基于转录组测序筛选影响从江香猪产仔数的候选基因
张福平 唐靓婷 王嘉福 冉雪琴 李升 黄世会


張福平(1978-),博士,副教授,贵州省生态家禽产业发展专班副班长,贵州省畜牧兽医学会家禽学分会理事长,贵州省畜禽遗传资源委员会委员,主要从事地方家禽遗传资源保护与开发利用研究工作。先后主持贵州省科技计划项目“贵州黄鸡种质资源创新利用研究”“优质赤水乌骨鸡培育及高效养殖HACCP体系研究”“黔东南小香鸡配套系选育及示范推广”“高产绿壳蛋鸡配套系选育及推广”等科研项目20余项;主要参与国家科技支撑计划项目1项、国家自然科学基金项目2项。获贵州省科学技术进步奖三等奖1项;制定地方标准3项;主编《蛋鸡养殖100问》养殖技术丛书1册;在《Theriogenology》《南方农业学报》《中国畜牧兽医》等科技期刊上发表学术论文60余篇。
摘要:【目的】筛选出影响从江香猪产仔性状的基因调控网络,揭示其繁殖分子机理,为后期开展从江香猪繁殖性能研究及良种选育提供理论依据。【方法】以高产仔家系(平均产仔数≥12头,遗传稳定)和低产仔家系(平均产仔数8~10头,遗传稳定)从江香猪为研究对象,选择初情期不同产仔家系从江香猪卵巢组织各3个,使用Illumina HiSeqTM 2000测序仪进行转录组测序(RNA-Seq),并对筛选获得的差异表达基因进行GO功能富集分析及KEGG信号通路富集分析,以寻找与从江香猪产仔数相关的基因调控网络。【结果】从高产仔家系和低产仔家系从江香猪卵巢组织中测序获得的纯净序列(Clean reads)均超过5000万条,且有96.00%以上的Clean reads能比对上猪参考基因组。以|log2FC|≥1.0且P<0.01为标准,筛选获得高产仔家系和低产仔家系从江香猪卵巢组织差异表达基因212个,其中上调表达基因138个、下调表达基因74个。采用实时荧光定量PCR对随机选择的10个差异表达基因进行定量分析,发现OSAP、MSMB、FGFBP1、RLN、HAS1和PLBD1基因在高产仔家系从江香猪卵巢中的相对表达量显著高于低产仔家系从江香猪(P<0.05,下同),而EDG7、PTX3、BSP1和MRO基因在低产仔家系从江香猪卵巢中的相对表达量显著高于高产仔家系从江香猪,与RNA-Seq测序结果一致。212个差异表达基因共富集在48个GO功能条目上,包含分子功能(Molecular function)、生物学过程(Biological process)和细胞组分(Cellular component);经KEGG信号通路分析发现共有70个差异表达基因注释到特定的代谢信号通路上,其中显著性富集的KEGG信号通路有类固醇生物合成通路(Steroid biosynthesis)、钙信号通路(Calcium signaling pathway)、卵巢类固醇生成通路(Ovarian steroidogenesis)、心肌细胞肾上腺信号通路(Adrenergic signaling in cardiomyocytes)和肥厚型心肌病通路(Hypertrophic cardiomyopathy,HCM)。在卵巢类固醇生成通路上发现SCARB1、STAR、COX2、CYP11A、CYP17A、17βHSD和CYP19A等7个基因与从江香猪的繁殖性能存在密切联系。【结论】从江香猪卵巢类固醇生成通路上的STAR、CYP11A、CYP17A、17βHSD和CYP19A基因与其发情排卵密切相关,于发情期上调表达能促进卵泡发育成熟及类固醇激素合成,通过增加排卵数量而促使从江香猪表现高产,故可作为从江香猪繁殖性状的候选基因。
关键词: 从江香猪;卵巢;产仔数;候选基因;转录组测序
中图分类号: S828.89 文献标志码: A 文章编号:2095-1191(2021)04-0847-10
Selection of candidate genes affecting litter size of Congjiang Xiang pig by transcriptome sequencing
ZHANG Fu-ping1,2, TANG Liang-ting1, WANG Jia-fu1*, RAN Xue-qin1,2,
LI Sheng1, HUANG Shi-hui2
(1Institute of Agro-bioengineering, Guizhou University/College of Life Sciences, Guizhou University/The Key Laboratory of Plant Resources Conservation and Germplasm Innovation in Mountainous Region(Ministry of Education), Guiyang 550025, China; 2College of Animal Sciences,Guizhou University, Guiyang 550025, China)
Abstract:【Objective】In order to provide theoretical basis for the breeding performance studies and breeding, gene regulatory networks and the molecular mechanism of reproduction traits on Congjiang Xiang pig were screened. 【Method】Three ovary tissues were selected from the high litter size family(number of average litter size≥12, stable inheri-tance) and the low litter size family(number of average litter size was 8-10, stable inheritance) at puberty, and transcriptome sequenced(RNA-Seq) by Illumina HiSeqTM 2000, and GO functional enrichment analysis and KEGG pathway enrichment analysis were performed on the differentially expressed genes(DEGs) to find the gene regulation networks related to litter size of Congjiang Xiang pigs. 【Result】More than 50 million Clean reads were obtained from sequencing results of ovaries from high litter size family and low litter size family , and more than 96.00% of the Clean reads could be mapped with the reference genome of pig. With |log2FC|≥1.0 and P<0.01 as the conditions,212 DEGs in ovary between high yield family and low yield family were found,and among which 138 were up-regulated genes, 74 down-regulated genes. Ten randomly selected DEGs was verified by real-time fluorescence quantitative PCR, the expression levels of OSAP, MSMB, FGFBP1, RLN, HAS1 and PLBD1 genes in the ovary of high litter size family were significantly higher than those in the low litter size family(P<0.05, the same below). However, the expression levels of EDG7, PTX3, BSP1 and MRO genes were in the ovary of low litter size family were significantly higher than those in the high litter size family. The results showed that the sequencing results were consistent with the results of qRT-PCR. A total of 212 DEGs were enriched in 48 GO functional items, including molecular function, biological process and cellular component. Through KEGG pathway analysis, a total of 70 DEGs were annotated to specific metabolic pathways. Significant enrichment pathways included steroid biosynthesis,calcium signaling pathway, ovarian steroidogenesis, adrenergic signaling in cardiom-yocytes and hypertrophic cardiomyopath(HCM). On the ovarian steroidogenesis pathway, seven genes including SCARB1, STAR, COX2, CYP11A, CYP17A, 17P17A and CYP19A1 genes were found to be closely related to reproductive performance of Congjiang Xiang pigs. 【Conclusion】STAR, CYP11A, CYP17, 17βHSD and CYP19A1 genes in the ovarian steroidogenesis pathway are closely related to estrus and ovulation in pigs. These genes which are up-regulated during estrus can promote follicle development and maturation, steroid hormone expression, enhance high reproduction of Congjiang Xiang pigs by increasing the number of ovulation. Thus these genes can be used as candidate genes for bree-ding traits in Congjiang Xiang pigs.
