The Ulva prolifera genome reveals the mechanism of green tides*
Yuan HE , Songdong SHEN , Dachun YU , Yehua WANG , Jiao YIN , Zongling WANG ,Yuantu YE ,**
1 Institute of Aquaculture, College of Biology and Basic Medical Sciences, Soochow University, Suzhou 215123, China
2 First Institute of Oceanography(FIO), Ministry of Natural Resources(MNR), Qingdao 266100, China
Abstract The genome of green microalgae has rarely been reported. Ulva prolifera is a green microalga that has received much attention. Despite research articles about U. prolifera in recent years, we know very little about its genome. Therefore, the 87.9-Mb haploid genome (containing 10 311 protein-coding genes)of U. prolifera was studied, and the genome was compared with that of U. mutabilis, which is the only published Ulva species. Results showed that the two species are closely related. A phylogenetic tree was constructed among U. prolifera and other green algae available in GenBank, revealing the evolutionary status of U. prolifera in Chlorophyta. To understand why U. prolifera could grow rapidly, we identified some genes related to growth, such as those involved in cell division, phosphorylation, and cell proliferation. In addition, genes related to stress resistance were found, which supports the notion that U. prolifera grows vigorously in nature. These results help to characterize green tides from a new perspective and reveal some important insight into the biology of U. prolifera.
Keyword: Ulva prolifera; genome; fast grow; evolution
1 INTRODUCTION
Chlorophyta is an important group of green algae,with approximately 350 genera and 5 000-8 000 species. The green algae group can be divided into two classes: Chlorophyceae and Conjugatophyceae.Green algae present a variety of forms, including single-cell individuals, filaments, and membranous bodies (Mine et al., 2008). Most green algae live in fresh water, whereas only approximately 10% grow in seawater. In China, seawater green algae belong to Chlorophyceae, which is divided into 11 orders,namely, Volvocales, Tetrasporales, Ulotrichales,Chlorococcales, Ulvales, Chaetophorales,Cladophorales, Siphonales, Siphonocladales,Prasiolales, and Dasycladales. The cell wall of green algae contains cellulose, and the cytological features share many similarities with those of higher vascular plants. Furthermore, the pigments produced are the same photosynthetic pigments of higher plants, such as chlorophylla, chlorophyllb, carotene, and lutein.The chloroplasts of green algae contain pyrenoids,which are surrounded by starch. Previous evolutionary analyses show that Chlorophyta is more closely related to higher plants than other algae, and higher plants are likely to have evolved from green algae(Herron et al., 2010).
Ulvamainly grows on rocks in intertidal zone on mud beaches or onto other floating seaweeds at the sea (Tan et al., 1999). There are approximately 11 species reported in China. The common species areU.pertusa,U.lactuca,U.prolifera,U.intestinalis,U.compressa,U.tubulosa,U.flexuosa, andU.linza.U.proliferabelongs to phylum Chlorophyta, class Ulvophyceae, order Ulvales, family Ulvaceae, and genusUlva(Gao et al., 2010). Algal bodies are bright green or light green, in height of 1-2 m and diameters 2-3 mm, with many branches. The main branches are distinct and elongated and have typical tubular structure of monolayer cells. The pigment body is not filled with cells and contains one starch core.
In life history,U.proliferaexhibits a variety of reproductive manners, such as asexual reproduction(vegetative reproduction and spore reproduction),sexual reproduction, and parthenogenesis.
Regarding biological characteristics,U.proliferacould tolerate wide ranges of temperature and salinity,and resist to strong light. Previous studies have shown that the adaptability ofU.proliferato the environment is within the following conditions: temperature 10-30 °C, salinity 7.2-53.5, light 1 000-10 000 lx, and pH 6-9 (Gao et al., 2016). Reports ofU.proliferacultured in China indicate the ash content of 8.2%-36.68%, which is lower than that of kelp and wakame and higher than that of seaweed, and the vitamin content of 0.174-0.206 mg/g, which is higher than that of kelp and wakame. Moreover,U.proliferais rich in protein and has high digestibility (98%).Therefore, it can be used as an important protein food source for humans.U.proliferaalso contains 18 amino acids, including all 8 essential amino acids (Xu et al., 2003). Sugars account for the largest proportion ofU.proliferacells, at 46.2%-63.9%, and become polymerized as dietary fiber in a certain structure that is a good source of dietary fiber. Moreover, as this species synthesizes a wide range of fatty acids,including linolenic acid, linoleic acid, eicosapentaenoic acid (EPA), arachidonic acid, and other important fatty acids, it can be used as a raw material for producing functional fats and oils. In addition, levels of three important mineral elements, Fe, Zn, and Cu,are significantly higher inU.proliferathan in other algae. Therefore, this species can be used as a good feed source (Fleurence et al., 1994).
Algae comprise an ancient group. These species have crossed the prokaryotic and eukaryotic boundary.To date, there are approximately 30 000 known species. Indeed, the evolutionary relationship of algae has been a heavily researched topic for many years(Ben Ali et al., 2002). With the development of genome sequencing technology and the decreasing cost, there have been some recent breakthroughs in genomics research of algae, and the whole genomes of several algae have been published.
