Prognostic role of sarcopenia in metastatic colorectal cancer patients during first-line chemotherapy: A retrospective study
Chiara Maddalena, Andrea Ponsiglione, Luigi Camera, Lidia Santarpia, Fabrizio Pasanisi, Dario Bruzzese,Camilla Panico, Giovanni Fiore, Simona Camardella, Tolomeo Caramia, Alessia Farinaro, Sabino De Placido,Chiara Carlomagno
Chiara Maddalena, Lidia Santarpia, Fabrizio Pasanisi, Giovanni Fiore, Simona Camardella,Tolomeo Caramia, Alessia Farinaro, Sabino De Placido, Chiara Carlomagno, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples 80131, Italy
Andrea Ponsiglione, Luigi Camera, Camilla Panico, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples 80131, Italy
Dario Bruzzese, Department of Public Health, University of Naples Federico II, Naples 80131,Italy
Abstract BACKGROUND Sarcopenia is a condition characterized by decreased skeletal muscle mass due to physiological ageing or to a concomitant disease such as neoplasia. In cancer patients, a low lean body mass is suggested to be a negative prognostic factor for survival and for the development of dose-limiting chemotherapy toxicities irrespective of disease stage.AIM To evaluate the prognostic role of sarcopenia in patients with metastatic colorectal cancer (mCRC) undergoing first-line chemotherapy.METHODS Our retrospective analysis included 56 mCRC patients who received first-line chemotherapy from 2014 to 2017 at the Medical Oncology Unit of our hospital.Computerized scans were performed before starting chemotherapy and at the first disease reassessment. Sarcopenia was assessed using the skeletal mass index =muscle area in cm2/(height in m2) calculated at the L3 vertebra. Overall survival and objective response rate were evaluated. Toxicities were analyzed during the first four cycles of therapy and graded according to Common Terminology Criteria for Adverse Events version 4.0. A loss of skeletal muscle mass ≥ 5% was considered indicative of deterioration in muscle condition.RESULTS Median age was 67 years and 35.7% of patients were ≥ 70 years old. Fourteen patients (25%) were sarcopenic at baseline computed tomography (CT) scan (7/33 men; 7/23 women); 5/14 sarcopenic patients were ≥ 70 years old. Median followup was 26.8 mo (3.8-66.8 mo) and median overall survival was 27.2 mo (95%CI:23.3-37.3). Sarcopenia was not correlated to overall survival (P = 0.362), to higher toxicities reported during the first 4 cycles of chemotherapy (P = 1.0) or to response to treatment (P = 0.221). At the first disease reassessment, a skeletal muscle loss (SML) ≥ 5% was found in 17 patients (30.3%) 3 of whom were already sarcopenic at baseline CT scan, while 7 patients became sarcopenic. SML was not correlated to overall survival (P = 0.961). No statistically significant correlation was found between baseline sarcopenia and age (P = 1.0), body mass index (P =0.728), stage at diagnosis (P = 0.355) or neutrophil/lymphocyte ratio (P = 0.751).CONCLUSION Neither baseline sarcopenia nor SML affected survival. In addition, baseline sarcopenia was not related to worse treatment toxicity. However, these results must be interpreted with caution due to the limited sample size.
Key Words: Sarcopenia; Lean body mass; Skeletal muscle mass; Metastatic colorectal cancer
INTRODUCTION
In medical oncology, the dose of cytotoxic drugs is calculated according to the patient’s body surface area (BSA) using formulae devised in the early twentieth century and validated on a limited number of subjects[1,2]. For instance, the formula proposed by Du Boiset al[2], which is routinely used in adult cancer patients, was based on data of only nine patients. Furthermore, individualized dosage of antineoplastic agents based on BSA does not necessarily equate to a patient’s drug exposure because the quantity of active drug circulating in the body and the duration of circulation may vary due to various factors[3,4]. In fact, pharmacokinetic parameters of a particular agent, such as area under the curve and clearance, may differ substantially among patients not only because of genetic factors, pharmacological interactions and the physiological characteristics of patients, but also because of body composition variations that are typical of the natural history of cancer patients[1,5].