Key words: Congjiang Xiang pig; ovary; litter size; candidate gene; transcriptome sequencing
Foundation item: National Natural Science Foundation of China(31672390); National High Technology Research and Development Program(863 Plan) of China(2013AA102503);Guizhou Science and Technology Talents Group Project(QKHPTRC〔2019〕-5615);Guizhou Agriculture Research Project(QKHZC〔2017〕2585)
0 引言
【研究意義】从江香猪是我国著名的地方猪种,主产于贵州省从江县的加榜、加鸠和宰便等8个乡(镇),1993年被列为国家二级保护畜种,2000年列入《国家级畜禽品种资源保护名录》(农业部130号公告),2006年被列入《国家级畜禽遗传资源保护名录》(农业部第662号公告)。从江香猪具有体型小、耐粗饲、适应性强、抗病能力强、肉质鲜美及性成熟早等优良特性(刘培琼等,2011;杨家大等,2016),经过长期的自然选择和人工选育,已形成近亲繁殖不退化且基因高度纯合的特征,是宝贵的猪种资源(黄仁建,1994)。但从江香猪存在繁殖力低的缺点,严重制约其产业的快速发展。2007和2014年的调查发现其平均产仔数为7~8头(申学林等,2007),远低于国内目前普遍饲养的外来猪种。此外,从江香猪的乳头数相对较少。刘培琼等(2011)研究发现56%的从江香猪乳头数为5对,25%的个体乳头数为6对,仅有1%的个体乳头数为7对。因此,提高从江香猪繁殖力是促进其养殖产业快速发展的重要保障。【前人研究进展】香猪是原始小型猪种,具有近交不退化、可放牧饲养的特点,也是用作试验动物的理想猪种(邱小田等,2013),包括从江香猪、剑白香猪、巴马香猪、环江香猪及藏香猪等品种,均属于矮小猪种(宋社果等,2011;李俊等,2015;莫家远等,2020)。近年来,国内针对从江香猪繁殖力的研究已有较多报道。刘金娟等(2008)采用RT-PCR扩增从江香猪卵巢和子宫中雌激素受体α、β基因cDNA序列,结果发现这2个亚基均存在碱基突变,可导致氨基酸改变,影响雌激素受体与ERs蛋白的应答元件,最终影响从江香猪繁殖系统的发育。谢健(2016)利用Illumina Porcine SNP60K芯片筛选获得从江香猪产仔数性状的潜在候选基因ZEB1、PDIA4、MARCKS、HDAC2、CDC42、FSH-β和PRSS21等,发现ADAMTS-1、AR、KIT、MED12、PN-1和SOD1基因的拷贝数变异仅出现在高产香猪群体中,并推测ADAMTS-1基因第7外显子5996位的多态性会影响其繁殖力。张笑等(2016)通过克隆从江香猪卵巢抑制素α(Inhibin-α,INHA)基因,检测高、低产从江香猪群体中INHA基因的SNP多态性及INHA基因在2个群体间的表达差异,结果表明从江香猪INHA基因结构保守,主要通过基因表达量变化来调节从江香猪的卵巢生长和卵泡发育。岑永秀(2017)对从江香猪miRNA表达谱测序结果进行预测,并通过实时荧光定量PCR进行验证,结果发现miR-449b-5p、miR-16-5p、let-7f-5p、miR-140-3p、miR-29c-3p及miR-1-3p等6个miRNA在其睾丸性成熟期起负调控作用。许瑶等(2017)研究发现卵泡刺激素受体(FSHR)基因锚定位点rs322800083和下游rs332115220位点构成的单倍型C-T在从江香猪群体中连锁,与其二胎、三胎总产仔数关联,可作为提高从江香猪产仔数的分子标记。卢圣菲(2018)对6头从江香猪的重测序数据进行分析,结果在8号染色体上获得8个结构变异位点,且均与繁殖相关QTL相重叠,其中MAN2B2-I3-sv100和NELFA-I1-sv40位点多态性与产仔数相关。【本研究切入点】目前,有关从江香猪繁殖性状的研究主要针对单个基因或单个位点进行分析,为阐明香猪繁殖调控机理提供了理论依据,但从江香猪发情早且产仔数差异明显的原因还有待进一步探究。【拟解决的关键问题】以高产仔家系和低产仔家系从江香猪为研究对象,分析2个家系初情期卵巢转录组的差异,筛选出影响产仔性状的基因调控网络,揭示其繁殖分子机理,为后期开展从江香猪繁殖性能研究及良种选育提供理论依据。
