Detection of short stature homeobox 2 and RAS-associated domain family 1 subtype A DNA methylation in interventional pulmonology
Jian Wu, Peng Li
Jian Wu, Department of Anesthesiology, Shengjing Hospital of China Medical University,Shenyang 110004, Liaoning Province, China
Peng Li, Department of Pulmonary and Critical Care Medicine, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China
Abstract One of the most important aspects of interventional pulmonology is to obtain tissue or liquid samples of the chest to diagnose a respiratory disease; however, it is still possible to obtain insufficient tissue or cytologic specimens. Indeed,methylation detection is an effective method by which to establish a diagnosis.This review focuses on the clinical application of short stature homeobox 2 and RAS-associated domain family 1 subtype A DNA methylation detection in interventional pulmonology, including bronchoscopic fluid biopsy, transbronchial needle aspiration, and pleural effusion.
Key Words: DNA methylation; Interventional pulmonology; Short stature homeobox 2;RAS-associated domain family 1 subtype A; Fluid biopsy; Transbronchial needle aspiration
INTRODUCTION
Lung cancer (LCA) is the most common cancer worldwide and has the highest mortality of all cancers[1]. Although the diagnosis and treatment of LCA have improved, there are still a large number of LCA cases in an advanced stage when diagnosed and the 5-year survival rate is only 16%[2]. Timely diagnosis and identification of recurrences can facilitate early intervention and improve prognosis in patients with LCA[3-5]. Interventional pulmonology is a method of diagnosis and treatment of respiratory diseasesviatracheoscopy and other instruments using minimally invasive technology, including many techniques to obtain tissue or liquid biopsy samples, such as biopsy, brushing, lavage, washing, and needle aspiration biopsy. The samples obtained using these methods can be further tested for tumorspecific biomarkers, which can be used for early warning and assistant diagnosis of cancer[6,7].
DNA methylation is the key by which gene expression and cell characteristics are maintained. Epigenetic changes through DNA methylation play an important role in the occurrence of multiple cancers[6,7]. Currently, methylation detection is considered to be a valuable auxiliary diagnostic method for LCA.
Studies have shown that short stature homeobox 2 (SHOX2) promoter methylation and RAS-associated domain family 1, subtype A (RASSF1A) DNA methylation have been identified as biomarkers for the diagnosis and prognosis of LCA[8,9]. The detection ofSHOX2and/orRASSF1Ain respiratory samples indicates that patients are highly likely to suffer from LCA. This method does not require complete tumor cells, thus it is suitable for application in interventional pulmonology, which often involves micro-tissue or liquid biopsy samples. Herein we review the application ofSHOX2andRASSF1Ain interventional pulmonology and introduce our research results.
SHOX2 AND RASSF1A DNA METHYLATION
SHOX2
SHOX2is a homologue of the short stature homeobox gene. TheSHOX2gene, also known as theOG12,OG12X, orSHOTgene, is another gene of theSHOXgene family and is located on chromosome 3q25-q26.1[10]. A meta-analysis showed thatSHOX2DNA methylation is an important diagnostic biomarker for LCA[11]. Studies involving lymph node samples obtained from bronchial needle aspiration,broncholavage fluid, pleural effusion (PE), plasma, and tumor tissue under the guidance of endobronchial ultrasound showed thatSHOX2DNA methylation is a useful and powerful biomarker for the detection of LCA[8,9,12-14].
RASSF1A
TheRASSF1gene consists of eight exons and seven transcripts that are produced by different promoters and alternative splicing (RASSF1A-G).RASSF1Ais the main subtype, which is transcribed from an independent CpG island promoter region[15].RASSF1Amethylation inactivation was initially described in LCA and breast cancer[16], and since thenRASSF1Ahas been one of the most common hypermethylated genes reported in human cancer. Inactivation is often observed in a wide range of tumors. Inactivation is absent in primary tumors of the lung, breast, bladder, stomach,bile duct, and esophagus[16-21].RASSF1Amethylation can also be detected in several body fluids from cancer patients, which highlights its potential as a disease marker[15]. Race subgroup analysis showed that methylatedRASSF1Agene was significantly associated with LCA in Caucasians and Asians[22].
High expression of SHOX2 and deletion of RASSF1A plays important regulatory roles in the occurrence, apoptosis, and transformation of LCA cells. The combination ofRASSF1AandSHOX2methylation has a high sensitivity and specificity for LCA detection, which can provide a valuable biomarker for LCA screening and progression monitoring[23].
METHYLATION IN LIQUID BIOPSY SPECIMENS UNDER BRONCHOSCOPY
We can usually obtain airway lavage fluid, bronchoalveolar lavage fluid, mucosal brushing, and mucosal biopsy specimens, including transbronchial lung biopsy specimens,viabronchoscopy. Mucosal brushing, biopsy, and TBLB specimens are not included in this review because they are direct tumor tissues, and detection of the methylation level does not significantly improve the LCA diagnosis rate. In this paper,methylation detection of bronchoalveolar and airway lavage fluid is reviewed.
