong S, Chen CLP, 2020. Multilayer neural networks-based control of underwater vehicles with uncertain dynamics and disturbances., 100(4):3555-3573. https://doi.org/10.1007/s11071-020-05720-5
Erkan Y, Saraç Z, Yılmaz E, 2019. Effects of astrocyte on weak signal detection performance of Hodgkin–Huxley neuron., 95(4):3411-3421. https://doi.org/10.1007/s11071-019-04764-6
Fitch AL, Yu DS, Iu HHC, et al., 2012. Hyperchaos in a memristor-based modified canonical Chua’s circuit., 22(6): 1250133. https://doi.org/10.1142/S0218127412501337
Furtak SC, Ahmed OJ, Burwell RD, 2012. Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination., 76(5):976-988. https://doi.org/10.1016/j.neuron.2012.10.039
Gabbiani F, Krapp HG, Koch C, et al., 2002. Multiplicative computation in a visual neuron sensitive to looming., 420(6913):320-324. https://doi.org/10.1038/nature01190
Ge MY, Jia Y, Xu Y, et al., 2019. Wave propagation and synchronization induced by chemical autapse in chain Hindmarsh–Rose neural network., 352:136-145. https://doi.org/10.1016/j.amc.2019.01.059
Guo SL, Tang J, Ma J, et al., 2017. Autaptic modulation of electrical activity in a network of neuron-coupled astrocyte., 2017:4631602. https://doi.org/10.1155/2017/4631602
Guo YT, Zhou P, Yao Z, et al., 2021. Biophysical mechanism of signal encoding in an auditory neuron., 1-12. https://doi.org/10.1007/s11071-021-06770-z
Heil P, 2004. First-spike latency of auditory neurons revisited., 14(4):461-467. https://doi.org/10.1016/j.conb.2004.07.002
Ibrahim O, Hassan SM, Abdulkarim A, et al., 2019. Design of wheatstone bridge based thermistor signal conditioning circuit for temperature measurement., 12(1):12-17. https://doi.org/10.25103/jestr.121.02
Jiao TC, Zong GD, Ahn CK, 2020. Noise-to-state practical stability and stabilization of random neural networks., 100(3):2469-2481. https://doi.org/10.1007/s11071-020-05628-0
Jin WY, Wang A, Ma J, et al., 2019. Effects of electromagnetic induction and noise on the regulation of sleep wake cycle., 62(12): 2113-2119. https://doi.org/10.1007/s11431-018-9423-x
Juzekaeva E, Nasretdinov A, Battistoni S, et al., 2019. Coupling cortical neurons through electronic memristive synapse., 4(1):1800350. https://doi.org/10.1002/admt.201800350
Koudafokê GN, Hinvi LA, Miwadinou CH, et al., 2021. Passive sensor with Josephson junction coupled to an electric resonator and a nanobeam., 318:112509. https://doi.org/10.1016/j.sna.2020.112509
Koundakjian EJ, Appler JL, Goodrich LV, 2007. Auditory neurons make stereotyped wiring decisions before maturation of their targets., 27(51): 14078-14088. https://doi.org/10.1523/JNEUROSCI.3765-07.2007
Kyprianidis IM, Papachristou V, Stouboulos IN, et al., 2012. Dynamics of coupled chaotic Bonhoeffer–van der Pol oscillators., 11(9):516-526.