In this study, the whole-genome sequence ofU.proliferawas studied with a focus on genes that involved in growth and stress resistance. In addition,our data were compared with those ofU.mutabilis,which is available in GenBank. The findings could not only enrich our knowledge ofUlvagenomes but also provide a foundation for understading theU.proliferaoutbreaks in terms of the cell division process.
2 MATERIAL AND METHOD
2.1 Strain and culture conditions
Floating green microalgaU.proliferawas collected in June 2018 at the Yellow Sea in Qingdao, Shandong,China (34°5′N, 122°50′E). The sample was placed on dry ice, transported to laboratory, and cultivated in seawater medium. The cultivation environment was 20 ℃, salinity 30, under cool-white fluorescent light in 12 h:12 h L:D cycle, illuminance 120 μmol/(m2·s)photons, and the length of culture 7 d. After the culture, they were frozen in liquid nitrogen for DNA extraction.
2.2 Genome sequencing and assembly
Total DNA was isolated using E.Z.N.A.®Plant DNA Kit (Omega BioTek, USA) as per the manufacturer’s protocol. A 20-kb DNA library was prepared according to PacBio DNA preparation protocols, and the whole genome was sequenced using the PacBio RS platform. De novo assembly of the genome was achieved using falcon software (Chin et al., 2016). The original reads were split into modules of specified sizes and compared. Line selfcorrection was employed, and overlapping data were searched. The Overlap-Layout-Consensus algorithm was applied to assemble the three generations of data after error correction through the overlap relationship among long reads. The original reads to the preliminary assembly results was mapped by correcting the latter to improve the accuracy. The assembly and annotation data were uploaded to NCBI under the accession number SDUY00000000.1.
2.3 Genome annotation and analysis
Genome annotation mainly includes three aspects:noncoding RNA prediction, gene structure prediction,and gene function annotation.
Noncoding RNAs include sRNA, rRNA, tRNA,snRNA, and miRNA. tRNAs were predicted using tRNAscan-SE (v1.3.1) (Schattner et al., 2005) and rRNAs using RNAmmer (v1.2) (Lagesen et al., 2007).Gene structure prediction of the genome was performed after masking repetitive sequences using the de novo prediction method with AUGUSTUS(Stanke et al., 2004). The reference genome used for the annotation was that ofU.mutabilis(De Clerck et al., 2018). Gene function annotation was carried out using the NR, COG/KOG, GO, KEGG, Swiss-Prot,and eggNOG databases. Alignment was performed using diamond software (Buchfink et al., 2015), with e<1e-5 annotations; proteins with the highest sequence similarity were screened to obtain functional annotation information.
2.4 Repeated sequence analysis
Repeated sequences can be divided into two major types, tandem repeats and interspersed repeats,depending on their arrangement in the genome(Wickstead et al., 2003). Repeated sequences were predicted using RepeatMasker (v4.0.7) software.
2.5 Phylogenetic tree reconstruction
The genomes of some green algae have been published in NCBI public databases. To compare homologous sequences, we first assembled each genome for alignment and then examined homologous sequences. We combined the genome ofU.proliferaobtained in this study with other green alga genomes and constructed a phylogenetic tree at the genome level. A maximum likelihood tree was constructed by using the software RAxML.
2.6 Analysis of genes related to growth and stress resistance
Genes involved in the cell cycle were analyzed using KEGG pathway annotation, and those involved in phosphorylation were identified using GO database annotation. Some genes related to stress resistance were found through the NR database.
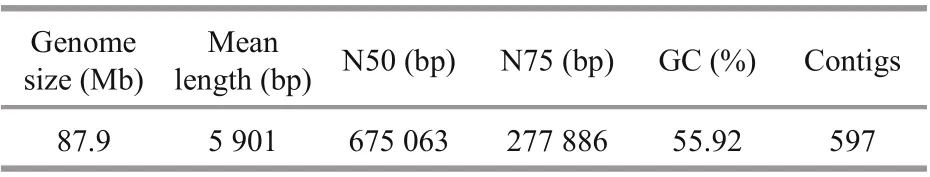
Table 1 Summary statistics of Ulva prolifera
3 RESULT
3.1 Genome sequencing and annotation
We first generated a total of 2 213 533 reads with a total size of 12 897 726 267 bp, a minimum length of 50 bp, a maximum length of 83 142 bp, and a mean length of 5 901 bp. After removing redundant scaff olds from the initial assembly, the N50 and N75 were 675 063 and 277 886 bp, respectively. The assembly of theU.proliferanuclear genome was 87.9 Mb, with contained 597 contigs (Table 1). The largest contig was 2 662 757 bp, with a GC content of 55.92%. The integrity of the genomic sequences and genetic predictions were analyzed using BUSCO software(Simão et al., 2015). The integrity of the genome was 75.3%.
Noncoding RNAs identified included rRNA,tRNA, and the other ncRNA. The total number of noncoding RNAs was 557. In addition, 185 rRNAs were found, accounting for 33.21%; 319 tRNAs,accounting for 57.27%; and 53 other ncRNAs,accounting for 9.52%. A total of 10 311 genes were detected, with a total length of 17 932 935 bp on average of 1 739 bp. 10 311 unigenes were annotated using diff erent databases (NR, Swiss-Prot, KEGG,KOG, eggNOG, and GO), with matches of 7 037(68.25%) in NR, 4 856 (47.10%) in Swiss-Prot, 3 315(32.15%) in KEGG, 4 495 (43.59%) in KOG, 6 180(59.94%) in eggNOG, and 4 770 (46.26%) in GO.