1 材料与方法
1. 1 试验材料
选择相同条件下饲养的高产仔家系(平均产仔数≥12头,遗传稳定)和低产仔家系(平均产仔数8~10头,遗传稳定)初情期从江香猪母猪(经发情鉴定,发情症状明显,且用公猪试情接受爬跨)各3头,高产仔家系组样本分别标记为H1、H2和H3,低产仔家系组样本分别标记为L1、L2和L3,宰杀后立即采集卵巢组织,液氮速冻,-80 ℃保存备用。
1. 2 转录组(RNA-Seq)测序分析
提取6头从江香猪卵巢总RNA,纯化后对其完整性和浓度进行检测,检测合格的样品采用磁珠法分离纯化mRNA,纯化样品经RNA Fragmentation Kit片段化后,构建11个样本的mRNA文库。构建好的文库经质检合格后,使用Illumina HiSeqTM 2000测序仪(美国)进行测序,回收测序数据,经FastQC软件质控获得纯净序列(Clean reads),然后对Clean reads进行基因和转录本定量分析;利用DESeq2_EBSeq对高产仔家系和低产仔家系转录本的表达量进行比较,以|log2FC|≥1.0且P<0.01为标准,筛选高产仔家系和低产仔家系从江香猪卵巢组织的差异表达基因。使用GOseq R软件包进行差异表达基因的基因本体论(GO)功能富集分析,并以基于KEGG数据库(http://www.genome.jp/kegg/)进行信号通路富集分析。
1. 3 差异表达基因实时荧光定量PCR验证
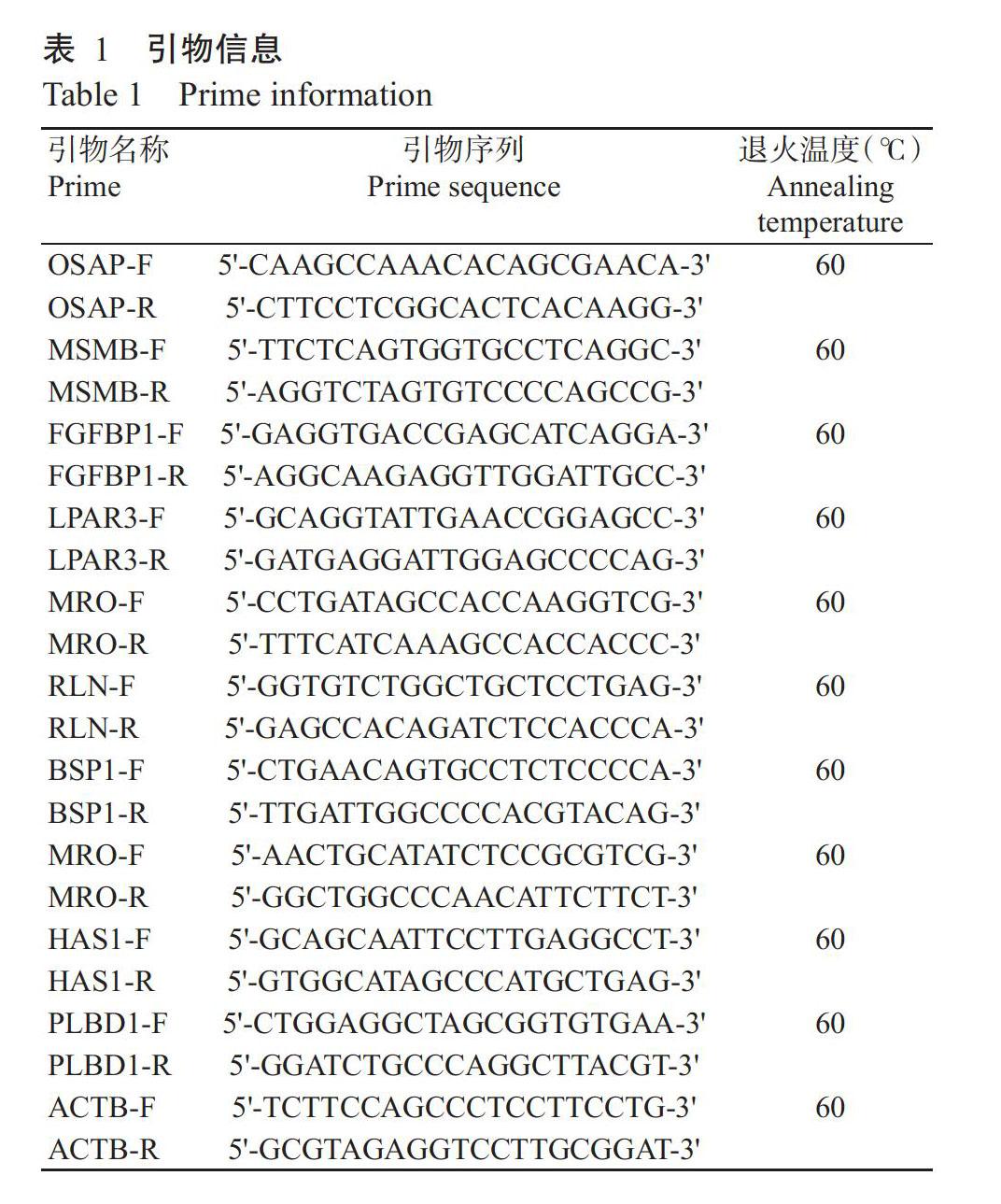
为进一步验证RNA-Seq测序获得高产仔家系和低产仔家系从江香猪发情期卵巢差异表达基因的准确性,随机选择10个差异表达基因,在Ensemble Gene ID下载相应基因cDNA序列,利用Primer 5.0设计特异性扩增引物(表1),委托生工生物工程(上海)股份有限公司合成。以β细胞骨架肌动蛋白基因(ACTB)(Ensembl:ENSG00000075624)为内参基因,对10个差异表达基因进行实时荧光定量PCR验证。
2 结果与分析
2. 1 原始数据过滤统计结果
由于RNA-Seq测序获得的原始序列(Raw reads)含有接头序(Adapter reads)和低质量序列(Low quality reads),因此需要对Raw reads进行过滤以获得Clean reads,确保后续的分析数据质量。由表2可知,在高产仔家系和低产仔家系从江香猪卵巢样品中发现有18280~36918条未知序列(Unknown reads),占Raw reads的0.04%~0.07%;去除Adapter reads和Low quality reads后,获得50011936~50413430条Clean reads,占Raw reads的96.45%~97.23%。
2. 2 高产仔家系和低产仔家系从江香猪卵巢转录组比对分析结果
将高产仔家系和低产仔家系从江香猪卵巢样本的Clean reads与猪参考基因(Sus_scrofa11.1)进行比对,结果发现有96.39%~96.81%的Clean reads能比对上猪参考基因组,其中比对到基因组唯一位置的Clean reads占94.22%~95.36%,而比对到基因组多个位置的Clean reads占1.46%~2.17%;比对到正链上的Clean reads占47.77%~48.06%,比对到负链上的Clean reads占47.97%~48.21%。
2. 3 差异表达基因筛选结果
以低产仔家系从江香猪(L组)为对照组,高产仔家系从江香猪(H组)为试验组。本研究在6个从江香猪卵巢组织样品共发现20561个表达基因,其中共表达基因19318个,H组特异性表达基因655个,L组特异性表达基因588个(图1)。以|log2FC|≥1.0且P<0.01为标准,筛选高产仔家系和低产仔家系从江香猪卵巢组织差异表达基因,最终筛选获得212个差异表达基因,其中上调表达基因138个、下调表达基因74个(图2)。
2. 4 10个差异表达基因的实时荧光定量PCR验证结果
为验证RNA-Seq测序结果的可靠性,選择10个差异表达基因(OSAP、MSMB、FGFBP1、EDG7、PTX3、RLN、BSP1、MRO、HAS1和PLBD1)进行实时荧光定量PCR检测,结果发现OSAP、MSMB、FGFBP1、RLN、HAS1和PLBD1基因在高产仔家系从江香猪卵巢中的相对表达量显著高于低产仔家系从江香猪(P<0.05,下同),而EDG7、PTX3、BSP1和MRO基因在低产仔家系从江香猪卵巢中的相对表达量显著高于高产仔家系从江香猪(图3),即实时荧光定量PCR检测结果与RNA-Seq测序结果趋势一致。
