Progress and Prospects of Hydrogen Production:Opportunities and Challenges
Bing Zhang| Sui-Xin Zhang | Rui Yao | Yong-Hong Wu | Jie-Shan Qiu
Abstract—This study presents an overview of the current status of hydrogen production in relation to the global requirement for energy and resources.Subsequently,it symmetrically outlines the advantages and disadvantages of various production routes including fossil fuel/biomass conversion,water electrolysis,microbial fermentation,and photocatalysis (PC),in terms of their technologies,economy,energy consumption,and costs.Considering the characteristics of hydrogen energy and the current infrastructure issues,it highlights that onsite production is indispensable and convenient for some special occasions.Finally,it briefly summarizes the current industrialization situation and presents future development and research directions,such as theoretical research strengthening,renewable raw material development,process coupling,and sustainable energy use.
1.lntroduction
Energy is a fundamental requirement for human survival,social civilization,and economic development.Traditional fossil fuels,such as coal,petroleum,and natural gas,have been used for over 20 decades,resulting in excessive energy consumption,unrestrained exploitation,and considerable waste.Consequently,these nonrenewable resources are rapidly approaching depletion and exhaustion.Particularly,the successive expansion of the global population and the rapid economic development have resulted in an increased daily energy demand,worsening the energy crisis issue[1].Additionally,the excessive exploitation and overuse of fossil fuels have caused serious environmental pollution.Hence,most countries are keen to find an alternative clean energy source[2],[3].
Hydrogen,as a clean energy carrier for heat and electricity,has many appealing characteristics,including a large storage capacity,high energy conversion,cleanliness and environmental friendliness,renewable production,vast specific energy,zero emission,wide sources,reliability,and easy storage and regeneration[4],[5].Thus,it is considered to be the cleanest and most promising energy source of the 21st century.Additionally,hydrogen is an essential chemical raw material,widely used in large-scale industrial processes including ammonia synthesis,petroleum refining,and the water-gas shift reaction.From the relationship between the supply and demand shown inFig.1,it can be inferred that hydrogen is extremely versatile,and it offers promising prospects.Thus,hydrogen production has received considerable global attention.

Fig.1.Relationship between the supply and demand of hydrogen.
Since hydrogen does not exist as a molecule in nature,it is produced by the conversion of certain source materials containing the hydrogen element (e.g.,water or carbohydrate).Presently,over 96% of the global hydrogen is produced from traditional fossil sources,of which steam reforming of natural gas contributes 48%,naphtha reforming 30%,and coal gasification 18%[6].Unfortunately,most of the traditional technologies for hydrogen production from fossil fuels are associated with serious environmental pollution and high energy consumption.Therefore,increasing attention has been paid to the application of new technologies for hydrogen production from renewable and nuclear sources,with increasingly strict and relevant environmental protection regulations worldwide[7].Such technologies include water electrolysis,biomass gasification,and nuclear thermal/chemical routes.Regardless,the hydrogen-production process must consume a certain amount of materials and energy via either a traditional or new technology,which will inevitably have an impact on the environment[8].Only by fully understanding each section of the hydrogen-production process can we objectively evaluate the economic and environmental benefits of various hydrogen-production technologies.
In the following section,the current hydrogen-production routes are discussed briefly.
2.Hydrogen-Production Routes
In the industrial chain of hydrogen energy (i.e.,hydrogen production,storage and transportation,fueling,and applications),hydrogen production is the most important segment that impacts the potential applications.Herein,we outline the technologies reported in the literature,including reforming,gasification,water electrolysis,microbial fermentation,and photocatalysis (PC),as well as some other conversion methods involving fossil fuels or biomass,which have been summarized inFig.2.
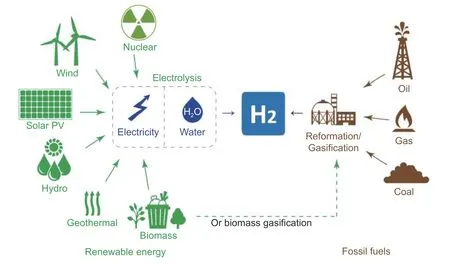
Fig.2.Hydrogen-production routes.
2.1.Source Materials
There are two major source materials for hydrogen production:Fossil fuels and biomass,which will be discussed detailedly in the following.
2.1.1.Fossil Fuel Source
A large variety of fossil fuels,such as natural gas,oil,and coal,can be applied to produce hydrogen via steam reforming or partial oxidation.The increasing demand for hydrogen,together with the growing coal chemical industry,deterioration of crude oil,upgrading of fuel oil quality,and progress in hydrogen energy technologies,has resulted in benefits and challenges for small-and large-scale coal-to-hydrogen production.
In China,where more coal is consumed than oil,coal gasification will remain the main resource for largescale hydrogen production.From the viewpoint of chemistry,coal is a complex material with a highly variable composition,which can be converted into a variety of products.Coal gasification is a method applied to generate power,liquid fuels,chemicals,and hydrogen.Specifically,hydrogen is produced by reacting coal with oxygen and steam at high pressure and temperatures to form synthesis gas,a mixture primarily comprised of carbon monoxide and hydrogen.Thereafter,hydrogen is recovered by pressure swing adsorption (PSA),which has the advantages of wide adaptability of feeding gas,cleanliness,energy saving,high-degree automation,good reliability and flexibility,convenient startup and shutdown,high hydrogen purity,etc.The future directions for this technique are as follows:1) Improving the performance of the adsorbent,2) optimizing the adsorption tower structure to strengthen the utilization rate of the adsorbent and eliminate the influence of pressure and temperatures on the stability,and 3) separating the gas mixture by coupling the low-temperature absorption and membrane technology[9].Additionally,it is very important to study high-efficiency catalysts (e.g.,iron-based oxides) for the water-gas shift reaction[10].