The total body mass consists of two major compartments, fat and lean, which are the major sites of distribution of lipophilic and non-lipophilic drugs, respectively[6].Therefore, the ratio of fat and lean tissue masses could be a better parameter than BSA with which to determine the dose of cytotoxic agents, as it affects metabolism, plasma concentration and the toxicity of chemotherapy drugs[6,7]. Moreover, patients with a similar or identical body weight, BSA or body mass index (BMI) may have a different lean body mass (LBM)[8,9]. Skeletal muscle tissue accounts for most of the LBM and is the predominant source of proteins which are essential for all cell processes[4,10].People with a low skeletal muscle mass may have a lower volume of drug distribution and reduced protein binding compared to people with a normal muscle mass thereby resulting in a higher plasma drug concentration and worse treatment toxicity[8,11].The skeletal muscle mass decrease due to physiological ageing or concomitant disease such as neoplasia is defined as “sarcopenia”[12]. In cancer patients, a low LBM and sarcopenia are negative prognostic factors for survival[8,9,13] and for the development of dose-limiting chemotherapy toxicities[6,14] irrespective of disease stage. The aim of the present study was to retrospectively analyze the prevalence of sarcopenia in patients with metastatic colorectal cancer (mCRC) and its prognostic role.
In 2018, the European Working Group on Sarcopenia in Older People 2 published an updated definition that uses low muscle strength as the primary parameter for recognizing sarcopenia, together with additional items of low muscle quantity or quality[15]. However, due to the retrospective nature of our analysis, we used the computed tomography (CT) scans performed at the time of first diagnosis of metastatic disease to evaluate the muscle area, and therefore the muscle quantity, at the level of the third lumbar vertebra.
MATERIALS AND METHODS
The primary end-point of this study was to assess the association between baseline sarcopenia, estimated before starting first-line chemotherapy, and overall survival(OS) in mCRC patients. The secondary end-points were: (1) to evaluate the potential correlation of baseline sarcopenia with the objective response rate (ORR) to first-line chemotherapy and with the development of side effects to antineoplastic therapy during the first four cycles of treatment; (2) to investigate the association between skeletal muscle loss (SML) at first disease reassessment and OS; and (3) to examine the relationship between sarcopenia and age, BMI, disease stage at the time of first diagnosis and the neutrophil/lymphocyte ratio (NLR) as an inflammation index.
Our retrospective analysis included 56 mCRC patients who received first-line chemotherapy for metastatic disease from 2014 to 2017 at the Medical Oncology Unit of the Federico II University Hospital. All patients had signed the informed consent document for the use of personal data in the medical record according to the Italian privacy legislation. The study was approved by the panel of scientists proposing the research and by all the collaborators who participated in the research and it was conducted in accordance with the 2013 Declaration of Helsinki.
Computerized scans were performed before starting chemotherapy (baseline) and at first disease reassessment (2-3 mo after starting therapy). The images were analyzed by a subspecialty trained abdominal radiologist. Sarcopenia was assessed using the skeletal mass index [SMI = muscle area in cm2/(height in m2)][16]. The cross-sectional area of all skeletal muscles was calculated at the third lumbar vertebra on pre-contrast axial CT images with a slice thickness of 5 mm, using the open-source Horos software(version 3.3.6)[16,17]. An attenuation threshold ranging from -29 to 150 Hounsfield units was set for muscle tissue[16].