2. 5 差异表达基因的GO功能富集分析结果
对筛选获得的212个差异表达基因进行GO功能富集分析,结果发现这些差异表达基因共富集在48个GO功能条目上,包含分子功能(Molecular function)、生物学过程(Biological process)和细胞组分(Cellular component)(图4)。在生物学过程组分中,细胞过程(Cellular process)类别所占比例最高,其次为单有机体过程(Single-organism process)类别,且包含生殖(Reproduction)及生殖过程(Reproductive process)2个与繁殖相关的生物学过程。在细胞组分中的19个分类中,以细胞(Cell)、细胞部分(Cell part)和细胞器(Organelle)类别所占比例较高,其次是膜组分(Membrane)。在分子功能组分涉及的18个分类中,以绑定分子功能(Binding)和催化活动(Catalytic activity)类别所占比例较高。
2. 6 差异表达基因的KEGG信号通路富集分析结果
KEGG信号通路分析发现,在高产仔家系和低产仔家系从江香猪卵巢组织中共有70个差异表达基因注释到特定的代谢信号通路上,富集到的前20条KEGG信号通路见图5,其中显著性富集的KEGG信号通路有5条(表4):类固醇生物合成通路(Steroid biosynthesis)、钙信号通路(Calcium signaling pathway)、卵巢类固醇生成通路(Ovarian steroidogenesis)、心肌细胞肾上腺信号通路(Adrenergic signa-ling in cardiomyocytes)和肥厚型心肌病通路(Hypertrophic cardiomyopathy,HCM)。此外,在卵巢类固醇生成通路上发现SCARB1、STAR、COX2、CYP11A、CYP17A、17βHSD和CYP19A等7个基因与从江香猪的繁殖性能存在密切联系。
3 讨论
卵巢是雌性哺乳动物的重要生殖器官。在发情周期中,卵巢上的卵母细胞增殖、分化及凋亡,会直接影响和决定雌性动物的排卵数量、受精率及其产仔数。卵巢功能的发挥必然伴随着大量基因表达转录调控,本研究通过构建高产仔家系和低产仔家系从江香猪卵巢mRNA文库,利用RNA-Seq测序技术从不同产仔家系猪卵巢中筛选出212个差异表达基因,发现许多上调表达基因参与类固醇激素代谢和生物合成、细胞黏附、有机物代谢及离子转运和信号转导,且部分上调表达基因为参与生殖的候选基因。NR5A1蛋白参与调控有性发育和生殖关键基因的转录(Jeyasuria et al.,2004),NR5A1基因失活则会导致卵巢发育不良,如NR5A1基因双敲除小鼠卵巢虽有卵泡产生,但缺乏黄体,而呈现排卵障碍(Louren?o et al.,2009);在肾上腺和性腺中发现STAR基因是胆固醇进入线粒体启动类固醇生成的关键基因(Clark and Hudson,2015);PAPSS2基因通过调控卵巢雄激素的表达效率,在调节卵巢功能方面发挥重要作用(Oostdijk et al.,2015);MTHFD2基因可能对快速增殖细胞中线粒体NADH和NADPH的产生有促进作用(Shin et al.,2017)。许多下调表达基因则直接参与纤毛运动、受体信号通路、激素分泌、蛋白修饰和凋亡过程的调控。如TEKT1基因是精子细胞特异性基因,在精子发生和变态过程中发挥重要作用(Larsson et al.,2000),但其在卵巢中的功能尚不清楚;TSPAN基因是四聚氰胺家族的新成员,具有抑制细胞增殖和迁移等功能作用(Wang et al.,2011);TGFβ家族具有控制卵泡发育的功能(Cela et al.,2016);fyn相关激酶(FRK)是一种无受体的酪氨酸激酶,通过抑制上皮细胞向间质转化而抑制细胞增殖、迁移和侵袭(Ogunbolude et al.,2017)。