A total of 3 315 unigenes were annotated in the KEGG database, involving 24 metabolic pathways(Fig.1). The pathway with the most unigenes was“translation” (324 unigenes), followed by “global and overview maps” (273 unigenes) and “energy metabolism” (262 unigenes). There were 114 unigenes predicted to participate in “cell growth and death”,including “cell cycle”, “cell cycle-yeast”, “cell cyclecaulobacter”, “meiosis-yeast”, “oocyte meiosis”,“p53 signaling pathway”, “apoptosis”, “apoptosisfly”, and “apoptosis-multiple species”. Genes ofinterested are involved in “cell cycle”.
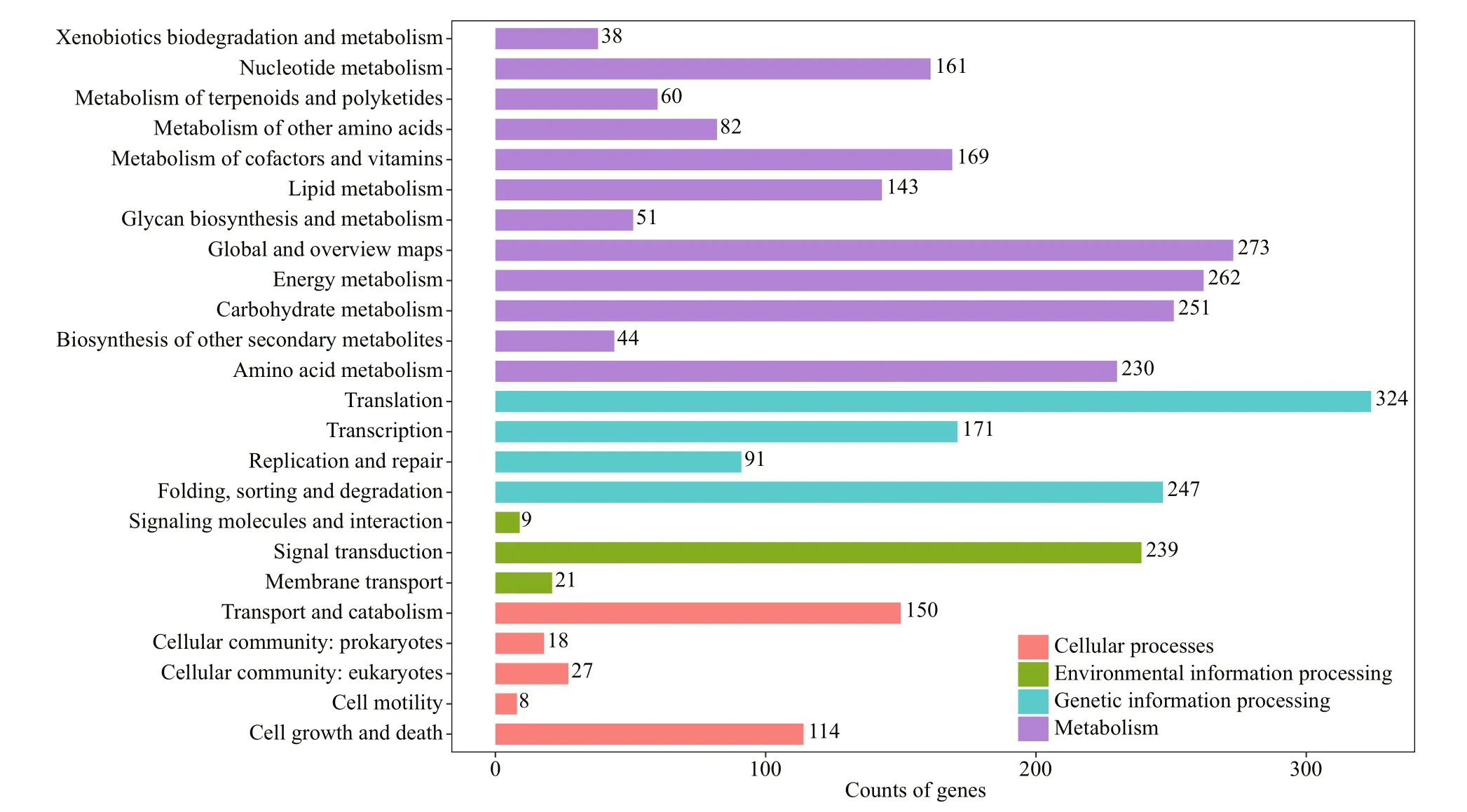
Fig.1 KEGG annotation of the nonredundant sequences of the sample
GO functional annotations consist of three ontologies: biological process, cellular component,and molecular function. In total, 4 770 annotated unigenes were categorized into the three ontologies with 64 GO terms (Fig.2). Most unigenes are involved in the “cellular component” category (4 453 unigenes)followed by “molecular function” (4 202 unigenes)and “biological process” (4 053 unigenes). For the cellular component category, “cell” and “cell part”were two most common terms; “binding” and“catalytic activity” were the two most common terms in the molecular function category and “cellular process” and “metabolic process” in the biological process category.