Sarcopenia was defined by Martin SMI cut-offs[8], that combined both sex-specific and BMI cut-offs: 43 cm2/m2for men with BMI < 25 kg/m2, 53 cm2/m2for men with BMI ≥ 25 kg/m2and 41 cm2/m2for women regardless of BMI. A loss of skeletal muscle mass ≥ 5% from baseline CT to first disease reassessment was considered indicative of a deterioration in muscle condition[18]. Patients’ characteristics were categorized as follows: age (< 70 yearsvs≥ 70 years), BMI (underweight < 18.5 kg/m2; normal weight 18.5-24.9 kg/m2; overweight 25-30 kg/m2and obese > 30 kg/m2) and disease stage at the time of first diagnosis (limitedvsmetastatic). A NLR ≥ 3 was considered as an inflammation index[19]. Toxicities were analyzed during the first four cycles of therapy and graded according to the Common Terminology Criteria for Adverse Events version 4.0. Survival was calculated from the date of baseline CT, at the time of metastatic disease diagnosis, to death or until the last outpatient visit. Disease status was assessed using the Response Evaluation Criteria in Solid Tumors version 1.1.
The statistical review of the study was performed by a biomedical statistician.Univariate and multivariate analysis and the calculation of the hazard ratio (95%CI)were carried out according to the Cox regression. Survival curves were estimated using the Kaplan–Meier method. The Chi-squared test was used to correlate sarcopenia and ORR, toxicities, age, BMI, disease stage at the time of first diagnosis and NLR. The statistical analysis was performed using the SPSS version 20.0 software(SPSS Inc.).
RESULTS
We examined 56 consecutive mCRC patients who had received first-line chemotherapy and whose CT-scans were available in our archive. Fourteen patients(25%) were sarcopenic at baseline CT scan (7/33 men; 7/23 women). The median age of patients was 67 years (37-85 years) and 20 of the 56 patients (35.7%) were 70 years or older. Five of the 14 sarcopenic patients were 70 years or older. BMI distribution was 0% underweight, 37.5% normal weight, 39.3% overweight and 23.2% obese. SMI varied within each BMI category: 6/21 normal weight patients, 6/22 overweight patients and 2/13 obese patients were sarcopenic at baseline CT scan. Eighteen patients (32.1%) had a NLR ≥ 3. At the time of first diagnosis, 23 patients (41.1%) had II or III stage disease according to the pTNM classification and they subsequently developed metastases; 33 patients (58.9%) received the diagnosis at the metastatic stage. Of the 14 sarcopenic patients at the time of first diagnosis of metastatic disease, 4 had metachronous metastases and 10 had synchronous metastases.
The median follow-up was 26.8 mo (3.8-66.8 mo) and the median OS was 27.2 mo(95%CI: 23.3-37.3) (Figure 1). Sarcopenia was not correlated to either OS (HR, 0.72 95%CI: 0.35-1.47,P= 0.362) (Figure 2) or higher toxicity during the first 4 cycles of chemotherapy (P= 1.0) (Table 1). Four of the 14 (28.6%) sarcopenic patients and 13 of the 42 (31%) non-sarcopenic patients had at least one reduction in drug dosage due to toxicity during the first four cycles of therapy (P= 1.0). Twenty-seven patients (48.2%)had a partial or complete response, the disease was stable in 24 patients (42.8%), and 5 patients (8.9%) had disease progression as best response to first-line treatment.Response rate was not correlated to baseline sarcopenia (P= 0.221) (Table 1).
At first disease reassessment, 17 patients had an SML ≥ 5% (30.3%); 3 of these patients were already sarcopenic at baseline CT scan, while 7 patients became sarcopenic. Of these 6 men and 1 woman, 4 were under the age of 70 years; at baseline,3/7 patients were normal weight, 3/7 were overweight and 1/7 was obese. One normal weight patient became overweight, while one overweight patient became normal weight at first disease reassessment. The median OS of these 7 patients was 27.93 mo, similar to that of the entire study population. Muscle mass loss was not correlated to OS (P= 0.961) (Figure 3).

Figure 1 Overall survival.

Figure 2 Overall survival according to baseline sarcopenia.

Figure 3 Overall survival according to muscle mass loss.
No statistically significant correlation was found between baseline sarcopenia and age (P= 1.0), BMI (P= 0.728), stage at diagnosis (P= 0.355) and NLR (P= 0.751)(Table 1).