Airway lavage fluid is the specimen obtained by injecting 10-20 mL of isotonic saline into the target bronchus, which is then aspirated. Bronchoalveolar lavage fluid is the fluid recovered after injecting 20-40 mL of normal saline into the target lung segment. Schmidtet al[9] detectedSHOX2methylation in 523 samples of airway lavage fluid obtained by bronchoscopy. Compared with benign diseases,SHOX2methylation detection showed a high sensitivity (68%) and specificity (95%) in the diagnosis of malignant lung disease. Compared with adenocarcinoma (47%), the sensitivity was increased in squamous (82%) and small cell carcinoma (97%). In a similar study involving 250 patients conducted by Dietrichet al[24], the sensitivity was 78% and the specificity was 96%. Compared with adenocarcinoma (77%), the sensitivity was increased in squamous (91%) and small cell carcinoma (93%). Ilseet al[25] testedSHOX2andRASSF1ADNA methylation in airway lavage fluid from 118 patients.SHOX2methylation had a sensitivity of 64% and specificity of 98%.RASSF1ADNA methylation had a sensitivity of 29% and specificity of 100%. The sensitivity(72%) and specificity (98%) were increased whenSHOX2andRASSF1ADNA methylation testing was combined. In a systemic analysis on methylation detection of airway lavage fluid by Niet al[22], the pooled sensitivity, specificity, and the area under the curve (AUC) of theSHOX2methylation status were 0.75, 0.94, and 0.94,respectively. The methylatedRASSF1Agene had a sensitivity of 40%, specificity of 99%, and AUC of 0.66.
Renet al[26] detected DNA methylation ofSHOX2andRASSF1Ain 305 bronchoalveolar lavage fluid samples. The sensitivity of the cytologic examination was only 5.7%, while the sensitivity of the combined detection was 71.5%. Zhanget al[27]detected DNA methylation ofSHOX2andRASSF1Ain bronchoalveolar lavage fluid samples from 322 patients. The sensitivity of combined detection was 81%. For early LCA (stage I), the sensitivity was as high as 85.7%, which was significantly higher than that of the carcinoembryonic antigen (10.7%) and cytology (46.4%). The specificity,positive predictive value, and negative predictive value (NPV) of combined detection among all enrolled patients were 97.4%, 99.6%, and 40.7%, respectively.
Based on methylation detection of liquid samples under bronchoscopy, we can predict LCA in patients without visualizing bronchoscopic abnormalities, and facilitate making full use of the samples acquired under bronchoscopy, shorten the diagnosis time, and reduce the need for additional examinations. Based on current research data, the combined detection ofSHOX2andRASSF1ADNA methylation has high predictive value for LCA. Patients may be unwilling to undergo multiple bronchoscopies because it is an invasive examination. While an airway lavage is easy to obtain during bronchoscopy, methylation detection of airway lavage is safe and fast,and has good predictive value for LCA, which can improve the diagnostic efficacy of patients with a negative bronchoscopy, so that more patients can get receive a timely diagnosis and early treatment.
METHYLATION OF TBNA SPECIMENS
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is the gold standard for N staging of LCA[28] and an important method for the pathologic diagnosis of mediastinal lymph node metastasis in some LCA patients[29-31]; however, micro-metastases may be missed by needle aspiration biopsy, which results in a low NPV and the need for further examination of some patients[32,33].Indeed, it may be useful to detect methylation in these patients. Darwicheet al[8]detectedSHOX2DNA methylation in 105 patients with TBNA and found that the accuracy of pathologic diagnosis alone was 52%. Combining pathology andSHOX2detection, the diagnostic accuracy could reach 99% and the NPV increased from 80%to 99%.Leiroet al[34]detectedSHOX2andRASSF1ADNA methylation in 218 lymph node samples of 112 patients acquired by EBUS-TBNA. Gender, age, lymph node short diameter, standardized uptake value of lymph nodes, and methylation ofSHOX2DNA were included in a multivariate regression model, which yielded a crossvalidation sensitivity of 82.7% and specificity of 82.4% in detecting malignant lymph node lesions in negative cytology samples. The proposed prediction method can supplement the application of EBUS-TBNA in mediastinal staging. It is also suggested that single methylation marker test is not suitable for the detection of malignant tumors.
During an EBUS-TBNA examination, it is necessary to flush the needle track with liquid[35,36]. We collected the syringe washing fluid and detected the methylation in small samples. The results ofSHOX2andRASSF1ADNA methylation detection of 18 needle tract washing fluid samples are shown in the Table 1 below. Although the number of cases is small, it is suggested that the syringe washing fluid can be used as a sample source for methylation detection, which provides a new way to make full use of TBNA samples.
