EPHA2抗体对结直肠癌西妥昔单抗耐药的逆转作用
王琪玮 张敬东


[摘要] 目的 明确结直肠癌(CRC)病人西妥昔单抗治疗前后EPHA2的表达水平,并探索EPHA2抗体能否逆转西妥昔单抗的耐药。
方法 收集8例KRAS野生型CRC病人在西妥昔单抗治疗前及疾病进展后的配对肿瘤组织标本,采用免疫组化方法检测EPHA2的表达水平。使用西妥昔单抗处理其敏感细胞系、原发及继发耐药的CRC细胞系,以MTT方法检测细胞增殖能力。使用EPHA2抗体DS-8895a、西妥昔单抗单药或联合处理原发及继发耐药的CRC细胞,检测各组细胞增殖能力。
结果 在西妥昔单抗治疗前及进展后的肿瘤组织中,存在治疗后EPHA2表达的上调(t=6.056,P<0.05)。在KRAS野生型CRC细胞系中,DS-8895a与西妥昔单抗有协同增敏作用;而在西妥昔单抗继发耐药CRC细胞系中,DS-8895a可逆转西妥昔单抗的耐药。
结论 EPHA2抗体与西妥昔单抗联合可能具有逆转西妥昔单抗继发耐药作用,以及潜在的协同增敏作用。EPHA2有可能成為CRC治疗策略的新突破。
[关键词] 结直肠肿瘤;受体,EphA2;西妥昔单抗;抗药性,肿瘤
[中图分类号] R735.34,R345.57
[文献标志码] A
[文章编号] 2096-5532(2021)03-0416-05
doi:10.11712/jms.2096-5532.2021.57.125
[开放科学(资源服务)标识码(OSID)]
[网络出版] https://kns.cnki.net/kcms/detail/37.1517.R.20210628.1638.006.html;2021-06-29 14:07:31
REVERSAL EFFECT OF EPHA2 ANTIBODY ON CETUXIMAB RESISTANCE IN COLORECTAL CANCER
WANG Qiwei, ZHANG Jingdong
(Medical Oncology Department of Gastrointestinal Cancer, Cancer Hospital of China Medical University, Shenyang 110042, China)
[ABSTRACT]Objective To investigate the expression level of EPHA2 before and after cetuximab treatment in patients with colorectal cancer (CRC), and to explore whether the EPHA2 antibody can reverse the resistance to cetuximab in CRC cell lines.
Methods Paraffin specimens of tumor tissues from eight KRAS wild-type CRC patients before and after treatment with cetuximab were collected, and the expression of EPHA2 was determined by immunohistochemistry. Cetuximab-sensitive, primary-resistant, and acquired-resistant CRC cell lines were treated with cetuximab, and the cell proliferation was measured by MTT method. The primary-resistant and acquired-resistant CRC cells were treated with EPHA2 antibody DS-8895a and cetuximab, alone or in combination, and the cell proliferation was assessed.
Results In the tumor tissues before and after cetuximab treatment, the expression of EPHA2 was up-regulated after treatment (t=6.056,P<0.05). In KRAS wild-type CRC cell line, DS-8895a and cetuximab had synergistic sensitization, while in CRC cell line with acquired resistance to cetuximab, DS-8895a reversed the resis-
tance to cetuximab.
Conclusion The combination of EPHA2 antibody and cetuximab may reverse the acquired resistance to cetuximab and have potential synergistic sensitization effects. EPHA2 may become a new target for the treatment of CRC.