Hydrogen production from natural gas occurs mainly via steam reforming,partial oxidation,autothermal reforming,and chemical chain reforming.The key factors influencing this technology are the catalyst,reactor type,impurities in the raw materials,choice of heating fuels during the reaction,carbon capture in the product,and choice of the hydrogen purification equipment.Osman investigated the two main mechanisms of partial oxidation of methane,namely direct partial oxidation and combustion and the reforming reaction,based on recent kinetic studies[11].Gao et al.discussed the preparation of catalysts,optimization of operation conditions,and influence of impurities in the dry reforming of biogas (methane and carbon dioxide),and they provided guidelines for its industrial applications in hydrogen energy generation[12].
Apart from methane,some other hydrocarbons can be used to produce hydrogen by thermo-catalytic decomposition (TCD).Muhammad et al.discussed the practice of co-feeding methane with other hydrocarbons,specifically ethylene,propylene,hydrogen sulfide,and ethanol,with a detailed overview of TCD reaction kinetics over an empirical model based on the power law[13].
Ethanol has also attracted significant attention from researchers and governments for hydrogen production in recent years,because it can be produced from either fossil or renewable biomass raw materials.Taghizadeh and Aghili recommended the integration of the catalytic reaction and separation process into a single process in a single membrane reactor to significantly improve the conversion and hydrogen yields and reduce the reactor volume.They also presented a brief overview of the effects of different catalysts (such as precious metals,non-precious metals,and bimetallic catalysts)[14].Ogo and Sekine developed a novel electrocatalytic reaction system involving conducting a catalytic reaction in an electric field for lowtemperature catalytic ethanol steam reforming with low-grade waste heat[15].In this electrocatalytic reaction system,hydrogen was produced efficiently from ethanol using a small amount of electricity,even at low temperatures.
2.1.2.Biomass Source
Compared with fossil fuels,biomass is more promising for hydrogen production because of its large reserves and supply,high annual output,and easy oxidation.
Thus far,hydrogen has been produced from various types of biomass via suitable methods,including PC of municipal solid waste,agricultural waste,forest residues[16],polyols,carboxylic acids,and saccharides[17];thermochemical conversion of wood waste,sawdust,olivine particles,lignin,cellulose,and biomass char[18];fermentation of microalgae and cassava (see subsection 2.3)[19],[20];steam reforming of gasified biomass tar[21],biomethane (biogas)[22],and bioethanol[23];supercritical water gasification of wastewater sludge[24];aqueous phase reforming of cellulose,sugars,and polyols[25].
However,most of these processes tend to form more complex and undegradable derivatives after hydrogen production.This limits the application of biomass for hydrogen production.Therefore,the selective conversion of biomass into chemicals or oil is essential for reducing the production cost of the desired product and achieving the full utilization of raw biomass materials.
2.2.Water Electrolysis
Electrolysis is the process of applying an electric current to dissociate water or a water-containing solution into hydrogen gas and oxygen gas,as illustrated inFig.3[26].The electric potential shifts positive ions (H+)toward the cathode and negative ions (OH-) toward the anode.There are different water electrolysis processes,i.e.,alkaline water electrolysis,proton exchange membrane water electrolysis,solid oxide water electrolysis,and alkaline anion exchange membrane (AEM) water electrolysis[27].Among them,alkaline water electrolysis has been successfully established on a pilot demonstration project by the Dalian Institute of Chemical Physics,Chinese Academy of Sciences,of which the highest energy efficiency that can be reached is~88%.
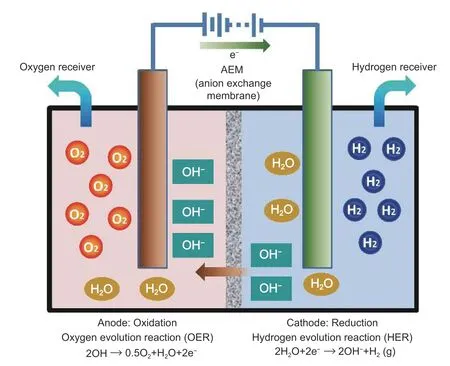
Fig.3.Schematic of water electrolysis[26].
Hydrogen production from electrolyzed coal slurry is superior to the traditional electrolysis water process in terms of energy consumption and production efficiency.This technology can simultaneously achieve the purification of the ore during the electrolysis process,which is worthy of promotion and development.The key future directions are as follows:A reduction of energy consumption in the electrolysis of coal water slurry using renewable energy;an in-depth study of the reaction mechanism to improve the efficiency of hydrogen production and achieve conversion via the coupling of chemical energy and electrical energy;an improvement of the electrode stability and corrosion resistance for improved durability to reduce the electrode cost;an enhancement of the reaction efficiency by developing new catalytic electrodes and catalysts[28],[29].
It is also believed that the utilization of AEM water electrolysis can enable the replacement of conventional noble metal electrocatalysts (Pt,Pd,Ru,and Ir) with low-cost transition metals.Although it is still a developing technology,AEM electrolysis has attracted special attention because of its high power efficiency,membrane stability,robustness,handling ease,and the low-cost hydrogen-production process[30].
Besides the expensive electrode materials,another bottleneck of hydrogen production from water electrolysis is high energy consumption because of the increase in the electrolysis voltage caused by the bubbles generated during the electrolysis process[31].The incorporation of hydrocarbons in water electrolysis can reduce energy consumption.The probable future direction for electrodes should be inexpensive metals or nonmetal composite materials,such as Ni.Moreover,the method of exhausting gas bubbles should be the focus of future research.
2.3.Microbial Fermentation
Biohydrogen generated from a wide variety of biomass has garnered substantial research interest in several fields.One of the major goals in this field is to develop technically sound and effective approaches for significantly improving bioconversion and biohydrogen yields.
Fermentative conversion of biomass gained significant interest during the last decade.Biohydrogen is not only an efficient transportation fuel but also a low-carbon source of heat and electricity.Microbe-assisted conversion (bioconversion) can occur either in the presence or absence of light,i.e.,photo fermentation or dark fermentation,respectively[32].The dark-/photo-fermentation technology is a new approach for increasing the hydrogen yield and energy recovery from organic waste[33].