Lee SJ, Kenyon C, 2009. Regulation of the longevity response to temperature by thermosensory neurons in., 19(9):715-722. https://doi.org/10.1016/j.cub.2009.03.041
Li JJ, Wang R, Du MM, et al., 2016. Dynamic transition on the seizure-like neuronal activity by astrocytic calcium channel block., 91:702-708. https://doi.org/10.1016/j.chaos.2016.08.009
Li YY, Gu HG, Jia B, et al., 2021. The nonlinear mechanism for the same responses of neuronal bursting to opposite self-feedback modulations of autapse., 64(7):1459-1471. https://doi.org/10.1007/s11431-020-1753-y
Liu Y, Xu WJ, Ma J, et al., 2020a. A new photosensitive neuron model and its dynamics., 21(9):1387-1396. https://doi.org/10.1631/FITEE.1900606
Liu Y, Xu Y, Ma J, 2020b. Synchronization and spatial patterns in a light-dependent neural network., 89:105297. https://doi.org/10.1016/j.cnsns.2020.105297
Lukić J, Denić D, 2015. A novel design of an NTC thermistor linearization circuit., 22(3):351-362. https://doi.org/10.1515/mms-2015-0035
Lv M, Ma J, Yao YG, et al., 2019. Synchronization and wave propagation in neuronal network under field coupling., 62(3):448-457. https://doi.org/10.1007/s11431-018-9268-2
Ma J, Qin HX, Song XL, et al., 2015. Pattern selection in neuronal network driven by electric autapses with diversity in time delays., 29(1):1450239.https://doi.org/10.1142/S0217979214502397
Ma J, Yang ZQ, Yang LJ, et al., 2019. A physical view of computational neurodynamics., 20(9):639-659. https://doi.org/10.1631/jzus.A1900273
Machens CK, Schütze H, Franz A, et al., 2003. Single auditory neurons rapidly discriminate conspecific communication signals., 6(4):341-342. https://doi.org/10.1038/nn1036
MacVicar BA, Newman EA, 2015. Astrocyte regulation of blood flow in the brain., 7(5):a020388. https://doi.org/10.1101/cshperspect.a020388
Nakayama T, 1985. Thermosensitive neurons in the brain., 35(3):375-389. https://doi.org/10.2170/jjphysiol.35.375
Perc M, 2007a. Effects of small-world connectivity on noise-induced temporal and spatial order in neural media., 31(2):280-291. https://doi.org/10.1016/j.chaos.2005.10.018
Perc M, 2007b. Fluctuating excitability: a mechanism for self-sustained information flow in excitable arrays., 32(3):1118-1124. https://doi.org/10.1016/j.chaos.2005.11.035
Peron S, Gabbiani F, 2009. Spike frequency adaptation mediates looming stimulus selectivity in a collision-detecting neuron., 12(3):318-326. https://doi.org/10.1038/nn.2259
Pikovskii AS, Rabinovich MI, 1978. A simple autogenerator with stochastic behavior.,23: 183-185.
Pountougnigni OV, Yamapi R, Filatrella G, et al., 2019. Noise and disorder effects in a series of birhythmic Josephson junctions coupled to a resonator., 99(3):032220. https://doi.org/10.1103/PhysRevE.99.032220
Qin HX, Ma J, Wang CN, et al., 2014. Autapse-induced target wave, spiral wave in regular network of neurons., 57(10):1918-1926. https://doi.org/10.1007/s11433-014-5466-5
Richter CP, Bayon R, Izzo AD, et al., 2008. Optical stimulation of auditory neurons: effects of acute and chronic deafening., 242(1-2):42-51. https://doi.org/10.1016/j.heares.2008.01.011
Ruchty M, Roces F, Kleineidam CJ, 2010. Detection of minute temperature transients by thermosensitive neurons in ants., 104(3):1249-1256. https://doi.org/10.1152/jn.00390.2010
Saira OP, Zgirski M, Viisanen KL, et al., 2016. Dispersive thermometry with a Josephson junction coupled to a resonator., 6(2):024005. https://doi.org/10.1103/PhysRevApplied.6.024005
Seung HS, Lee DD, Reis BY, et al., 2000. The autapse: a simple illustration of short-term analog memory storage by tuned synaptic feedback., 9(2):171-185. https://doi.org/10.1023/A:1008971908649
Song XL, Wang HT, Chen Y, 2019. Autapse-induced firing patterns transitions in the Morris–Lecar neuron model., 96(4):2341-2350. https://doi.org/10.1007/s11071-019-04925-7
Tang J, Zhang J, Ma J, et al., 2017. Astrocyte calcium wave induces seizure-like behavior in neuron network., 60(7):1011-1018. https://doi.org/10.1007/s11431-016-0293-9
Vourkas I, Sirakoulis GC, 2016. Emerging memristor-based logic circuit design approaches: a review., 16(3):15-30. https://doi.org/10.1109/MCAS.2016.2583673