[KEY WORDS]colorectal neoplasms; receptor, EphA2; cetuximab; drug resistance, neoplasm
结直肠癌的发病率居我国恶性肿瘤的第2位,死亡率居第5位[1]。以西妥昔单抗为代表的抗细胞生长因子受体(EGFR)单抗,被广泛用于KRAS基因野生型转移性结直肠癌(mCRC)病人的转化[2-5]及姑息治疗中[6-7]。然而,西妥昔单抗与化疗联合的客观有效率仅为55%~65%,中位无进展生存期为10~11月,这意味着有近半数的病人未能获益,并且有效的病人多在1年内出现进展[6,8]。因此,探讨西妥昔单抗的原发及继发耐药机制并尝试进行逆转具有重要的基础及临床意义。除了研究较充分的耐药机制,如KRAS、NRAS、BRAF、PI3K等基因突变导致下游通路的持续激活[9],近年来的研究显示,结直肠癌中EPHA2的过表达与肿瘤转移和更差的预后相关,且可能与西妥昔单抗的疗效相关[10-14]。EPHA2是促红细胞生成素产生肝细胞受体(EPH)家族的成员之一,目前关于EPHA2靶向治疗能否逆转西妥昔单抗的耐药尚无深入研究。本研究旨在明确CRC病人西妥昔单抗治疗前后EPHA2的表达水平,并在原发及继发CRC耐药细胞系中探索EPHA2抗体能否逆转西妥昔单抗的耐药。
1 材料与方法
1.1 实验材料
1.1.1 抗体与试剂 BCA蛋白浓度检测试剂盒(由美国Thermo Fisher Scientific公司提供);β-actin抗体、EPHA2抗体、pEPHA2抗体(美国Sigma公司);RPMI 1640培养基(美国Hyclon公司);胎牛血清(BI公司);胰蛋白酶(杭州四季青生物工程材料有限公司);MTT试剂(美国Sigma公司);山羊抗兔二抗(美国Abcam公司),DS-8895a(日本Daiichi Sankyo公司)。
1.1.2 细胞系 人结直肠癌细胞系HCT116购自中国科学院上海细胞库,人结直肠癌细胞系HKH-2由日本国际医学中心研究所的SHIRASAWA博士转赠。
1.2 实验方法
1.2.1 细胞培养 使用含体积分数0.10胎牛血清的RPMI 1640培养基分别对HKH-2和HCT116细胞进行培养,每2~3 d传代1次,于37 ℃、含体积分数0.05的CO2培养箱中培养,实验时取对数生长期细胞。
1.2.2 细胞活性检测 细胞用不同药物处理72 h后,用MTT方法检测细胞活性。酶标仪检测细胞在450 nm波长处的吸光度值,根据吸光度值计算不同药物浓度对应的细胞活性,绘制细胞增殖曲线。
1.2.3 Western blot检测蛋白表达 细胞用不同药物处理24 h后,收取细胞,加入胰蛋白酶和磷酸酶抑制剂,加入CytoBuster蛋白提取试剂提取总蛋白后,用BCA法测定蛋白浓度。取等量蛋白样品于含体积分数0.10的SDS-PAGE中电泳后转移到硝酸纤维素膜上,加入一抗4 ℃摇床上过夜,加入二抗室温孵育1 h,ECL法显色。在凝胶成像系统中成像拍照,以β-actin标定。
1.2.4 免疫组化检测 选取2017年7月—2017年12月在辽宁省肿瘤医院行转化治疗(西妥昔单抗联合化疗)后出现疾病进展的8例结直肠癌病人,年龄43~65岁,8例病人在转化治疗前经肠镜活检病理确诊为结直肠腺癌,在疾病进展后均接受姑息性手术治疗,术后病理均诊断为结直肠腺癌。本研究选取该8例病人在转化治疗前的肠镜活检标本蜡块,以及转化治疗后的结直肠癌原发灶手術标本蜡块,标本的组织蜡块均保存于我院病理科。使用切片机对石蜡包埋的肿瘤组织进行切片,厚度4~5 μm,置于60 ℃恒温干燥箱中烘烤4 h。切片脱蜡水化,之后于体积分数0.03 H2O2中室温摇床孵育10 min,PBS清洗3次。