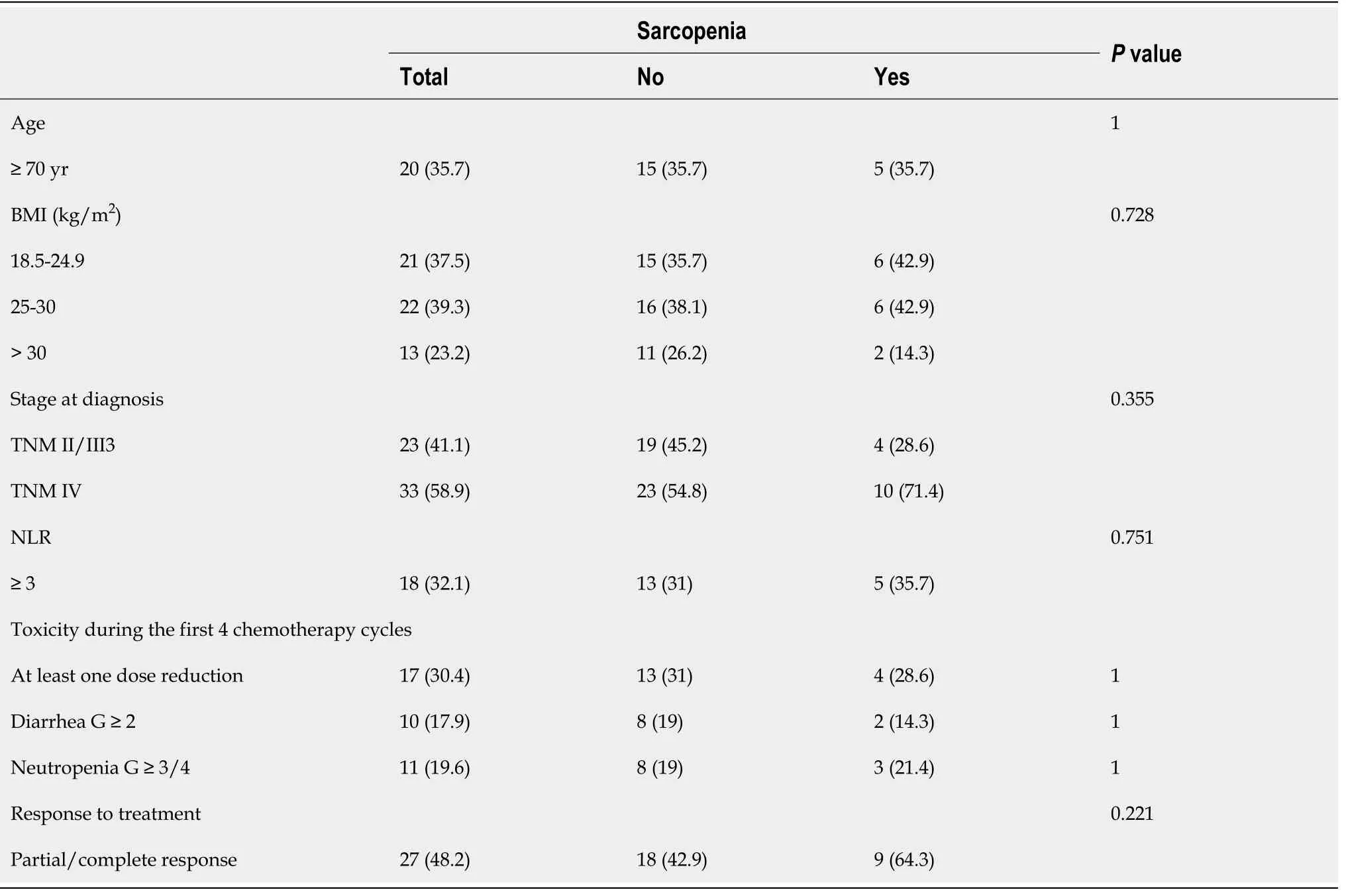
Table 1 Correlation between sarcopenia, age, body mass index, stage at diagnosis, neutrophil/lymphocyte ratio, toxicity and response to treatment, n (%)
DISCUSSION
Skeletal muscle decrease is generally associated with physiological ageing. The probable mechanism of sarcopenia is an imbalance in muscle protein turnover due to endocrine changes (e.g., reduction of sex hormones and growth factors), age-related cell damage and mitochondrial dysfunction, oxidative stress, low-grade systemic inflammation, physical inactivity and malnutrition[20-23]. In cancer patients the loss of muscle mass can occur earlier than in healthy people due to the synergy between physiologic and tumor factors (e.g., production of inflammatory cytokines that induces a catabolic state)[24]. Depending on histology and disease stage, the prevalence of sarcopenia varies greatly among patients affected by neoplasia; for example,sarcopenia has been diagnosed in 30%-65% of patients with pancreatic neoplasia[25,26]; in 15.9%-66.9% of women with breast cancer[27,28]; in 47.9%-89% of gastric cancer patients[29-31]; in 27.5% of patients with advanced hepatocellular carcinoma[32]; in 52.5%-54.5% of patients with metastatic renal cell carcinoma[33,34]; in 19.4%-39% of patients with colorectal cancer[35,36].