Fig.4gives the two pathways of biohydrogen production[34].Generally,photo fermentation is associated with a high yield and low productivity,while dark fermentation is associated with a low yield and high productivity.Moreover,the hydrogen-production efficiency is tightly associated with the selected process factors,including the pretreatment conditions,raw materials,and enzymes or bacteria.
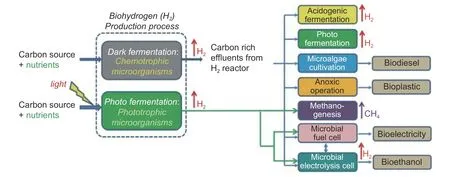
Fig.4.Two microbial pathways for biohydrogen production[34].
So far,several fungi species and enzymes have been exploited for hydrogen production from a large variety of biomass or organic substances.These include microalgae (including cyanobacteria and green algae)[35],[FeFe]-hydrogenases[36],nitrogenase[37],Escherichia coli[38],cyanobacteria[39],and the genus Clostridium[40].Among them,biomass is particularly interesting because of its abundant availability,low costs,mild reaction conditions (usually at ambient temperature and pressure),environmental friendliness,energy saving,and zero consumption of mineral resources.To date,several types of biomass have been validated in the literature,including organic waste[41],food waste[42],carbohydrate-rich biomass[43],sugars[44],lignocellulose[45],sewage sludge[46],macroalgal[47],and crop residues[48].
Moreover,other factors,such as the fermentation mode[32],culture system pretreatment[49],and additives[50],influence the hydrogen-production efficiency.
Presently,the research trends of this technology are mainly focused on the following points:1) Breeding bacterial sources with anti-inhibition and excellent performance to improve the yield and efficiency of hydrogen production;2) expanding more biomass types as raw materials,particularly for annual large-volume production (moreover,the simultaneous realization of multiple objectives,such as environmental protection and high values of biomass resources,by considering the process of hydrogen production from renewable raw materials,industrial and agricultural wastewater,and waste materials,can be achieved);3) developing cleaner and efficient production processes by optimizing the configuration of the fermentative reactor and process.Thus,the economic feasibility of this technique can be effectively improved.
2.4.PC
Solar water splitting is a promising approach for transforming sunlight into renewable,sustainable,and green hydrogen energy using photocatalytically active materials[51].There are three representative methods for transforming solar radiation into molecular hydrogen:PC,photoelectrochemical (PEC),and photovoltaicphtotoelectrochemical (PV-PEC) routes (Fig.5)[52]-[54].
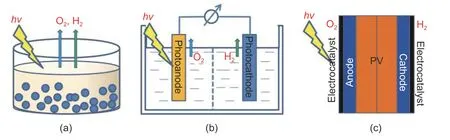
Fig.5.Illustrations for hydrogen production via solar water splitting:(a) PC,(b) PEC,and (c) PV-PEC[54].
PEC cells can directly convert solar energy to hydrogen fuels by water photoelectrolysis,where the functions of both light-harvesting and electrolysis are combined.In such devices,photocathodes and photoanodes carry out the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER),respectively.The focus of such a device is on photocathodes for HER,which are traditionally metal oxides,III-V group and II-VI group semiconductors,silicon,and copper-based chalcogenides as photoactive materials[55].Fereidooni et al.demonstrated the viability of hydrogen production through electrolysis,supported by a photovoltaic power system for economic feasibility,with an annual output of 20 kW (the photovoltaic power station is located at Yazd City in Iran) by both experimental studies and simulations[56].Ahmed and Dincer discussed the possible commercialization of viable PEC reactors[57].Varas-Concha et al.[58]analyzed the effects of five factors on photocatalytic hydrogen production by photoreforming organic compounds.The analyzed factors were the presence of Au as a cocatalyst,type of alcohol applied as the electron donor,intensity of ultraviolet (UV) light,electron donor concentration,and nanoparticle concentration.A main and interaction effect analysis is presented with reduced fixed-effect models for three responses:Total hydrogen generation,catalyst productivity,and electron donor productivity.The presence of Au as a cocatalyst,the intensity of UV light,and their interaction had the highest effect.The best configuration allowed us to achieve the catalyst productivity of 2925 μmol(H2)g–1h–1.
Regardless,the most important factor for realizing hydrogen production is the photocatalyst.The current catalysts used for the photolysis of water to produce hydrogen mainly include metal oxides,metal sulfides,metal nitrogen (nitrogen oxide) compounds,graphite carbonitrides,and new heterostructure photocatalysts[59]-[62].Most of them have a low utilization rate of solar energy and low recombination of photogenerated electron-hole pairs.Moreover,another vital limitation of photocorrosion or cycling stability must be tackled before practical applications[63].These problems lead to low hydrogen-production efficiency,which severely limits the actual photocatalyst use.Therefore,the development of inexpensive,environmentally friendly,and high-performance photocatalysts with a relatively long wavelength spectral response is essential for photocatalytic hydrogen production[64].
TiO2,as a typical metal oxide,has been extensively studied for water photolysis to produce hydrogen.Additionally,metal sulfides,such as CdS,ZnS,and their solid solutions,show good catalytic activity in hydrogen production by water photolysis,because of their narrow and suitable bandgaps.Metal nitrogens(nitrogen oxides) have an ideal visible-light water-splitting energy band structure,which requires modification to reflect the water-splitting activity.Graphite carbonitrides,a new type of nonmetallic visible-light catalyst,present stupendous potential in hydrogen production.Recently,carbon nanomaterial-modified photocatalysts(e.g.,fullerenes,carbon nanotubes,graphene,carbon nanofibers,and carbon quantum dots) have gained significant interest because of their peculiarly controllable morphology[65].Moreover,the use of earth-abundant and inexpensive co-catalysts as replacements of noble metals is necessary for photocatalytic hydrogen production to overcome the main challenges of the high photocatalyst cost and low efficiency for photocatalytic hydrogen production[66].