将其放入含有EDTA抗原修复液的锅中,煮沸、冷却并清洗。使用50 g/L的BSA抗原封闭1 h,滴加一抗后于湿盒中4 ℃孵育过夜。复温后二抗孵育1 h,以DAB显色、苏木精复染,盐酸乙醇分化、梯度脱水及封片,风干后保存。免疫组化结果判定由病理科医生按照如下标准独立进行。染色强度被分为4级:未着色评分为0,着色较弱评分为1分,染色中等和高强度分别评分为2分和3分。染色强度乘以染色细胞的百分比(1%~100%)获得H-score,H-score范围为0~300。见图1A。
1.3 统计学分析
采用SPSS 20.0统计软件对数据进行分析。计量资料数据以±s表示,两组均数间比较使用配对t检验,多组均数间比较使用双因素方差分析。以P<0.05为差异有统计学意义。
2 结 果
2.1 结直肠癌组织中西妥昔单抗耐药后EPHA2表达上调
本研究收集了8例KRAS野生型结直肠癌病人在西妥昔单抗治疗前及疾病进展后的配对的肿瘤组织石蜡标本,使用免疫组化方法检测了EPHA2的表达水平,以免疫组化评分表示EPHA2表达水平。见图1A。结果显示,在西妥昔单抗耐药后的结直肠癌组织中,存在EPHA2表达的明显上调(t=6.056,P<0.05),EPHA2上调可能与西妥昔单抗的继发耐药相关。见图1B、C。
2.2 西妥昔单抗对3种不同CRC细胞的增殖活力和EPHA2及pEPHA2表达影响
本研究前期选用KRAS野生型(KRAS-WT)的西妥昔单抗敏感CRC细胞系HKH-2,给予递增浓度的西妥昔单抗处理并多次传代5个月后,已成功构建对西妥昔单抗耐药的CRC细胞系HKH-2/CR,以及KRAS突变型(KRAS-MT)的CRC细胞系HCT116。分别使用递增浓度的西妥昔单抗处理HKH-2细胞、HKH-2/CR细胞和HCT116细胞,采用MTT方法检测细胞增殖活力,使用GraphPad Prism绘制细胞增殖曲线。结果表明,药物浓度和不同细胞系有交互作用(F药物=407.50,P<0.05;F细胞系=4 981.00,P<0.05;F药物×细胞系=67.57,P<0.05)。加入西妥昔单抗处理细胞后,HKH-2/CR细胞和HCT116细胞存在明显耐药性;而HKH-2细胞则对西妥昔单抗的处理相对敏感,加入西妥昔单抗后,细胞生长受到抑制。见图2A。在HKH-2细胞中,使用Western blot法分别检测细胞在西妥昔单抗(10 nmol/L)处理前及其处理后1、12、24 h的EPHA2和pEPHA2表达水平,结果显示,西妥昔单抗给药后EPHA2及pEPHA2表达水平均明显上调。见图2B。
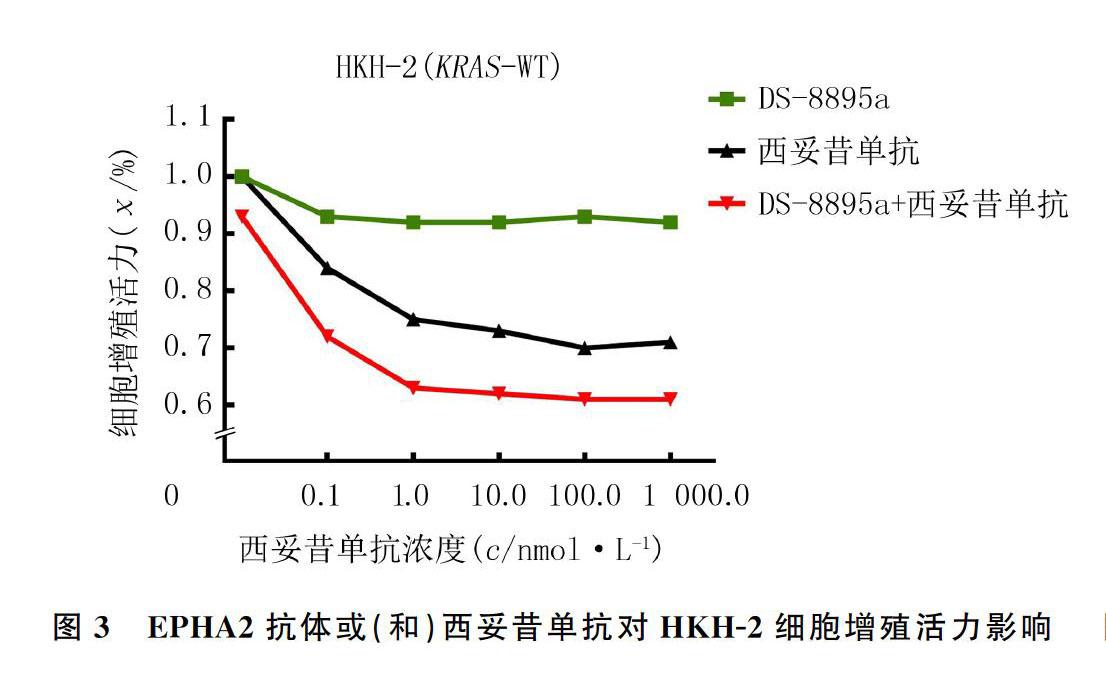
2.3 EPHA2抗体或(和)西妥昔单抗对HKH-2细胞增殖活力影响
在KRAS-WT的西妥昔单抗敏感CRC细胞系HKH-2中,分别用不同浓度的EPHA2抗体DS-8895a、西妥昔单抗、DS-8895a联合西妥昔单抗处理细胞,其中联合用药组中DS-8895a的浓度固定为10 nmol/L,采用MTT法检测各组细胞增殖能力。结果显示,药物浓度以及不同细胞系有交互作用(F药物=1 936.00,P<0.05;F细胞系=4 137.00,P<0.05;F药物×细胞系=228.70,P<0.05)。DS-8895a与西妥昔单抗具有明显的协同作用。见图3。
2.4 EPHA2抗体或(和)西妥昔单抗对HKH-2/CR细胞继发耐药影响