In our study population, the prevalence of sarcopenia was 25%: 14 of the 56 patients were sarcopenic at baseline CT and most of them (9/14, 64%) were under the age of 70 years, which indicates that it is not uncommon to find a low skeletal mass in young adults. In this context, it is notable that Miyamotoet al[37] found that young CRC patients (< 65 years) with sarcopenia had a significantly shorter OS than those without sarcopenia, while the prognostic role of sarcopenia was lost in patients above 65 yearsof age. Consequently, it is also important to assess muscle mass in young CRC patients upon diagnosis, to better define the prognosis of each patient, and possibly to tailor anticancer treatment and improve the correction of sarcopenia. Indeed, various strategies have been reported to improve muscle mass and strength, namely exercise[38,39], dietary supplementation of proteins[40] and long-term intake of omega-3 fatty acids, which have anti-inflammatory and anabolic activities[41].
Clinicians should determine whether patients have sarcopenia not only regardless of age, but also regardless of BMI. In fact, a feature of sarcopenia that differentiates it from cachexia, is that it can occur without a concomitant loss of adipose tissue. In our study, no patient was underweight, and 8 of the 14 (57%) sarcopenic patients were overweight or obese (Figure 4). Notably, not all sarcopenic patients have a low BMI:unlike other causes of muscle loss, sarcopenia can be associated with normal or even excessive body weight,i.e., the so-called “sarcopenic obesity”[8,9]. The loss of muscle tissue can be associated with increased intramuscular fat, which results in a reduction in strength and muscle mass[40]. Martinet al[8] found that high weight loss, a low muscle index and low muscle attenuation due to fat infiltration, independently worsened survival in 1473 patients with lung or gastrointestinal cancer. Patients with all three of these poor prognostic variables survived 8.4 mo (95%CI: 6.5 to 10.3)regardless of BMI, in contrast to patients who had none of these features, who survived 28.4 mo (95%CI: 24.2 to 32.6;P< 0.001)[8]. In addition, BMI was predictive of survival, with the heaviest patients showing the longest survival. However, obese patients without any risk factorS survived 35.6 mo, which is twice longer than the median survival of the entire population (16.7 mo), while obese patients with three poor prognostic variables survived only 8.5 mo[8].
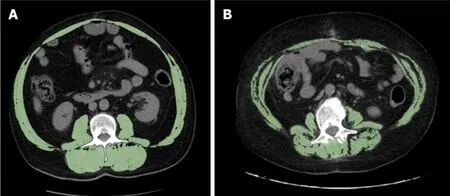
Figure 4 Representative examples of two obese patients with different skeletal mass index values. A: The patient is a 56-year-old man with body mass index (BMI) = 32.91 kg/m2 and normal skeletal mass index (SMI) value = 62.70 cm2/m2; B: The patient is a 61-year-old woman showing BMI = 32.54 kg/m2 and reduced SMI value = 39.45 cm2/m2.