本研究对筛选得到的差异表达基因进行KEGG信号通路分析,结果发现2条与繁殖相关的重要KEGG信号通路,即类固醇生物合成通路(Steroid biosynthesis)和卵巢类固醇生成通路(Ovarian steroidogenesis)。其中,卵巢类固醇生成通路是调控猪发情排卵的重要通路,与生物过程中激素相关的GO功能富集分析结果相符,进一步证实高产仔家系和低产仔家系从江香猪发情期卵巢间存在激素调节系统方面的差异。在卵巢类固醇生成通路上,高产仔家系从江香猪卵巢组织中的STAR、CYP11A、CYP17、17βHSD和CYP19A1基因均呈上调表达趋势,即促进雌二醇表达,而雌二醇表达水平又直接影响母猪产仔数(Robic et al.,2014)。类固醇激素是一系列生殖生理活动所必需的激素,其作为机体化学信使,在生物体的发育和成熟过程中发挥重要作用。目前,已发现与类固醇激素合成相关的限速酶基因有STAR基因、细胞色素P450家族及羟基类固醇脱氢酶类基因(HSDs)。细胞色素P450是一类存在于肝细胞微粒体上的超基因大家族,对动物机体内源性物质和外源性化合物具有重要的生物转化作用(Da-nielson,2002)。细胞色素P450还参与固醇和类固醇激素的生物合成,尤其是在生殖激素调节中扮演着重要角色(Kandiel et al.,2010)。与猪繁殖性能相关的细胞色素P450家族基因有CYP11A1、CYP11B、CYP17、CYP19A1、CYP19A2、CYP19A3和CYP21。本研究发现,CYP17、CYP11A和CYP19A1基因在卵巢类固醇生成通路上均高表达,说明这3个基因对高产仔家系从江香猪发情期的排卵功能有显著影响。已有的研究表明,哺乳动物卵巢CYP19A1是雌激素合成相关的限速酶,除了参与类固醇激素的合成外,还能调节卵泡生成(Hatey et al.,1992)。应诗家等(2013)利用同期发情技术处理绵羊后,发现其卵巢黄体后期直径>2.5 mm卵泡中的CYP17A1和CYP19A1基因表达水平极显著高于直径为2.5 mm卵泡中的表达水平,说明CYP17A1和CYP19A1基因对绵羊的卵泡发育有直接影响。此外,CYP11A1基因突变会导致卵巢多囊综合症(Zhang et al.,2012)。
17β-羥基类固醇脱氢酶(17β-HSDs)是一类NAD(P)H/NAD(P)+依赖的氧化还原酶家族,在哺乳动物中发现该基因共有14个成员,其中17β-HSD1(Saloniemi et al.,2009)、17β-HSD2(Rantakari et al.,2008)、17β-HSD3(Adamski and Jakob,2001)、17β-HSD4(Baes,2000)、17β-HSD5(Penning et al.,2000)和17β-HSD12(Rantakari et al.,2010)6种17β-HSDs与雌激素的氧化、还原和代谢密切相关。本研究检测到17β-HSD1基因在高产仔家系从江香猪卵巢组织中呈上调表达。在诸多物种中,17β-HSD1在雌酮转化为雌二醇(E2)的过程中发挥重要作用,而HSD17B1基因异常表达与许多雌激素依赖性疾病相关,包括乳腺癌、子宫内膜癌、子宫内膜异位症和子宫内膜息肉等(Feigelson et al.,2006;Cong et al.,2012;刘博等,2014)。沈峻宇等(2015)利用PCR-RFLP对新西兰兔17β-HSD1基因进行分型,并对该基因突变位点与繁殖性状进行最小二乘法分析,结果发现17β-HSD1基因与母兔繁殖性状显著关联,其中c.1509C>T突变位点与家兔繁殖性能有较强的相关性,可作为家兔遗传改良的分子标记。卢圣菲等(2018)研究发现在从江香猪17β-HSD12基因的SV225位点存在2个ESE和3个ISE可变剪接调控元件,而这些剪接调控元件可能有助于17B-HSD12基因的转录本加工,提高基因转录效率,促进雌激素生成,进而影响从江香猪发情排卵。综上所述,在发情期,高产仔家系从江香猪卵巢组织中的STAR和17βHSD等基因上调表达,促进卵泡发育成熟及类固醇激素合成,最终通过增加排卵数量而促使从江香猪表现高产。
4 结论
从江香猪卵巢类固醇生成通路上的STAR、CYP11A、CYP17A、17βHSD和CYP19A基因与其发情排卵密切相关,于发情期上调表达能促进卵泡发育成熟及类固醇激素合成,通过增加排卵数量而促使从江香猪表现高产,故可作为香猪繁殖性状的候选基因。
参考文献:
岑永秀. 2017. 12个MicroRNAs及其靶基因在香猪睾丸组织中的表达[D]. 贵阳:贵州大学. [Cen Y X. 2017. Expression of 12 MicroRNAs and target genes in Xiang pig testis tissue[D]. Guiyang:Guizhou University.]