在西妥昔单抗继发耐药的CRC细胞系HKH-2/CR中,分别使用不同浓度的EPHA2抗体DS-8895a、西妥昔单抗、DS-8895a联合西妥昔单抗处理细胞,其中联合用药组中DS-8895a的浓度固定为10 nmol/L,采用MTT法检测各组细胞的增殖能力。结果显示,药物浓度和不同细胞系存在交互作用(F药物=600.70,P<0.05;F细胞系=8 031.00,P<0.05;F药物×细胞系=91.13,P<0.05)。在对西妥昔单抗继发耐药的CRC细胞HKH-2/CR中,EPHA2抗体DS-8895可能有逆转西妥昔单抗继发耐药的作用。见图4。
3 讨 论
EPH家族是人类基因组中最大的酪氨酸激酶受体家族,而EPHA2是EPH家族中的一个重要成员,其在多种上皮来源的组织和细胞系中广泛表达[11]。EPHA2在正常细胞的恶性转化、血管生成、肿瘤转移和治疗耐药中的作用已在多种肿瘤中得到广泛研究,特别是在乳癌[15]、胰腺癌[16,17]、非小细胞肺癌[18]、黑色素瘤[19]和头颈部鳞癌[20]等肿瘤中。在非小细胞肺癌中,EPHA2的过表达与EGFR酪氨酸激酶抑制剂(EGFR-TKI)的治疗耐药相关,抗EPHA2治疗可逆转EGFR-TKI耐药[11]。在头颈部鳞癌中EPHA2的过表达与西妥昔单抗的疗效相关[19]。既往我们对于EPHA2在结直肠癌组织中的表达及意义并不十分了解,但近年来研究结果显示,EPHA2可能是促进结直肠癌侵袭转移的驱动基因,结直肠癌中EPHA2表达与西妥昔单抗的疗效呈负相关[10-11],为西妥昔单抗提供了潜在的疗效预测标志物。然而,关于EPHA2靶向治疗能否逆转西妥昔单抗的耐药尚无深入研究。
本研究在西妥昔单抗治疗前及疾病进展后配对的肿瘤组织中,观察到继发耐药后存在EPHA2的表达上调。而既往研究多为检测治疗前EPHA2表达水平对西妥昔单抗疗效影响[13,21],即原发耐药与EPHA2的关系,罕见报道EPHA2水平与西妥昔单抗继发耐药的关系。此外,本研究首次报道了新型EPHA2抗体DS-8895a与西妥昔单抗有协同增敏作用,而且在继发耐药细胞系中可逆转西妥昔单抗的耐药,结果与MARTINI等[21]报道的EPHA2抗体ALW-II-41-27可逆转西妥昔单抗的结论一致。因此,我们认为EPHA2靶点可能成为结直肠癌中逆转西妥昔单抗耐药的一个新方向。目前,EPHA2已逐渐成为多种肿瘤治疗的潜在靶点[22],EPHA2抗体DS-8895a在实体瘤的Ⅰ期临床研究中表现出了良好的安全性[23],我们期待未来在结直肠癌病人中验证DS-8895a对西妥昔单抗的逆转耐药及增敏作用。然而,本研究尚未對EPHA2抗体逆转西妥昔单抗耐药的机制做进一步的探讨。有研究表明,EPHA2受体及配体Ephrin-A1,可形成一个重要的细胞通讯系统[24],EPHA2可通过调节配体依赖性和非配体依赖性信号传导来抑制和促进肿瘤细胞的迁移和侵袭[20]。在无配体情况下,非配体依赖性的EPHA2通路在RAS/ERK/RSK下游信号通路中起关键作用[10]。EPHA2抗体逆转结直肠癌西妥昔单抗耐药的作用机制仍有待深入探讨。
综上所述,EPHA2抗体与西妥昔单抗联合应用可能有逆转西妥昔单抗继发耐药的作用,以及潜在的协同增敏作用。本研究为结直肠癌治疗中逆转西妥昔单抗耐药提供了新的理论依据,EPHA2有可能成为结直肠癌治疗策略的新突破。
[参考文献]
[1]BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: a Cancer Journal for Clinicians, 2018,68(6):394-424.