A low LBM and sarcopenia have been correlated to a worse prognosis and a worse quality of life in patients with solid tumors[6,7,13]. A meta-analysis of 38 studies,involving a total of 7843 patients, showed that subjects with a reduced SMI had a shorter OS, cancer-specific survival and disease-free survival than subjects with a normal SMI[13]. However, it included studies of various tumor types (e.g., pancreaticobiliary cancer, hepatocellular carcinoma and esophageal cancer that have worse outcomes than other malignancies, such as colorectal cancer), disease stages (limited and advanced), therapeutic strategies, imaging techniques and sarcopenia cut-off values.
In our patient cohort undergoing first-line chemotherapy for mCRC, sarcopenia was not related to either survival or response rate. Previous studies reported that low muscle mass was a negative prognostic factor both in resectable[37,42] and in advanced[43-45] colorectal cancer. However, those studies included patients with clinical and disease-related characteristics different to our patients. For example, Vashiet al[43] studied patients younger than ours (median age 53.3 years) who were at different disease stages (early and metastatic disease), some of whom had already been treated for metastatic disease. Moreover, they used cut-off values that did not consider gender or BMI in their definition of sarcopenia. Also the reports by Kurket al[44] and Charetteet al[45] were based on data derived from clinical trials designed for different endpoints, and included patients undergoing maintenance chemotherapy after the first therapeutic line or heavily pretreated patients. The latter two groups of patients have a better and worse prognosis, respectively, than our patients.
Differently, other studies did not find a correlation between basal sarcopenia and survival, but they suggested that muscle mass loss during treatment plays a negative prognostic role. For example, Miyamotoet al[18] analyzed 182 Asian patients with unresectable CRC. Female gender (P< 0.001) and BMI < 25 kg/m2(P< 0.001) were significantly associated with a lower SMI. There were no significant associations between baseline skeletal muscle mass, progression-free survival (PFS) and OS[18].However, 22 patients with SML > 5% after first-line chemotherapy had significantly shorter PFS and OSvsthose without SML (PFS, log-rankP= 0.029; OS, log-rankP=0.009)[18]. Sasakiet al[46] found sarcopenia in 135 of 219 Asian mCRC patients (mostly male, older, with a lower BMI, lower visceral and subcutaneous fat content and a lower waist circumference than patients without sarcopenia). Baseline sarcopenia was not associated with prognosis, but SML ≥ 9% at 3 mo was associated with a high incidence of adverse events (P= 0.01), poor ORR (P< 0.01) and poor PFS (P= 0.03)[46]. Also Blauwhoff-Buskermolenet al[47] observed that the muscle area of 67 patients with mCRC (78% at first-line treatment and 22% at second-line treatment) decreased by 6.1% (95%CI: 28.4% to 23.8%;P< 0.001) during 3 mo of chemotherapy. Changes in muscle area were not associated with any treatment dosage modifications (dose reduction, delay or discontinuation), but patients with a muscle loss of 9% or more during treatment had significantly lower survival rates (at 6 mo, 33%vs69% of patients alive; at 1 year, 17%vs49% of patients alive; log-rankP= 0.001)[47].
We found no association between muscle loss during first-line treatment and survival, but it is interesting to note that a SML ≥ 5% occurred in 17 patients (30.3%);only 3 of whom were already sarcopenic at baseline CT scan, while 7 patients became sarcopenic during therapy. Chemotherapy probably induces progressive muscle damage both directlyviaa cytotoxic mechanism, and indirectly consequent to a more sedentary lifestyle because of the development of toxicity and asthenia.