3.2 Analysis of repeated sequences
The proportion of repeated sequences in the genome was 21.92% by calculation. Among the sequences, 84 small RNAs are present in tandem repeats, accounting for 0.07% of the total number of sequences. There are 19 009 simple repeats,accounting for 1.23%, and 908 low-complexity sequences, accounting for 0.05%. The total length of the interspersed repeat is 18 100 769 bp, with the largest number of the total number of sequences being long terminal repeats (LTRs), at 4 353, accounting for 6.43%, followed by DNA elements, at 2 653,accounting for 0.78%, and long interspersed nuclear elements (LINEs), at 1 026, accounting for 0.48%.Short interspersed nuclear element sequences (SINEs)represented the least, at 205, accounting for 0.41%,and there were 35 564 unclassified tandem repeats.
3.3 Phylogenetic tree analysis
To better understand the evolutionary status ofU.proliferaamong green algae, the genomes of other green algae present in GenBank, such asVolvox,Chlamydomonas,Chlorella,Dunaliella, andGonium,were used to construct phylogenetic trees.Arabidopsisand rice clustered together and acted as an outgroup.Eighteen species of green algae were divided into four categories: Chlorophyta, Ulvophyceae,Trebouxiophyceae, and Prasinophyceae (Fig.3). The genomes ofU.proliferaandU.mutabiliswere the closest, clustering together with Ulvophyceae. The result also supports the general notion that Chlorophyceae and Ulvophyceae are evolutionarily close within Chlorophyta.
3.4 Genomic comparisons of Ulva mutabilis
Ulvais the representative of Ulvophyceae. At present, only oneUlvaspecies, namely,U.mutabilis,has been fully sequenced (De Clerck et al., 2018). To explore the features of theUlvagenome, we compared our data with that ofU.mutabilis.