黄仁建. 1994. 香猪的特点及其成因(综述)[J]. 养猪,(4):31-32. [Huang R J. 1994. Characteristics and genesis of Xiang pig[J]. Swine Production,(4):31-32.]
李俊,陈美娟,许钟峯,吕冠霖,赵子榆,石德顺,刘庆友. 2015. 环江香猪FSHβ、ESR及ZAR1基因多态性分析[J]. 基因组学与应用生物学,34(3):495-499. doi:10.13417/j.gab.034.000495. [Li J,Chen M J,Xu Z F,Lü G L,Zhao Z Y,Shi D S,Liu Q Y. 2015. Polymorphism analysis on FSHβ、ESR and ZAR1 in Huanjiang pig[J]. Genomics and Applied Biology,34(3):495-499.]
刘博,马伟,陈博,甘莉,刘冬梅,陈柯宇,张录顺. 2014. HSD17B1和HSD17B2基因多态性与四川汉族人群肝癌的关联性研究[J]. 河南科技大学学报(医学版),32(3):164-166. doi:10.15926/j.cnki.issn1672-688x.2014.03.002. [Liu B,Ma W,Chen B,Gan L,Liu D M,Chen K Y,Zhang L S. 2014. Association of HSD17B1 and HSD17B2 gene polymorphisms with hepatocellular carcinoma in Sichuan Han Population[J]. Journal of Henan University of Science and Technology(Medical Sciences),32(3):164-166.]
刘金娟,王嘉福,冉雪琴. 2008. 香猪雌激素受体α、β基因cDNA的克隆及分析[J]. 动物学报,54(4):733-738. [Liu J J,Wang J F,Ran X Q. 2008. Identification and characte-rization of cDNA encoding estrogen receptor alpha and betain Xiang pigs[J]. Acta Zoologica Sinica,54(4):733-738.]
刘培琼,申学林,刘若余,杨廷模,陈警,谌洪光. 2011. 香猪的生产性能及开发利用[J]. 养猪,(6):49-51. doi:10.3969/j.issn.1002-1957.2011.06.035. [Liu P Q,Shen X L,Liu R Y,Yang T M,Chen J,Chen H G. 2011. Production performance and development and utilization of Xiang pig[J]. Swine Production,(6):49-51.]
卢圣菲. 2018. 香猪8号染色体繁殖相关QTL区域结构变异的研究[D]. 贵阳:贵州大学. [Lu S F. 2018. Studies on the structural variation of QTL region on chromosome 8 related to reproductive traits of Xiang pig[D]. Guiyang:Guizhou University.]
卢圣菲,冉雪琴,刘畅,牛熙,李升,黄世会,王嘉福. 2018. 5个猪种HSD17B12基因结构变异SV225的多态性研究[J]. 畜牧与兽医,50(7):10-13. [Lu S F,Ran X Q,Liu C,Niu X,Li S,Huang S H,Wang J F. 2018. Polymorphism of structural variation SV225 in the HSD17B12 gene of five pig breeds[J]. Animal Husbandry & Veterinary Medi-cine,50(7):10-13.]
莫家遠,高九昱,奉玲丽,李月月,田威龙,刘笑笑,程锋,梁靓,雷树桥,文蔚,梁晶,兰干球. 2020. 巴马香猪产活仔数性状全基因组关联分析[J]. 中国畜牧兽医,47(12):3965-3975. doi:10.16431/j.cnki.1671-7236.2020.12.019. [Mo J Y,Gao J Y,Feng L L,Li Y Y,Tian W L,Liu X X,Cheng F,Liang L,Lei S Q,Wen W,Liang J,Lan G Q. 2020. Genome-wide association study on alive litter size trait in Bama Xiang pigs[J]. China Animal Husban-dry & Veterinary Medicine,47(12):3965-3975.]
邱小田,张芸,刘培琼. 2013. 贵州香猪遗传资源保护利用进展[J]. 中国猪业,8(S1):161-162. doi:10.16174/j.cnki. 115435.2013.s1.015. [Qiu X T,Zhang Y,Liu P Q. 2013. Progress in protection and utilization of genetic resources of Guizhou Xiang pig[J]. China Swine Industry,8(S1):161-162.]
申学林,杨秀江,韦胜权. 2007. 从江香猪生长发育繁殖性能测定[J]. 种业研究,(11):39-41. doi:10.3969/j.issn.1673-4556. 2007. 11.020. [Shen X L,Yang X J,Wei S Q. 2007. Determination of growth and reproduction performance of Congjiang Xiang pig[J]. The Chinese Livestock and Poultry Breeding,(11):39-41.]