In our analysis, sarcopenia was not related to higher toxicity reported during the first four cycles of chemotherapy, but 30.4% of all patients had at least one reduction in drug dosage due to toxicity, which indicates that approximately one-third of our patients did not receive an adequate drug dosage as calculated based on BSA. In this context, it is interesting to refer to the data reported by Pradoet al[14], who examined 62 patients with stage II/III colorectal cancer receiving adjuvant treatment with 5-fluorouracil (5-FU). Exposure to 5-FU was then normalized per kilogram of LBM. In women, the 5-FU dose/kg LBM varied from 12.8 to 23 mg/kg LBM and, in men, from 12 to 20.1 mg/kg LBM[14]. Levels greater than or equal to 20 mg of 5-FU/kg of LBM were associated with an increased risk of developing dose-limiting toxicities (any grade 3/4 toxicity, dose delay or reduction) at first therapy cycle, especially in women[14]. The population analyzed in the latter study differed greatly from our patients as it included only early-stage colon cancer patients who had undergone surgery on the primary tumor and they were treated with a single adjuvant drug, administered with an obsolete schedule (5-FU and leucovorin by i.v. bolus for 5 d every 28 d); however,these data illustrate how drug exposure varies widely among patients and how this variation affects treatment tolerability.
Other studies investigated the correlation between low muscle mass and worse toxicity during chemotherapy. For instance, Aliet al[6] assessed data from one prospective (n= 80 patients) and one retrospective study (n= 58 patients) that included patients at different stages of CRC, treated with different therapeutic regimens with one or more drugs. They observed that a low LBM was an independent determinant of toxicity and neuropathy in patients administered a FOLFOX-based regimen (5-FU + oxaliplatin) using conventional BSA dosing[6]. Gökyeret al[48]evaluated 36 patients with mCRC who received regorafenib. Dose-limiting toxicity(DLT), defined as toxicity requiring dose reduction or drug withdrawal, occurred in 13 of the 23 patients (56.5%) with basal sarcopenia, whereas only 1 of the 13 patients(7.6%) without sarcopenia experienced DLT (P= 0.005)[48]. Kurket al[44,49], using data of the randomized phase 3 CAIRO3 study[50], found that sarcopenia at the start of maintenance capecitabine + bevacizumab was not associated with DLT, whereas patients with > 2% SMI loss had a significantly higher risk of DLT. When capecitabine+ oxaliplatin + bevacizumab was reintroduced due to disease progression, 25% of patients started the treatment at a reduced dose and most of them were patients with previous SMI loss[49]. Interestingly, after drug dose adjustment, no further DLT was observed in the subgroup of patients with SMI loss[49].
Currently, data on the prognostic and predictive role of sarcopenia are based mostly on retrospective studies or on clinical trials designed for other endpoints. Conflicting results highlight the need to investigate further the role of low muscle mass in cancer patients. Indeed, there is a need for prospective studies of more homogeneous populations in terms of age, sex, tumor histology, stage of disease, treatment setting,and mono- or polychemotherapy regimens. In the future, clinicians might evaluate the body composition of cancer patients before starting chemotherapy in order to select the drug (e.g.lipophilic, hydrophilic, immunotherapy or biological) with the shortest regime (for example, shortening induction therapy in favor of a weakened therapy in sarcopenic patients), the most adequate dosage, and ancillary support strategies (e.g.exercise, specific nutrition supplements, drugs,etc.).
CONCLUSION
In our study, neither baseline sarcopenia nor muscle mass loss during first-line chemotherapy influenced survival in mCRC patients. Moreover, baseline sarcopenia did not worsen treatment toxicities during first-line chemotherapy. However, these results must be interpreted with caution given the limited sample size. Further prospective studies are needed to investigate the actual role of sarcopenia in prognosis and therapeutic decision-making. Greater efforts should be made to diagnose sarcopenia upon cancer diagnosis to correct strength and muscle mass as early as possible and thus improve the patient’s tolerability to treatment and survival.
ARTICLE HIGHLIGHTS
Research background
People with a low skeletal muscle mass, defined as “sarcopenia”, may have a lower volume of drug distribution and reduced protein binding compared to people with a normal muscle mass thereby resulting in a higher plasma drug concentration and worse treatment toxicity. In cancer patients, sarcopenia is considered a negative prognostic factor for survival and for the development of dose-limiting chemotherapy toxicities.