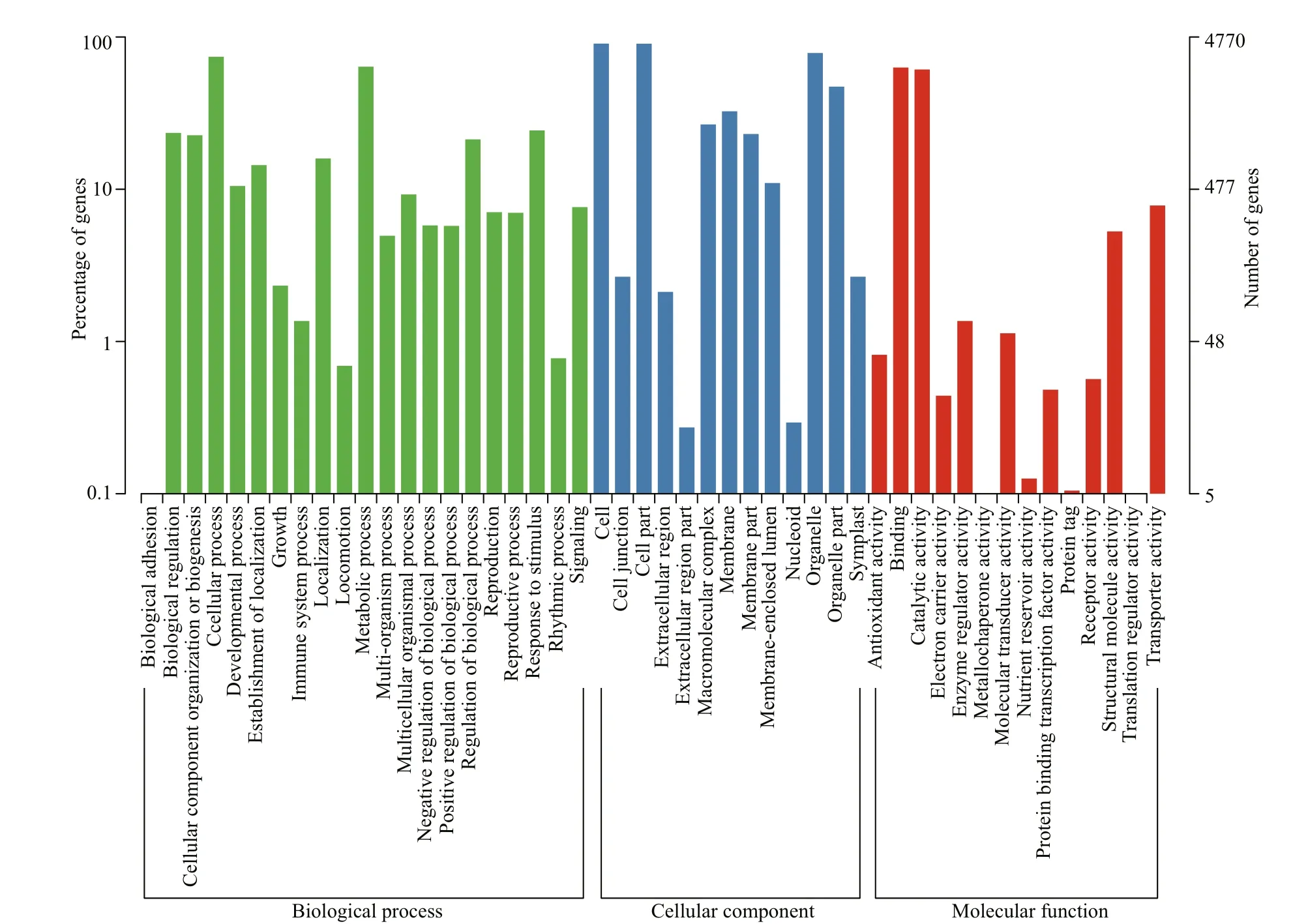
Fig.2 GO annotation of the nonredundant sequences of the sample
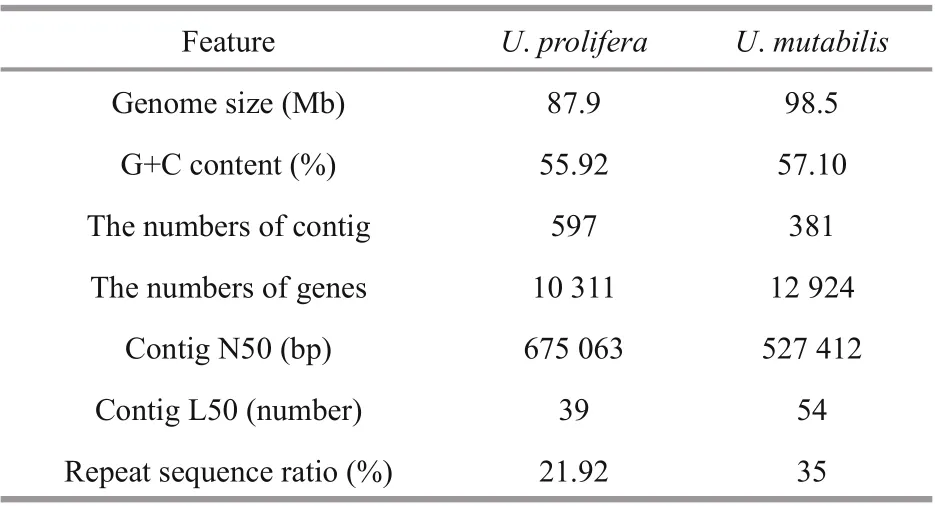
Table 2 Comparison of general features of U. prolifera andU. mutabilis genome
The size of the genome ofU.mutabilisis 98.5 Mb,which is more than 10.6 Mb larger than the genome ofU.prolifera. The number of protein-coding genes is 12 924, which is also more than that obtained forU.prolifera(10 311). However, the N50 ofU.proliferais 675 063 bp, which is longer than that ofU.mutabilis(527 412 bp). In addition, the GC content ofU.mutabilisis 57.1%, which is similar to that ofU.prolifera(55.92%). Based on the GC content and morphological complexity, the morphological features of the two species are very similar. In terms of repetitive sequences, LTRs and LINEs inU.mutabiliscomprise 15.3 Mb and 9.3 Mb,respectively, which is considerably longer than the values forU.prolifera(5.9 Mb and 0.5 Mb). A comparison of the general features of theU.proliferaandU.mutabilisgenomes is presented in Table 2. The result of collinear analysis also showed that the genetic type and relative sequences of the two algae are relatively conserved (Fig.4).
3.5 Analysis of rapid growth-related genes
Fifty genes are involved in the cell cycle according to KEGG annotation, and we found 27 cell cycle pathway-related genes in algae via comparison with data in GenBank (Table 3). These genes included some cell cycle factors, such as CDC14, CCNA,CDC2, and CDC23, cyclin-dependent kinase proteins,such as CDKD, other genes involved in the cell cycle,such as MCM2-7, and anaphase-promoting complexes, such as APC1 and APC6.