Different from inorganic semiconductor photocatalysts,organic semiconductor materials have the advantages of diverse synthesis methods,easy functional modification,facile adjustment of energy band and electronic structure,etc.[67].These properties enable their applications in the field of photocatalytic hydrogen production[68].Particularly,the organic conjugated microporous polymer materials developed in recent years have both the semiconductor characteristics of traditional conjugated polymers and the porous characteristics of high specific-surface-area materials[69].
Apart from the frequently used water,some other resources can be adopted as raw materials for photocatalytic hydrogen production,such as liquid waste[70],glycerol[71],and many other types of biomass.
2.5.Other Storage or Conversion Methods
In practice,the transportation and storage of hydrogen are difficult because of its hard compression and liquefaction,as well as its flammable and explosive properties.This restricts its application range.The use of hydrogen-containing compounds (hydrocarbons,alcohols,ethers,etc.) for in situ online reforming for hydrogen production is considered the most realistic solution.As discussed,there are many reforming technologies for hydrogen production,such as methane,methanol,ethanol,and dimethyl ether (DME)reforming methods.Among them,the temperature required for the reforming reaction and energy consumption of methane and ethanol to produce hydrogen is relatively high.Additionally,methanol is toxic and detrimental to the environment and human beings.Moreover,methanol is a liquid;thus,it requires additional gasification equipment,which increases the operating cost.Differently,DME is nontoxic,noncorrosive,environmentally friendly,and easy to store and transport.
Recently,some other species,including acetic acid,formic acid,hydrous hydrazine,ammonia borane,ammonia,and sulfuric acid,were proposed as good candidates for the applications in hydrogen conversion.
Acetic acid is the major component of bio-oil derived from biomass and has been extensively studied for hydrogen generation via catalytic steam reforming[72].The catalyst selection is one of the most important processes in this technology.The heterogeneous catalysts include the supported and unsupported types.The supported catalysts are far superior to the unsupported catalysts in terms of catalytic performance for hydrogen production.
Formic acid,the simplest carboxylic acid,has emerged as one of the most promising candidates for the application as liquid organic hydrogen carriers.Similarly,the preparation of high-efficiency catalysts is the most concerning hurdle in the conversion of hydrogen from formic acid[73]-[75].
Subsequently,the desired hydrogen can be fed to a fuel cell,again producing electricity.There are many methods for improving the efficiency of this process,particularly for the combination of the electrolytic process with other non-electrochemical processes.One of them is the thermochemical hybrid sulfur cycle (also known as the Westinghouse cycle).This cycle combines a thermochemical step (H2SO4decomposition) with the electrochemical one,where hydrogen is produced from the oxidation of SO2and H2O (SO2depolarization electrolysis,conducted at a considerably lower cell voltage compared with that of conventional electrolysis)[76].
Hydrous hydrazine (N2H4·H2O) is a promising hydrogen storage material for fuel cells because of its high hydrogen content and easy transportation and storage;furthermore,N2is the only byproduct of its complete decomposition.Over the past two decades,in response to the global clean and sustainable energy strategies,nanocatalysts have been intensively investigated for the decomposition of hydrous hydrazine with high selectivity for hydrogen production[77].
Ammonia borane is another satisfactory hydrogen storage material with the characteristics of high hydrogen storage density (152.9 g/L),mild hydrogen release conditions,nontoxicity,stable storage at room temperature,and easy storage and transportation.The release of hydrogen from ammonia borane can be fulfilled by three protocols,i.e.,pyrolysis,alcoholysis,or hydrolysis.For the pyrolysis of ammonia borane,attention is paid to reducing the temperature and inhibiting the formation of gaseous byproducts.The research hot spot of hydrogen production by hydrolysis or alcoholysis is the binary or ternary non-noble metal nanocore shell or supported catalysts.Compared with the pyrolysis of ammonia borane,hydrolysis or alcoholysis is more practical because of the mild conditions and fast hydrogen release.The largest challenge associated with ammonia borane as a hydrogen storage material is its regeneration,because the derived byproducts after its decomposition cannot be directly hydrogenated again[78].
The first step of the ammonia decomposition route is the evaporation of liquid ammonia to ammonia gas through a preheater.This is followed by decomposition into the mixed gas containing 75% hydrogen and 25%nitrogen through a decomposition furnace packed with a catalyst at a certain temperature.The catalysts mainly include supported ruthenium,nickel,and iron,as well as carbides and nitrides.Ruthenium-based catalysts exhibit high catalytic activity only under the condition of high loading and high alkalinity on the catalyst surface.Consequently,more precious metal ruthenium and an anticorrosion reactor are required,which largely elevates the production cost.Researchers have explored some inexpensive catalysts,such as iron,nitride,and nickel.It has been found that nickel-based catalysts have the advantages of low costs,easy preparation,good stability,and broad application prospects,although nickel exhibits slightly less catalytic activity than ruthenium[79].The research direction of this technology is on designing new catalysts for ammonia decomposition at low pressure,low temperatures,and high activity to reduce energy consumption.
Nuclear hydrogen production is one of the most prospective methods for the efficient production of CO2-free hydrogen on a large scale with water electrolysis or by thermochemical processes[80].In today’s lightwater reactors,the total process efficiency of converting nuclear heat into hydrogen is about 25%,while the total efficiency of power generation by reactors used for water electrolysis is about 33%.High-temperature reactors can produce hydrogen with up to 50% efficiency using the combination of steam electrolysis and thermochemical or hybrid processes[81],[82].One typical iodine-sulfur (IS) process includes the Bunsen reaction and the characteristic separation of an HI/I2/H2SO4/H2O system,the purification of HIxand sulfuric acid phases,the electro-electrodialysis stacks for HI acid preconcentration,and the catalysts used for HI and SO3decomposition[83].Fig.6presents a simple schematic of the IS cycle[84].The current operating light-water or heavy-water reactors and gas-cooled reactors have a maximum temperature of 350 °C.Thus,these reactors can be used for lowtemperature electrolysis.Other than the economic issues,the nuclear hydrogen technology does not have any major issues for hydrogen production.