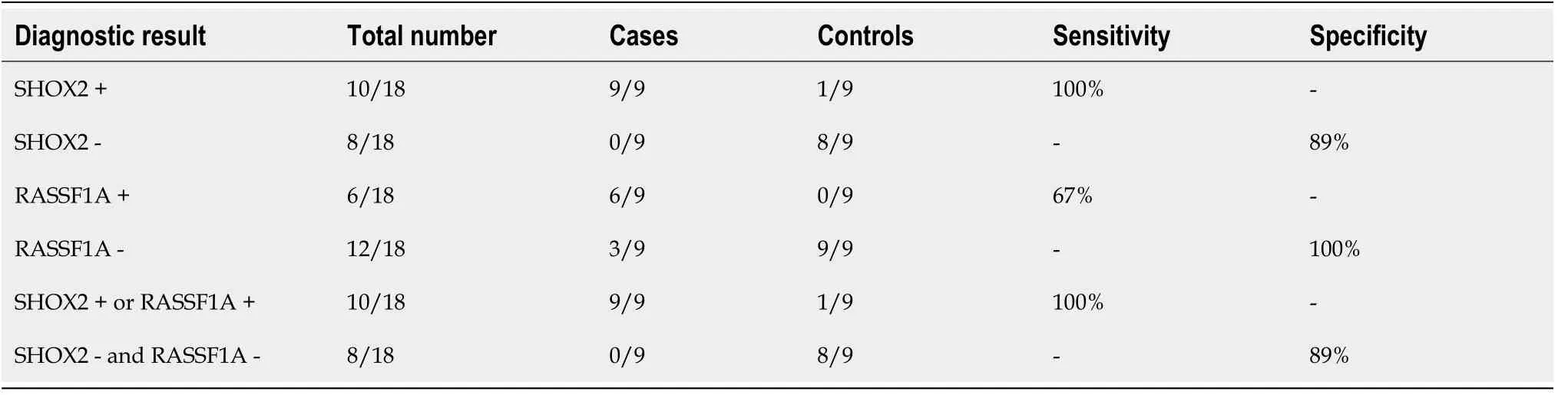
Table 1 Short stature homeobox 2 and RAS-associated domain family 1 subtype A DNA methylation detection of needle tract washing fluid samples during transbronchial needle aspiration
METHYLATION IN PE
Medical thoracoscopic biopsies of abnormal pleural tissue have adequate diagnostic rates. Thus, there is no need for methylation testing to establish a diagnosis; however,when there is no apparent abnormality under the microscope, methylation testing of a PE may have clinical significance. Dietrichet al[37] detectedSHOX2methylation in 114 patients with PEs and found that the combined analysis of cytology and DNA methylation increased the positive PEs by 71% compared with cytologic analysis alone. The specificity ofSHOX2methylation was 100% for benign PEs. A study by Ilseet al[12] showed that among 1270 patients, the sensitivity and specificity ofSHOX2DNA methylation for benign and malignant PEs were 39.5% and 96.2%, respectively.Based on the results, the sensitivity of methylation detection for PEs was lower than that of liquid biopsy by bronchoscopy; the underlying reason was not clear.
CONCLUSION
The above is a summary of the application ofSHOX2andRASSF1ADNA methylation detection in interventional respiratory diseases. Interventional pulmonology has many indications in the diagnosis and treatment of respiratory diseases, including percutaneous lung biopsy and electromagnetic navigation bronchoscopy. At present,there is no data on methylation detection of samples obtained by percutaneous lung biopsy due to the high diagnosis rate of this type of specimen. The significance of methylation detection has mainly focused on the cases in which samples are difficult to obtain and few samples are obtained. In view of this situation, further research is urgently needed. In addition, electromagnetic navigation bronchoscopy provides the feasibility for tunnel biopsy of peripheral nodules and extrabronchial lesions, and the research on the diagnostic rate of methylation detection for these specimens is still lacking. In conclusion,SHOX2andRASSF1ADNA methylation detection may help interventional respiratory physicians improve the accuracy of LCA screening and guide personalized clinical medication in a timely fashion to improve the 5-year survival rate of LCA patients.
 World Journal of Clinical Cases2021年20期
World Journal of Clinical Cases2021年20期
- World Journal of Clinical Cases的其它文章
- Obesity in people with diabetes in COVID-19 times: Important considerations and precautions to be taken
- Revisiting delayed appendectomy in patients with acute appendicitis
- Borderline resectable pancreatic cancer and vascular resections in the era of neoadjuvant therapy
- Esophageal manifestation in patients with scleroderma
- Exploration of transmission chain and prevention of the recurrence of coronavirus disease 2019 in Heilongjiang Province due to inhospital transmission
- Effects of nursing care in fast-track surgery on postoperative pain,psychological state, and patient satisfaction with nursing for glioma