3.6 Analysis of stress-resistant genes
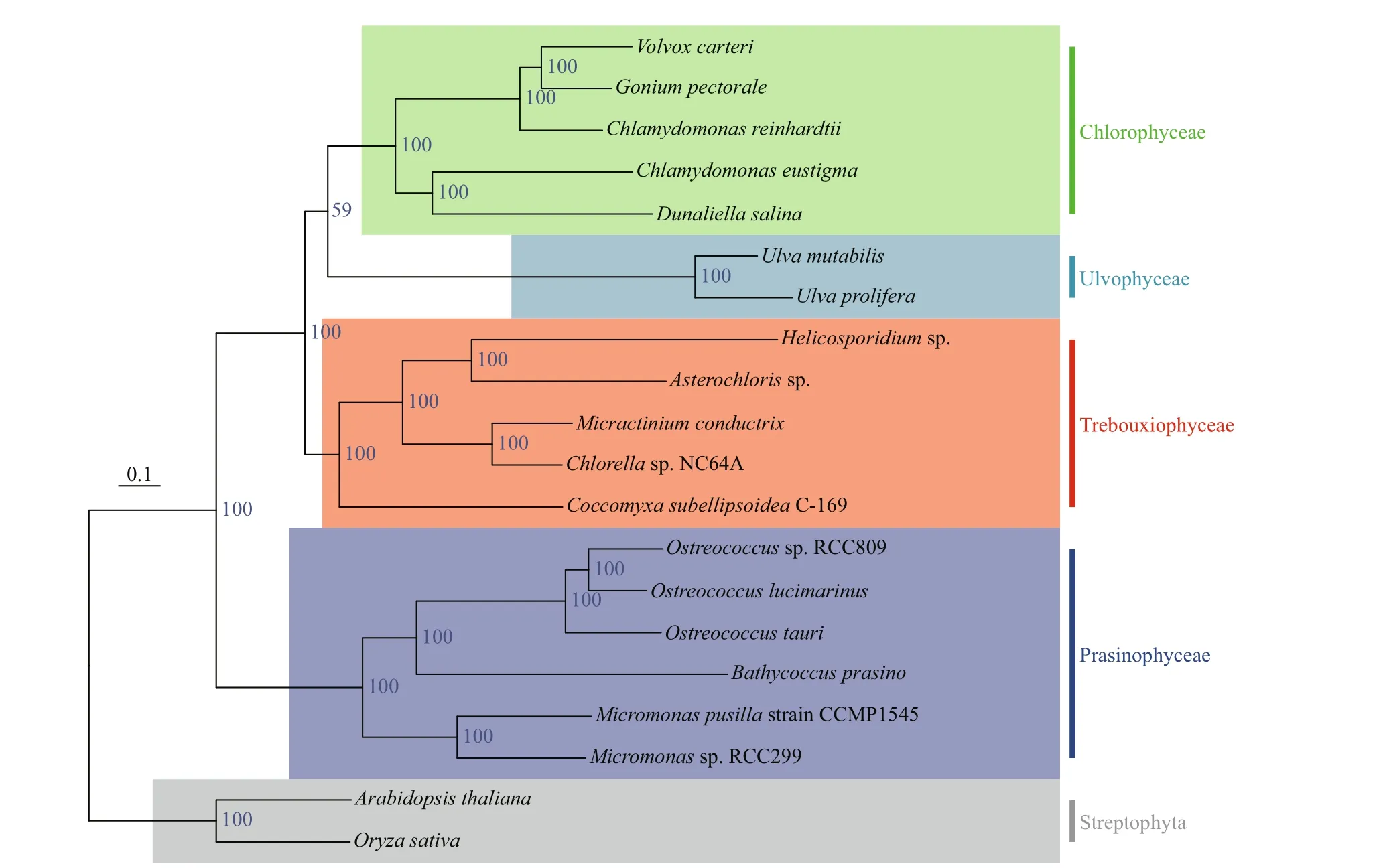
Fig.3 The phylogenetic tree was constructed based on the genomes of U. prolifera and the other green algae using maximumlikelihood methods

Fig.4 Collinear analysis of U. prolifera and U. mutabilis
Based on annotations, a large number of genes were identified as being responsive to stress, as follows: heat shock protein 40 in response to hightemperature stress; a cold shock protein in response to low-temperature stress; phytoene desaturase and carotenoid isomerase in response to high light; and catalase, ascorbate peroxidase, glutathione peroxidase, thioredoxin reductase, dehydroascorbate reductase, peroxiredoxin-5, peroxiredoxin-2B, ironsuperoxide dismutase 2, iron-superoxide dismutase 1,glutathione reductase, and iron reductase related to antioxidants. Some ion channel proteins were also identified, including chloride channel protein,voltage-gated channel, cadmium channel protein, and sodium channel protein.
4 DISCUSSION
The first species in Rhodophyta with a sequenced genome wasCyanidioschyzonmerolae, and the results indicate that theC.merolaegenome is an ideal model for studying the origin, evolution, and basic mechanisms of eukaryotes (Matsuzaki et al., 2004).Genome-sequencing research ofPyropiayezoensishas also been completed, with a size of 43 Mb. In total,10 327 genes were predicted, similar to the number of genes inU.proliferain the present results. Sequence homology analysis indicated an unknown function for 3 611 genes, of which 2 069 genes were identical tothose ofC.merolae, and the light-trapping pigment phycobilin was predicted determinant of the pigment characteristics of red algae. Thus, the genome ofP.yezoensisis an ideal model of Rhodophyta(Nakamura et al., 2013). As shown in our data, the genome ofU.proliferais larger than that ofP.yezoensis. The genome ofP.umbilicalisis 87.7 Mb,similar to that ofU.proliferaincluding 13 125 genetic loci, although this number is greater than that ofU.prolifera. The findings provide an important reference for the comprehensive understanding of the evolutionary relationship of red algae with other algae associated with secondary endosymbiosis (Brawley et al., 2017). The genome ofChondruscrispushas also been sequenced, with a size of 105 Mb and 9 606 annotated genes. Although theChondruscrispusgenome size is larger than that ofU.prolifera, the number of genes is slightly smaller than that inU.prolifera. These genomic data provide insight into the metabolic characteristics of marine red algae and their ability to adapt to the marine environment,including halogen metabolism, hydroxyl phospholipids, and multicellularity (Collén et al.,2013).