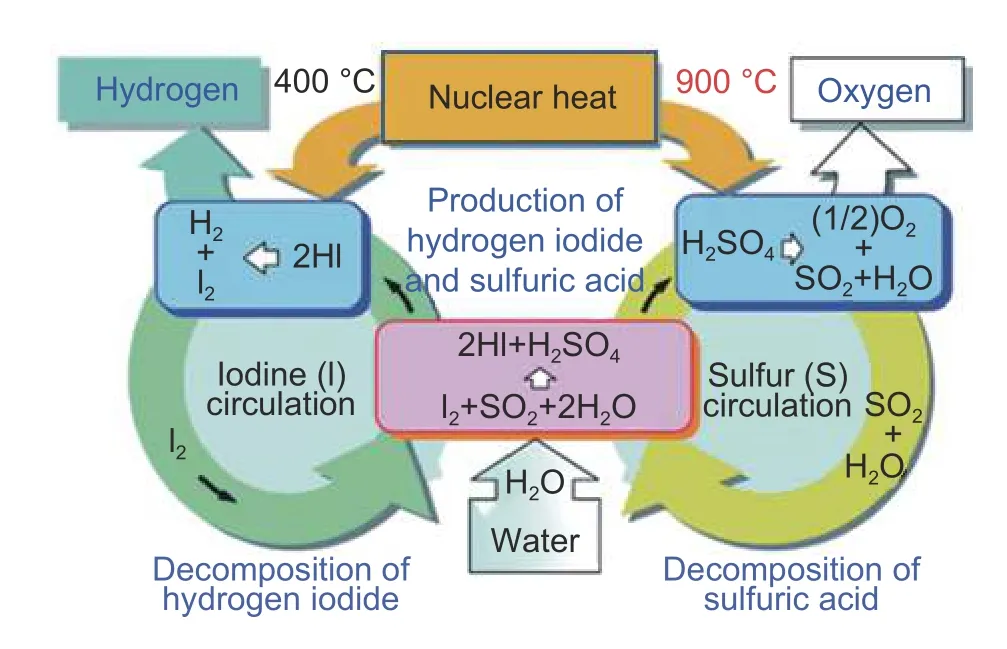
Fig.6.Scheme of the sulfur-iodine cycle[84].
Chemical looping reforming is also applicable for direct hydrogen production in the presence of solid metal oxides as oxygen carriers to replace the water vapor or pure oxygen required in traditional reforming processes.As exemplified inFig.7[85],this method can directly convert the fuel into high-purity synthesis gas or carbon dioxide and water.Furthermore,the reduced metal oxides can be regenerated with the water vapor and directly generate hydrogen[86].The whole process realizes near-zero energy consumption for the in situ hydrogen separation.According to the different products and heating methods,the direct hydrogenproduction system of chemical looping reforming can be categorized into two types:Double-bed system and three-bed system[85].The key factor for this method is the selection of oxygen carriers and the reactors to ensure the appropriate gas-solid contact mode,according to the characteristics of different raw materials and products.
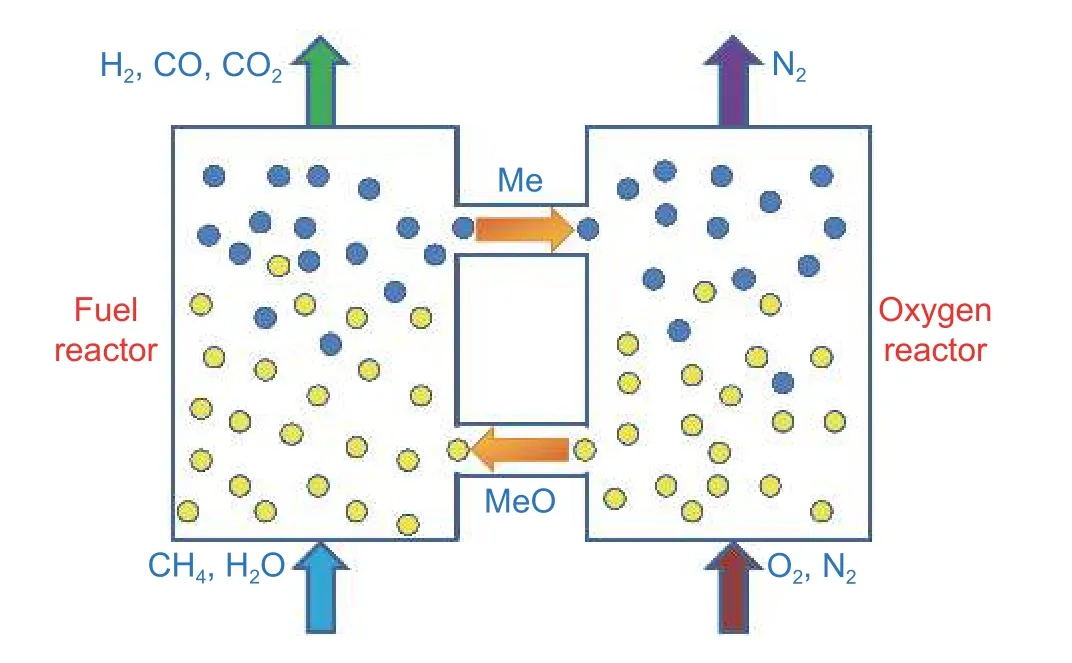
Fig.7.Scheme of the chemical looping reforming of methane[85].
3.Current Status of lndustrialization
According to the public reports,the total global demand for hydrogen has rapidly increased from 255.3 billion cubic meters in 2013 to 324.8 billion cubic meters in 2020,an increase of 27.2%[87].From the previous analysis,it is clear that hydrogen,as a secondary energy source,should be extracted from substances,such as hydrocarbons and water,through an energy conversion process,such as thermochemistry and water electrolysis.Among them,thermochemical processes using fossil as raw materials are particularly used in many industries,mainly including steam reforming of hydrocarbons,partial oxidation of heavy oil,coal gasification,and water electrolysis.