沈峻宇,杨嵩,陈定超,冉强. 2015. 家兔HSD17B4基因与繁殖性能的关联性分析[J]. 四川畜牧兽医,(4):34-37. doi:10.3969/j.issn.1001-8964.2015.04.026. [Shen J Y,Yang S,Chen D C,Ran Q. 2015. Correlation analysis between HSD17B4 gene and reproductive traits in rabbit[J]. Si-chuan Animal & Veterinary Sciences,(4):34-37.]
宋社果,安小鹏,赵海波,刘海艳,曹斌云. 2011. 藏香猪屠宰特性及肉品质的分析[J]. 西北农业学报,20(12):26-32. doi: 10.3969/j.issn.1004-1389.2011.12.007. [Song S G,An X P,Zhao H B,Liu H Y,Cao B Y. 2011. Analysis of slaughter traits and meat quality in Zangxiang pig[J]. Acta Agriculturae Boreali-Occidentalis Sinica,20(12):26-32.]
谢健. 2016. 基于高密度SNP芯片的香猪产仔数性状基因筛选和鉴定[D]. 贵阳:贵州大学. [Xie J. 2016. Screening and identification of genes related with the litter size of Xiang pig based on SNP chip[D]. Guiyang:Guizhou University.]
許瑶,刘畅,牛熙,冉雪琴,王嘉福. 2017. 香猪FSHR基因外显子10的多态性及其与产仔数间的关联分析[J]. 中国畜牧兽医,44(3):799-806. doi:10.16431/j.cnki.1671-7236. 2017.03.025. [Xu Y,Liu C,Niu X,Ran X Q,Wang J F. 2017. Polymorphism of FSHR gene Exon 10 and its relationship with the litter size of Xiang pigs[J]. China Animal Husbandry & Veterinary Medicine,44(3):799-806.]
杨家大,任琼,吴声榕. 2016. 从江香猪CYP2A19基因单核苷酸多态性的群体遗传学分析[J]. 南方农业学报,47(7):1209-1215. doi:10.3969/j:issn.2095-1191.2016.07.1209. [Yang J D,Ren Q,Wu S R. 2016. Population genetics of single nucleotide polymorphisms of CYP2A19 gene in Congjiang Xiang-pig[J]. Journal of Southern Agriculture,47(7):1209-1215.]
应诗家,彭中友,李燕,蔡柳萍,施振旦. 2013. 绵羊黄体期卵巢类固醇激素调节基因的表达研究[J]. 畜牧兽医学报,44(11):1775-1780. doi:10.11843/j.issn.0366-6964.2013. 11.011. [Ying S J,Peng Z Y,Li Y,Cai L P,Shi Z D. 2013. Study on ovarian sterol-regulatory genes expression in sheep during luteal phase[J]. Acta Veterinaria et Zootechnica Sinica,44(11):1775-1780.]
张笑,苏艳,杨世彬,王嘉福,冉雪琴. 2016. 从江香猪抑制素α基因克隆及其卵巢表达研究[J]. 中国畜牧兽医,43(1):191-196. doi:10.16431/j.cnki.1671-7236.2016.01.028. [Zhang X,Su Y,Yang S B,Wang J F,Ran X Q. 2016. Cloning of inhibin-α gene and its mRNA expression pa-ttern in the ovary of Congjiang Xiang pig[J]. China Animal Husbandry & Veterinary Medicine,43(1):191-196.]
Adamski J,Jakob F J. 2001. A guide to 17 beta-hydroxyste-roid dehydrogenases[J]. Molecular and Cellular Endocrinology,171(1-2):1-4. doi:10.1016/s0303-7207(00)00383-x.
Baes M. 2000. Mouse models for peroxisome biogenesis disor-ders[J]. Cell Biochemistry and Biophysics,32:229-237. doi:10.1385/cbb:32:1-3:229.
Cela P,Hampl M,Fu K K,Bosakova M K,Krejci P,Richman J M,Buchtova M. 2016. MORN5 expression during craniofacial development and its interaction with the BMP and TGFβ pathways[J]. Frontiers in Physiology,7:378. doi:10.3389/fphys.2016.00378.
Clark B J,Hudson E A. 2015. StAR protein stability in Y1 and Kin-8 mouse adrenocortical cells[J]. Biology(Basel),4(1):200-215. doi:10.3390/biology4010200.
Cong R J,Huang Z Y,Cong L,Ye Y,Wang Z,Zha L,Cao L P,Su X W,Yan J,Li Y B. 2012. Polymorphisms in genes HSD17B1 and HSD17B2 and uterine leiomyoma risk in Chinese women[J]. Archives of Gynecology and Obste-trics,286(3):701-705. doi:10.1007/s00404-012-2328-0.
Danielson P B. 2002. The cytochrome P450 superfamily:Biochemistry,evolution and drug metabolism in humans[J]. Current Drug Metabolism,3(6):561-597. doi:10.2174/1389200023337054.
Feigelson H S,Cox D G,Cann H M,Wacholder S,Kaaks R,Henderson B E,Albanes D,Altshuler D,Berglund G,Berrino F,Bingham S,Buring J E,Burtt N P,Calle E E,Chanock S J,Clavel-Chapelon F,Colditz G,Diver W R,Freedman M L,Haiman C A,Hankinson S E,Hayes R B,Hirschhorn J N,Hunter D,Kolonel L N,Kraft P,LeMarchand L,Linseisen J,Modi W,Navarro C,Peeters P H,Pike M C,Riboli E,Setiawan V W,Stram D O,Tho-mas G,Thun M J,Tjonneland A,Trichopoulos D. 2006. Haplotype analysis of the HSD17B1 gene and risk of breast cancer:A comprehensive approach to multicenter analyses of prospective cohort studies[J]. Cancer Research,66(4):2468-2475. doi:10.1158/0008-5472.CAN-05-3574.