[2]MODEST D P, HEINEMANN V, FOLPRECHT G, et al. Factors that influence conversion to resectability and survival after resection of metastases in RAS WT metastatic colorectal cancer (mCRC): analysis of FIRE-3- AIOKRK0306[J]. Annals of Surgical Oncology, 2020,27(7):2389-2401.
[3]KURRECK A, GEISSLER M, MARTENS U M, et al. Dynamics in treatment response and disease progression of metastatic colorectal cancer (mCRC) patients with focus on BRAF status and primary tumor location: analysis of untreated RAS-wild-type mCRC patients receiving FOLFOXIRI either with or without panitumumab in the VOLFI trial (AIO KRK0109)[J]. Journal of Cancer Research and Clinical Oncology, 2020,146(10):2681-2691.
[4]HU H B, WANG K, HUANG M J, et al. Modified FOLFO-XIRI with or without cetuximab as conversion therapy inpatients with RAS/BRAF wild-type unresectable liver meta-stases colorectal cancer: the FOCULM multicenter phase Ⅱ trial[J]. The Oncologist, 2021,26(1):e90-e98.
[5]CIARDIELLO F, NORMANNO N, MARTINELLI E, et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase Ⅱ trial of FOLFOX plus cetuximab versus FOLFOX[J]. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 2016,27(6):1055-1061.
[6]STINTZING S, MODEST D P, ROSSIUS L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial[J]. The Lancet Oncology, 2016,17(10):1426-1434.
[7]VENOOK A P, NIEDZWIECKI D, LENZ H J, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial[J]. JAMA, 2017,317(23):2392-2401.
[8]MARTINELLI E, CIARDIELLO D, MARTINI G, et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: challenges and future perspectives[J]. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 2020,31(1):30-40.