Unlike laboratory research,industrial applications should comprehensively consider various factors,including the source of raw materials,technical environmental protection,investment,and operation costs[88].Presently,the representative processes are natural gas steam reforming,coal gasification,and water electrolysis.Basically,the production cost of natural gas steam conversion is lower than that of water electrolysis for hydrogen production under the conditions of different scales,particularly for large-scale plants.When the scale of hydrogen production is small,the investment required for water electrolysis is lower than that for natural gas steam reforming.When the scale of hydrogen production is large,the conversion of natural gas steam reforming is lower than that of water electrolysis.Therefore,it is appropriate to use water electrolysis to produce hydrogen when the raw material supply is not limited,and the demand for hydrogen is low.Oppositely,it is better to use natural gas steam reforming to produce hydrogen when the demand for hydrogen is large.Additionally,considering the hydrogen-production cost only,coal gasification is superior to natural gas steam reforming[89].
Currently,hydrocarbon steam reforming is the most used method globally,utilized in more than 90% of industrial hydrogen-production plants.The technology was originally invented by Badische Anilin-und-Soda-Fabrik (BASF) in 1926,and Imperial Chemical Industries (ICI) first realized industrialization in the 1930s[90]-[92].The raw materials include natural gas,refinery gas,naphtha,liquefied gas,and various kinds of hydrocarbonrich gas.In a reaction system,multiple parallel reactions and series reactions occur simultaneously.Hydrogen is produced through detoxification purification,water steam conversion,high-temperature shift reactions,low-temperature shift reactions,decarbonization,and methanation of the raw materials.Because the process is rather mature,the hydrogen yield over a unit of raw material consumption is relatively high.Nevertheless,the process route is too long,and the purity of the generated hydrogen is only~95%.
Heavy oil is the residue produced in petroleum refining,which mainly includes atmospheric residues,vacuum residues,and fuel oil produced by petroleum deep processing.Hydrogen is generated by the oxidation of heavy oil with oxygen and steam at certain pressure.Compared with methane,heavy oil has a higher hydrocarbon-to-hydrogen ratio.Therefore,the hydrogen produced by the partial oxidation of heavy oil mainly originates from steam and carbon monoxide,of which steam contributes 69%[93].Different from natural gas steam reforming,the partial oxidation of heavy oil requires additional air separation equipment to produce pure oxygen.
Coal gasification is conducted via the reaction of coal or coke with steam,air,or oxygen at a high temperature to yield a gas mixture of hydrogen,carbon monoxide,and carbon dioxide.After cooling,dust removal,and desulfurization,the gas mixture undergoes a shift reaction with steam.Thereafter,a high percentage of the carbon monoxide content is converted into hydrogen and carbon dioxide.Finally,highly purified hydrogen is recovered by PSA[94].The representative coal gasification technologies include the dry coal powder gasification process of Royal Dutch Shell,the coal water slurry gasification process of American General Electric Company,and the GSP (a trademark owned by Siemens Ltd.) gasification technology.All these companies utilize the process of pressurized pure oxygen fluidized bed liquid slag gasification.
Water electrolysis has been applied industrially for nearly 120 years since 1900.When an aqueous solution of an inorganic acid or alkali metal hydroxide conducts a direct current using two electrodes that do not react chemically,the cathode generates hydrogen gas,and the anode generates oxygen gas.Representative companies are German Lurgi Company,Canada Toronto Electrolyzer Co.,Ltd.,and Norway Hydro Company.The efficiency of hydrogen production by water electrolysis is 75% to 85%.The process is simple and environmentally benign;however,the energy consumption is significant,approximately 4.5 kWh·m-3to 5.5 kWh·m-3.The electricity expenditure accounts for~80% of the hydrogen-production cost[95].
Hydrogen production from methanol conversion includes methanol decomposition and steam reforming.It involves the dehydrogenation of methanol at a low temperature to produce methyl formate,formic acid,and hydrogen.Furthermore,the intermediates,methyl formate and formic acid,decompose to carbon monoxide and hydrogen at high temperatures[96].The commonly used catalysts for the reaction include Ni-based catalysts,Cu-based catalysts,and supported precious metal catalysts.Ni-based catalysts have good stability,a wide application range,and good anti-poison properties;however,they are associated with low activity at low temperatures,poor selectivity,and high contents of carbon monoxide and methane in the product.Cubased catalysts have good catalytic activity and selectivity,although they are easily poisoned.For supported precious metal catalysts,alumina is mostly used as a carrier,platinum and palladium as active components,and rare earth metals,cerium and lanthanum,as modifiers;furthermore,they have the advantages of excellent catalytic activity,selectivity,and stability,and high resistance to poisons and heat,although they are expensive[97].Typically,such technologies have been supplied by Haldor Topsoe,Air Liquide,Catalysts and Chemicals Europe,IHI Corporation,Compañía Española de Sistemas Aeronáuticos (CESA),and Technipetrol.
4.Research and Development Directions
4.1.Comprehensive Evaluation
Besides the rapid increase in demand for hydrogen,the market shows a strong trend toward the diversification of the hydrogen-production scale.This drives the development of new hydrogen-production technologies,such as photocatalytic water splitting,solar energy water splitting,nuclear electrolysis water,biomass conversion,hydrogen sulfide decomposition,photochemical cells,aluminum-alkali redox reactions,phenol steam conversion,and sodium borohydride hydrolysis.Although these technologies are not advanced enough to replace the present industrial hydrogen-production technologies,they have significant scope for the improvement from the perspective of long-term development.Future research should focus on achieving green,efficient,convenient,safe,and low-cost hydrogen-production technologies[98],[99].
Overall,microbial fermentation is associated with low efficiency,PC with harsh conditions to initiate the reaction,and water electrolysis with high energy consumption and costs[100],[101].Table 1summarizes the capital and hydrogen costs of various methods,where the data are adapted from [82].