Research motivation
Pharmacokinetic parameters of a given drug, such as area under the curve and clearance, may differ substantially among patients depending on body composition.The ratio of fat to lean tissue mass could be a better tool than body surface area with which to determine the dose of cytotoxic agents as it affects metabolism, plasma concentration and the toxicity of drugs.
Research objectives
The primary end-point of this study was to assess the association between baseline sarcopenia, evaluated before starting first-line chemotherapy, and overall survival in metastatic colorectal cancer patients. The secondary end-points were to investigate: (1)the potential correlation of baseline sarcopenia with the objective response rate to firstline chemotherapy and with the development of side effects during the first four cycles of treatment; (2) the association between skeletal muscle loss (SML) at first disease reassessment and overall survival (OS); and (3) the relationship between sarcopenia and age, body mass index (BMI), disease stage at the time of first diagnosis and the neutrophil/lymphocyte ratio as an inflammation index.
Research methods
Computed tomography (CT)-scans were performed before starting chemotherapy and at the first disease reassessment. Sarcopenia was assessed using the skeletal mass index [SMI = muscle area in cm2/(height in m2)] calculated at the L3 vertebra.Sarcopenia was defined by Martin SMI cut-offs that combined both sex-specific and BMI cut-offs: 43 cm2/m2for men with BMI < 25 kg/m2, 53 cm2/m2for men with BMI ≥25 kg/m2, and 41 cm2/m2for women regardless of BMI. OS and objective response rate were evaluated. Toxicities were analyzed during the first four cycles of therapy and graded according to Common Terminology Criteria for Adverse Events version 4.0. A loss of skeletal muscle mass ≥ 5% was considered indicative of deterioration in muscle condition.
Research results
The prevalence of sarcopenia was 25%: 14 of the 56 patients were sarcopenic at baseline CT and most of them (9/14, 64%) were under the age of 70 years, which indicates that it is not uncommon to find a low skeletal mass in young adults. No patient was underweight, and 8 of the 14 (57%) sarcopenic patients were overweight or obese. Sarcopenia was not correlated to overall survival (P= 0.362), to higher toxicities reported during the first 4 cycles of chemotherapy (P= 1) or to response to treatment (P= 0.221). At the first disease reassessment, a SML ≥ 5% was found in 17 patients (30.3%) 3 of whom were already sarcopenic at baseline CT scan, while 7 became sarcopenic. SML was not correlated to overall survival (P= 0.961).
Research conclusions
Although this is a negative study, our results must be interpreted with caution given the limited sample size. Moreover, the body composition of cancer patients should be evaluated before starting chemotherapy to better select the drug (e.g.lipophilic,hydrophilic, immunotherapy or biological) with the shortest regime (for example,shortening induction therapy in favor of a weakened therapy in sarcopenic patients),the most adequate dosage, and ancillary support strategies (e.g.exercise, specific nutrition supplements, drugs,etc.).
Research perspectives
There is a need for prospective studies of more homogeneous populations in terms of age, sex, tumor histology, stage of disease, treatment setting, and mono- or polychemotherapy regimens, to investigate the actual role of sarcopenia in prognosis and therapeutic decisions. Greater efforts should be made to diagnose sarcopenia upon cancer diagnosis in order to correct strength and muscle mass as early as possible and thus improve the patient’s treatment tolerability and survival.
ACKNOWLEDGEMENTS
We thank Jean Ann Gilder (Scientific Communication Srl., Naples, Italy) for language assistance.
 World Journal of Clinical Oncology2021年5期
World Journal of Clinical Oncology2021年5期
- World Journal of Clinical Oncology的其它文章
- Cellular based treatment modalities for unresectable hepatocellular carcinoma
- COVID-19: Where is the treatment?
- Immune response evaluation criteria in solid tumors for assessment of atypical responses after immunotherapy
- Hepatic Langerhans cell histiocytosis: A review
- Thymoquinone anticancer activity is enhanced when combined with royal jelly in human breast cancer
- Reduction of muscle contraction and pain in electroporation-based treatments: An overview