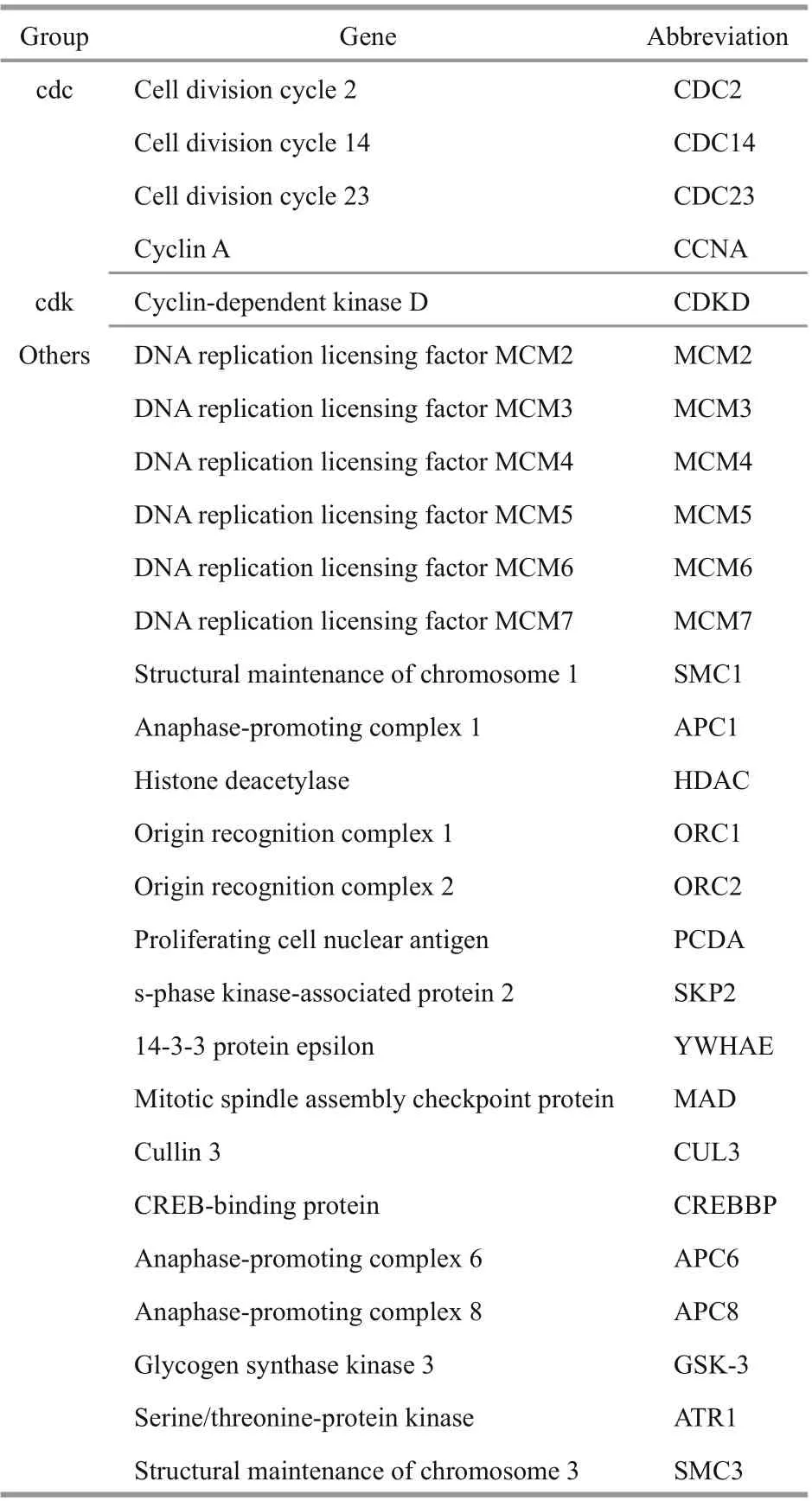
Table 3 Genes involved in cell cycle according to the KEEG annotation
The genome ofEctocarpussiliculosuswas the first sequenced in Phaeophyta, and its size was 214 Mb reportedly, much larger than that ofU.prolifera. The discovery oflight-harvesting and pigment-synthesizing genes and new metabolic processes, including halide metabolism, can help explain whyE.siliculosusis able to adapt to the high degree of change in tidal environment. Overall, previous studies indicate thatE.siliculosusis an ideal model for studying the genome of brown algae (Cock et al., 2010). In the future, we will explore whether similar metabolic processes exist inU.proliferato adapt to various sea environment. The complete kelp genome in size of 537 Mb and 18 733 annotated genes, has also recently been obtained. The size of the genome and number of genes are much larger than that inU.prolifera. This research made important contributions to plant genome research (Ye et al., 2015).Ectocarpusconfervoideshas a genome size of 140 Mb, containing 13 640 protein-coding genes; this genome is smaller than those ofE.siliculosusand kelp but larger than that ofU.prolifera(Nishitsuji et al., 2016).
With a size of 34 Mb, the genome ofThalassiosirapseudonanawas the first to be sequenced in Bacillariophyta, and 24 chromosomes were obtained by sequence assembly (Mock et al., 2008).Phaeodactylumtricornutum, with a genome size of 27.4 Mb, is a model organism in Bacillariophyta, and it was found significantly diff erent from the genomic structure ofT.pseudonana(Bowler et al., 2008). The genome sizes of algae in Bacillariophyta are generally smaller than that ofU.prolifera, and the structures are relatively simple.