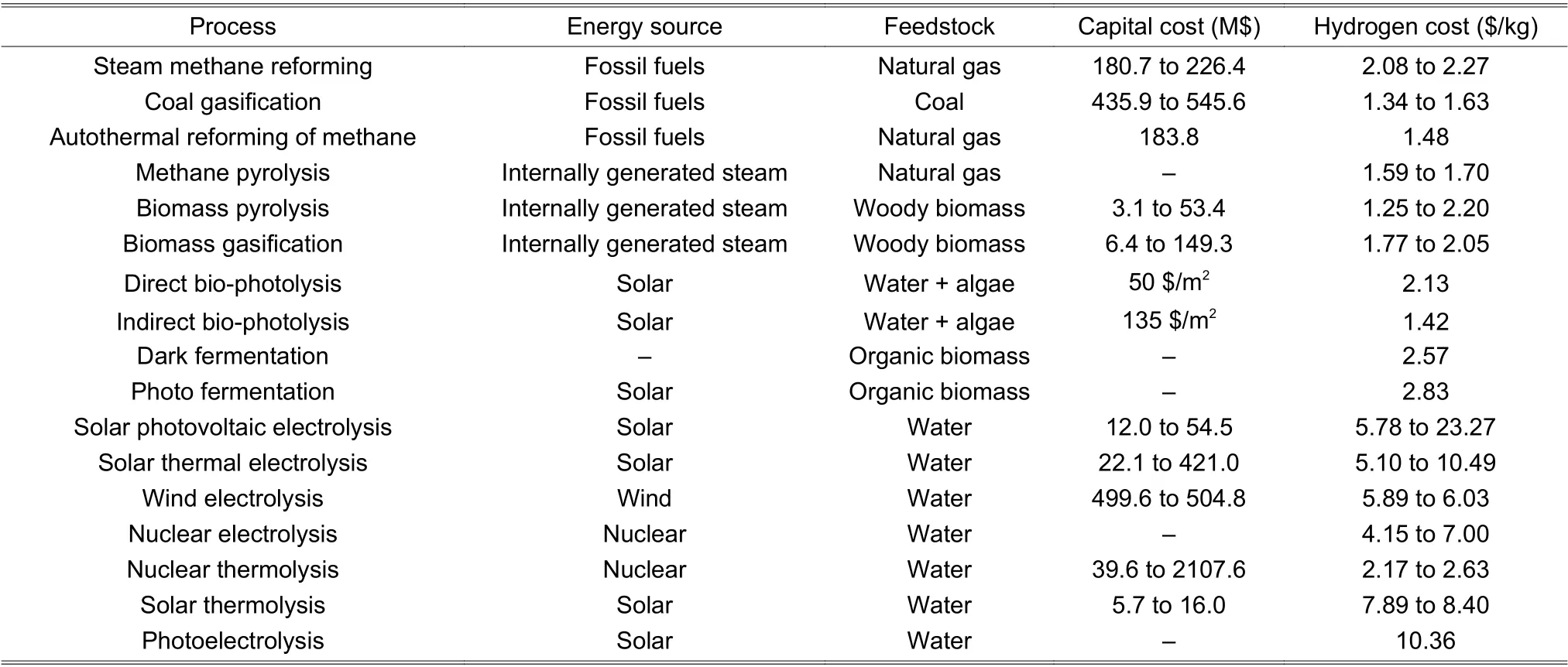
Table 1:Comparison of the capital and hydrogen costs of various hydrogen-production methods
Each technology needs to be comprehensively evaluated according to some general indicators,such as the cleanness index and life cycle cost,considering the technique,economy,and environment aspects[102],[103].It is particularly important to do an objective comparative study of various technologies to provide a technical guide for the healthy development of the hydrogen-production industry.
Xie et al.[104]discussed the life cycle assessment of traditional hydrogen-production technologies (coal gasification,natural gas,etc.) and new hydrogen-production technologies (thermochemistry,renewable energy power generation,biomass gasification,etc.) based on the situation in China.As shown inTable 2with data being adapted from [104] based on unit hydrogen production,the wind power technology is the most environmentally friendly method for minimal equivalent greenhouse gas emission,and nuclear thermal chemistry has the potential for large-scale applications in the future for the lowest energy consumption.
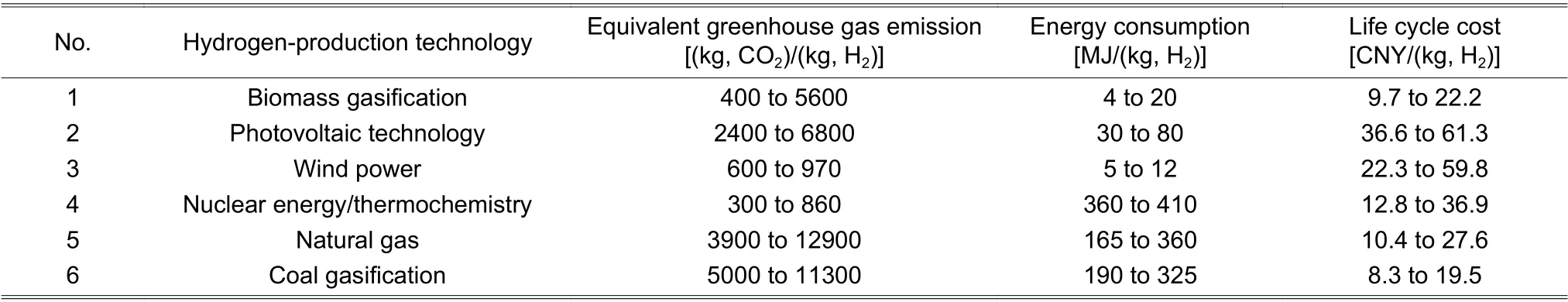
Table 2:Evaluation indexes of several hydrogen-production technologies
4.2.Special Requirements
Although more than 90% of the global hydrogen is manufactured from well-developed,low-cost methane and other gaseous fossil hydrocarbon fuels,inevitably,the subsequent compression,storage,transportation,and distribution of the technology will cause a steady increase in the selling price to consumers.Moreover,many basic supporting facilities are inadequate,and the technologies cannot meet the requirements for smallscale distribution of hydrogen sources in power supply and other fields,including transportation,military submarines,and individual combat at the present stage[105],[106].