[9]SEPULVEDA A R, HAMILTON S R, ALLEGRA C J, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American society for clinical pathology, college of American pathologists, association for molecular pathology, and American society of clinical oncology[J]. Archives of Pathology & Laboratory Medicine, 2017,141(5):625-657.
[10]DUNNE P D, DASGUPTA S, BLAYNEY J K, et al. EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer[J]. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research, 2016,22(1):230-242.
[11]STRIMPAKOS A, PENTHEROUDAKIS G, KOTOULA V, et al. The prognostic role of ephrin A2 and endothelial growth factor receptor pathway mediators in patients with advanced colorectal cancer treated with cetuximab[J]. Clinical Colorectal Cancer, 2013,12(4):267-274.e2.
[12]DE ROBERTIS M, LOIACONO L, FUSILLI C, et al. Dysregulation of EGFR pathway in EphA2 cell subpopulation significantly associates with poor prognosis in colorectal cancer[J]. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research, 2017,23(1):159-170.
[13]DE ROBERTIS M, MAZZA T, FUSILLI C, et al. EphB2 stem-related and EphA2 progression-related miRNA-based networks in progressive stages of CRC evolution: clinical significance and potential miRNA drivers[J]. Molecular Cancer, 2018,17(1):169.
[14]CUY S E, QUERALT B, MARTIN-CASTILLO B, et al. EphA2 receptor activation with ephrin-A1 ligand restores cetu-ximab efficacy in NRAS-mutant colorectal cancer cells[J]. Oncology Reports, 2017,38(1):263-270.
[15]TORRES-ADORNO A M, VITRAC H, QI Y, et al. Eicosa-
pentaenoic acid in combination with EPHA2 inhibition shows efficacy in preclinical models of triple-negative breast cancer by disrupting cellular cholesterol efflux[J]. Oncogene, 2019,38(12):2135-2150.
[16]MARKOSYAN N, LI J, SUN Y H, et al. Tumor cell-intrinsic EPHA2 suppresses anti-tumor immunity by regulating PTGS2 (COX-2) [J]. Journal of Clinical Investigation, 2019,130:3594-3609.
[17]RODRIGUES M, RICHARDS N, NING B, et al. Rapid lipid-based approach for normalization of quantum-dot-detected biomarker expression on extracellular vesicles in complex biological samples[J]. Nano Letters, 2019,19(11):7623-7631.
[18]TAN Y H Carol, SRIVASTAVA S, WON B M, et al. EPHA2 mutations with oncogenic characteristics in squamous cell lung cancer and malignant pleural mesothelioma[J]. Oncogenesis, 2019,8:49.
[19]SAKAMOTO A, KATO K, HASEGAWA T, et al. An agonistic antibody to EPHA2 exhibits antitumor effects on human melanoma cells[J]. Anticancer Research, 2018,38(6):3273-3282.
[20]IEGUCHI K, MARU Y. Roles of EphA1/A2 and ephrin-A1 in cancer[J]. Cancer Science, 2019,110(3):841-848.
[21]MARTINI G, CARDONE C, VITIELLO P P, et al. EPHA2 is a predictive biomarker of resistance and a potential therapeutic target for improving antiepidermal growth factor receptor therapy in colorectal cancer[J]. Molecular Cancer Therapeutics, 2019,18(4):845-855.
[22]HUANG C H, YUAN W J, LAI C, et al. EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance[J]. International Journal of Cancer, 2020,146(7):1937-1949.
[23]SHITARA K, SATOH T, IWASA S, et al. Safety, tolerabi-
lity, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: a first-in-human phase Ⅰ dose escalation and dose expansion study in patients with advanced solid tumors[J]. Journal for Immunotherapy of Cancer, 2019,7(1):219.
[24]LI J Y, XIAO T, YI H M, et al. S897 phosphorylation of EphA2 is indispensable for EphA2-dependent nasopharyngeal carcinoma cell invasion, metastasis and stem properties[J]. Cancer Letters, 2019,444:162-174.
(本文編辑 于国艺)