Wang CN, Guo SL, Xu Y, et al., 2017. Formation of autapse connected to neuron and its biological function., 2017:5436737. https://doi.org/10.1155/2017/5436737
Wang CN, Tang J, Ma J, 2019. Minireview on signal exchange between nonlinear circuits and neurons via field coupling., 228(10):1907-1924. https://doi.org/10.1140/epjst/e2019-800193-8
Wiederman SD, O’Carroll DC, 2013. Selective attention in an insect visual neuron., 23(2):156-161. https://doi.org/10.1016/j.cub.2012.11.048
Wu FQ, Zhang Y, Zhang XJ, 2019. Regulating firing rates in a neural circuit by activating memristive synapse with magnetic coupling., 98(2):971-984. https://doi.org/10.1007/s11071-019-05239-4
Wu FQ, Ma J, Zhang G, 2020. Energy estimation and coupling synchronization between biophysical neurons., 63(4):625-636. https://doi.org/10.1007/s11431-019-9670-1
Xu Y, Liu MH, Zhu ZG, et al., 2020a. Dynamics and coherence resonance in a thermosensitive neuron driven by photocurrent., 29(9):098704. https://doi.org/10.1088/1674-1056/ab9dee
Xu Y, Guo YY, Ren GD, et al., 2020b. Dynamics and stochastic resonance in a thermosensitive neuron., 385:125427. https://doi.org/10.1016/j.amc.2020.125427
Yao CG, 2020. Synchronization and multistability in the coupled neurons with propagation and processing delays., 101(4):2401-2411. https://doi.org/10.1007/s11071-020-05922-x
Yao CG, He ZW, Nakano T, et al., 2019. Inhibitory-autapse-enhanced signal transmission in neural networks., 97(2):1425-1437. https://doi.org/10.1007/s11071-019-05060-z
Zandi-Mehran N, Jafari S, Golpayegani SMRH, et al., 2020. Different synaptic connections evoke different firing patterns in neurons subject to an electromagnetic field., 100(2):1809-1824. https://doi.org/10.1007/s11071-020-05576-9
Zhang L, Jones S, Brody K, et al., 2004. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse., 286(6):G983-G991. https://doi.org/10.1152/ajpgi.00441.2003
Zhang Y, Xu Y, Yao Z, et al., 2020. A feasible neuron for estimating the magnetic field effect., 102(3):1849-1867. https://doi.org/10.1007/s11071-020-05991-y
Zhang Y, Zhou P, Tang J, et al., 2021. Mode selection in a neuron driven by Josephson junction current in presence of magnetic field., 71:72-84. https://doi.org/10.1016/j.cjph.2020.11.011
Zhao ZG, Li L, Gu HG, 2020. Excitatory autapse induces different cases of reduced neuronal firing activities near Hopf bifurcation., 85:105250. https://doi.org/10.1016/j.cnsns.2020.105250
Zhou P, Yao Z, Ma J, et al., 2021a. A piezoelectric sensing neuron and resonance synchronization between auditory neurons under stimulus., 145: 110751. https://doi.org/10.1016/j.chaos.2021.110751
Zhou P, Hu XK, Zhu ZG, et al., 2021b. What is the most suitable Lyapunov function?, 150:111154. https://doi.org/10.1016/j.chaos.2021.111154
Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering)
ISSN 1673-565X (Print); ISSN 1862-1775 (Online)
www.jzus.zju.edu.cn; link.springer.com
E-mail: jzus_a@zju.edu.cn
© Zhejiang University Press 2021
July 13, 2021;
Revision accepted Aug. 5, 2021;
Crosschecked Sept. 4, 2021
https://doi.org/10.1631/jzus.A2100323
O59; TN710
*Project supported by the National Natural Science Foundation of China (No. 12072139)
 Journal of Zhejiang University-Science A(Applied Physics & Engineering)2021年9期
Journal of Zhejiang University-Science A(Applied Physics & Engineering)2021年9期
- Journal of Zhejiang University-Science A(Applied Physics & Engineering)的其它文章
- Correspondence:Centrifuge model testing to ascertain vertical displacements of a pile under cyclic lateral loads*#
- Dynamic response analysis of airport pavements during aircraft taxiing for evaluating pavement bearing capacity*
- Compressive behavior of hybrid steel-polyvinyl alcohol fiber-reinforced concrete containing fly ash and slag powder: experiments and an artificial neural network model*#
- A method to avoid the cycle-skip phenomenon in time-of-flight determination for ultrasonic flow measurement*
- Carbon self-doped polytriazine imide nanotubes with optimized electronic structure for enhanced photocatalytic activity*#