Hatey F,Gasparoux J P,Mulsant P,Bonnet A,Gasser F. 1992. P450scc regulation in pig granulosa cells:Investigation into the mechanism of induction[J]. The Journal of Steroid Biochemistry and Molecular Biology,43(8):869-874. doi:10.1016/0960-0760(92)90314-9.
Jeyasuria P,Ikeda Y,Jamin S P,Zhao L P,de Rooij D G,Themmen A P N,Behringer R R,Parker K L. 2004. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function[J]. Molecular Endocrinology,18(7):1610-1619. doi:10.1210/me.2003-0404.
Kandiel M M M,Watanabe G,Taya K. 2010. Ovarian expression of inhibin-subunits,3β-hydroxysteroid dehydrogenase,and cytochrome P450 aromatase during the estrous cycle and pregnancy of shiba goats(Capra hircus)[J]. Experimental Animals,59(5):605-614. doi:10.1538/expanim.59.605.
Larsson M,Norrander J,Gr?slund S,Brundell E,Linck R,St?hl S,H??g C. 2000. The spatial and temporal expression of Tekt1,a mouse tektin C homologue,during spermatogenesis suggest that it is involved in the development of the sperm tail basal body and axoneme[J]. European Journal of Cell Biology,79(10):718-725. doi:10. 1078/0171-9335-00097.
Louren?o D,Brauner R,Lin L,de Perdigo A,Weryha G,Muresan M,Boudjenah R,Guerra-Junior G,Maciel-Guerra A T,Achermann J C,McElreavey K,Bashamboo A. 2009. Mutations in NR5A1 associated with ovarian insufficiency[J]. The New England Journal of Medicine,360(12):1200-1210. doi:10.1056/NEJMoa0806228.
Ogunbolude Y,Dai C L,Bagu E T,Goel R K,Miah S,Mac-Ausland-Berg J,Ng C Y,Chibbar R,Napper S,Raptis L,Vizeacoumar F,Vizeacoumar F,Bonham K,Lukong K E. 2017. FRK inhibits breast cancer cell migration and invasion by suppressing epithelial-mesenchymal transition[J]. Oncotarget,8(68):113034-113065. doi:10.18632/oncotarget.22958.
Oostdijk W,Idkowiak J,Mueller J W,House P J,Taylor A E,O'Reilly M W,Hughes B A,de Vries M C,Kant S G,Santen G W E,Verkerk A J M H,Uitterlinden A G,Wit J M,Losekoot M,Arlt W. 2015. PAPSS2 deficiency causes androgen excess via impaired DHEA sulfation—in vitro and in vivo studies in a family harboring two novel PAPSS2 mutations[J]. The Journal of Clinical Endocrinology and Metabolism,100(4):E672-E680. doi:10.1210/ jc.2014-3556.
Penning T M,Burczynski M E,Jez J M,Hung C F,Lin H K,Ma H,Moore M,Palackal N,Ratnam K. 2000. Human 3-alpha-hydroxyste-roid dehydrogenase isoforms(AKR1C1-AKR1C4) of the aldo-keto reductase superfamily:Functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones[J]. The Biochemical Journal,351(1):67-77. doi: 10.1042/0264-6021:3510067.
Rantakari P,Lagerbohm H,Kaimainen M,Suomela J P,Strauss L,Sainio K,Pakarinen P,Poutanen M. 2010. Hydroxysteroid(17{beta}) dehydrogenase 12 is essential for mouse organogenesis and embryonic survival[J]. Endocrinology,151(4):1893-1901. doi:10.1210/en.2009-0929.
Rantakari P,Strauss L,Kiviranta R,Lagerbohm H,Paviala J,Holopainen I,Vainio S,Pakarinen P,Poutanen M. 2008. Placenta defects and embryonic lethality resulting from disruption of mouse hydroxysteroid(17-beta) dehydrogenase 2 gene[J]. Molecular Endocrinology,22(3):665-675. doi:10.1210/me.2007-0257.
Robic A,Faraut T,Prunier A. 2014. Pathways and genes involved in steroid hormone metabolism in male pigs:A review and update[J]. The Journal of Steroid Biochemistry and Molecular Biology,140:44-55. doi:10.1016/j.jsbmb.2013.11.001.
Saloniemi T,Welsh M,Lamminen T,Saunders P,M?kel? S,Streng T,Poutanen M. 2009. Human HSD17B1 expression masculinizes transgenic female mice[J]. Molecular and Cellular Endocrinology,301(1-2):163-168. doi:10.1016/ j.mce. 2008.10.047.
Shin M,Momb J,Appling D R. 2017. Human mitochondrial MTHFD2 is a dual redox cofactor-specific methylenete-trahydrofolate dehydrogenase/methenyltetrahydrofolate cy-clohydrolase[J]. Cancer & Metabolism,5:11. doi:10.1186/ s40170-017-0173-0.
Wang H X,Kolesnikova T V,Denison C,Gygi S P,Hemler M E. 2011. The C-terminal tail of tetraspanin protein CD9 contributes to its function and molecular organization[J]. Journal of Cell Science,124(16):2702-2710. doi:10.1242/jcs.085449.
Zhang C W,Zhang X L,Xia Y J,Cao Y X,Wang W J,Xu P,Che Y N,Wu X K,Yi L,Gao Q,Wang Y. 2012. Association between polymorphisms of the CYP11A1 gene and polycystic ovary syndrome in Chinese women[J]. Mole-cular Biology Reports,39(8):8379-8385. doi:10.1007/s11033-012-1688-7.
(責任编辑 兰宗宝)