These fields often require onsite hydrogen production or conversion for convenience and safety.Fig.8lists the existing production methods from the points of onsite and offsite.Among them,the conversion of methanol to hydrogen has attracted significant attention because of its characteristics of liquid carrier,high energy density,safety and reliability,low storage and transportation costs,relatively mild conversion conditions for hydrogen production,no sulfur,low toxicity,and relatively easy realization of the technical process[107].Conversely,methanol can be derived from either fossils or renewable sources.Presently,the major research direction of this technology is toward the replacement for precious metal catalysts,which are expensive and susceptible to poisoning.One of the developed candidates is Cu-based catalysts,which are inexpensive and exhibit outstanding activity at low temperatures.The research mostly addresses the structural composition,spatial distribution,coating form,etc.,in the determination of the methanol conversion,reaction rate,and hydrogen yields.
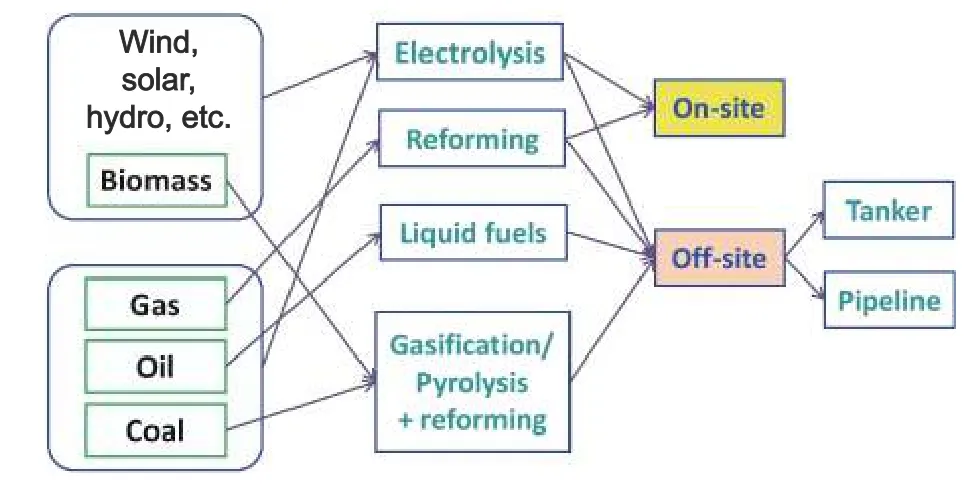
Fig.8.Hydrogen-production methods with respect to onsite and offsite.
4.3.Process Coupling/Enhancement
Since most of the present hydrogen-production technologies have the problems of high energy consumption,severe pollution,poor efficiency,and low purity of hydrogen products,the chemical process enhancement is frequently proposed for various renewable sources or sustainable technologies.The former case has been reviewed in the previous section of this paper,whereas the latter case has been exploited by coupling with sonochemistry and sonoelectrochemistry[108],geothermal energy[109],microwave heating[110],carbon-assisted water electrolysis[111],etc.
Another direction is to integrate multiple processes,e.g.,reaction and separation.This could overcome most of the existing problems while reducing the investment and preparation costs and simplifying the technical processes.Such processes include photovoltaic-thermoelectric systems[112],adsorption-enhanced carbonate looping processes[113],membrane coupling with thermochemical processes[114],and membraneassisted gas switching reforming[115].For example,previously,our team developed a kind of catalytic carbon membrane that exhibits outstanding properties for improving the methanol conversion and hydrogen yields during the steam reforming of methanol by integrating the reaction with a membrane separation process[116].
In summary,all the abovementioned protocols have been verified to be effective for improving the efficiency of the current hydrogen-production technologies.
5.Outlook and Remarks
Hydrogen has been recognized as the cleanest and most promising energy source in the 21st century.Additionally,it is an important chemical raw material widely used in industry.Presently,a large variety of feasible techniques have been developed for hydrogen production,including reforming/oxidation/gasification of fossil fuels,decomposition of hydrogen-containing resources,water electrolysis,microbial fermentation,and PC[117].However,most of them have several drawbacks,including significant energy consumption,serious pollution,inferior conversion efficiency,and high content of impurities in products.Therefore,the following aspects should be considered in future research to conquer the confronted issues.
1) Theoretical research.To achieve substantial progress in theory,the interface engineering technology can be applied to investigate the steam reforming of hydrocarbons from the microscopic perspective of atomic engineering methods[118].Conversely,computer modeling and calculations are also helpful for predicting,evaluating,and optimizing different types of hydrogen-production methods[119].This aspect can guide the development of experimental routes and the optimization of process conditions,thereby improving the efficiency and reducing the hydrogen-production costs.
2) Development of raw materials.The production cost would be greatly reduced by fully exploiting renewable resources,biomass,and even waste as raw materials.This will certainly result in significant economic,social,and environmental benefits.In fact,several attempts have been made to utilize such raw materials to produce hydrogen,including biomass and residual waste[120],plastic waste[121],and seawater[122].These studies inspired our new ideas and directions.
3) Process coupling and sustainable energy use.Besides the numerous raw materials,current hydrogenproduction technologies are associated with complicated processes and high energy consumption.Therefore,the process coupling strategy is recommended for combining some essential processes to enhance the efficiency of hydrogen-production technologies,e.g.,PC-electrolysis hybrid systems[123].Moreover,the use of sustainable energy is recommended,including solar energy,geothermal energy,nuclear power,and wind power[124].These measures will significantly improve the efficiency of hydrogen-production technologies and operational flexibility and convenience,as well as the future market competition and application prospects.
Disclosures
The authors declare no conflicts of interest.
 Journal of Electronic Science and Technology2021年2期
Journal of Electronic Science and Technology2021年2期
- Journal of Electronic Science and Technology的其它文章
- Perovskite Single Crystals:Synthesis,Properties,and Applications
- Improved Active Islanding Detection Technique for Multi-Inverter Power System
- Big Data-Based Transformer Substation Fault Prediction Method
- Comparison of Khasi Speech Representations with Different Spectral Features and Hidden Markov States
- Neural Network Based Adaptive Tracking of Nonlinear Multi-Agent System