In Chlorophyta, the genomes of many species have been successfully sequenced. A model organism of green algae, the genome size ofChlamydomonasreinhardtiiis 120 Mb. In general, studying the genome can deepen our understanding of primitive eukaryotic cells and reveal previously unknown genes associated with photosynthesis and the function of flagella;indeed, a link between ciliary diseases and the components and functions of the flagella has been established, showing the characteristics of the last common ancestor of plants and animals and identifying many cilia- and plastid-related genes (Merchant et al.,2007). Moreover, the genomes of three species ofChlorellahave been determined.ChlorellavariabilisNC64A, with a genome size of 46 Mb, is an ideal model for studying the interaction between algae and viruses. The symbiotic protein family was found to have greatly improved our understanding of the evolution of green algae, including interactions with viral genomes and symbiotic relationships with other eukaryotes (Blanc et al., 2010).Chlorellaprotothecoidessp. 0710 is considered one of the best candidates for industry production of microalgal biofuel. Its genome size is 22.9 Mb, which is only half of that ofC.variabilis. Genomic studies ofC.protothecoideshave revealed the mechanism underlying its capacity to produce large amounts oflipids, and genome analysis has facilitated evaluating algae as a model for the production of oils. With a genome size of 56.8 Mb,C.pyrenoidosaFACHB-9 is a microalga that rapidly converts intracellular stored energy to lipids. These studies may provide a basis for investigating metabolism and optimizing food and fuel production (Fan et al., 2016). The genome size ofMonoraphidiumneglectumis 68 Mb. Its carbohydrate metabolism and fatty acid biosynthesis processes,which are used in biotechnology for carbohydrate conversion, exhibit high metabolic diversity and provide new insight into the metabolic diversity of microalgal lipids (Bogen et al., 2013). Additionally,Parachlorellakessleriis a high-effi ciency unicellular green alga in genome size of 62.5 Mb. Upregulation of synthesis and autophagy is a potential key mediator oflipid hyperaccumulation under nutrient stress conditions (Ota et al., 2016).Volvoxcarteriis a multicellular green alga that is suitable for studying the evolution and development of multicellular organisms; its genome size is 138 Mb. There are only a small number of specific protein-coding genes inV.cartericompared withC.reinhardtii; theC.reinhardtiigenome encodes some proteins involved in the degree of expansion and height during division as well as the extracellular matrix. In general, the increased complexity of organisms is associated with modification of pedigree-specific proteins, rather than a large increase in protein-coding ability (Prochnik et al., 2010).Goniumpectoraleis another species of Volvocales, with a genome size of 148.8 Mb, similar to that ofVolvoxcarteri. The evolution of cell cycle regulation is important for population evolution and may reveal the evolutionary history of other multicellular evolutionary transitions (Hanschen et al., 2016).OstreococcustauriandOstreococcuslucimarinusare two small eukaryotic green algae.O.taurihas a genome size of 12.56 Mb, and some special features and a streamlined gene family makeO.taurian ideal model for studying the evolution of eukaryotic genes, including the origins of specific chromosomes and green algae families (Derelle et al., 2006). At 13.2 Mb, the genome size ofO.lucimarinusis similar to that ofO.tauri, and the study of theO.lucimarinusgenome has off ered insight into the biospecific metal metabolism of these organisms, which contain large amounts of selenocysteine proteins (Palenik et al.,2007).Micromonasis a genus of very small green algae with diameters ofless than 2 microns. The genomes ofMicromonasRCC299 andMicromonasCCMP1545 were sequenced simultaneously, with sizes of 20.9 Mb and 21.9 Mb, respectively, which are considerably smaller than that ofC.reinhardtiibut larger than that ofOstreococcus. This finding highlights the dynamics of the evolution of marine algae and provides a platform for further exploring the functions of phytoplankton (Worden et al., 2009).Dunaliellasalinais a single-celled green alga that is extremely tolerant to high salt. The genome ofD.salinaCCAP19/18, 343.7 Mb, was recently published, and the study of this genome facilitated the investigation of metabolic processes involved in the regulation of stress responses, including carotenoid production and growth in high-salt environments(Polle et al., 2017). The genomes ofGuillardiathetaandBigelowiellanatansare 87.2 Mb and 94.7 Mb,respectively (Curtis et al., 2012).Coccomyxasubellipsoideais a green alga that grows in the polar region and has strong cold adaptability. Its genome size is 48.8 Mb, and this is the first determination of polar eukaryotic microorganisms. Moreover, it was found that prokaryotes and eukaryotes followed a similar evolutionary route to achieve survival under cold conditions (Blanc et al., 2012). The genome size ofU.proliferais moderate among members of Chlorophyta, and it is very close to that ofGuillardiatheta.
In this study, we sequences the genome ofU.prolifera, with a size of 87.9 Mb, and the data obtained were compared with those forU.mutabilis.U.proliferais the main species that causes green tides(Wu et al., 2018), which have negative impacts on the environment and local economy in China every year.Therefore, the reasons whyU.proliferacan cause green tides have been a heavily researched topic in the study of green algae. We identified the genes involved in the cell cycle, which includes the entire process from the completion of one division to the end of the next division and is divided into a division phase and an interphase. Regulation of the cell cycle is an important aspect of biological growth and development, and many important life processes are related to the cell cycle. However, aberrant regulation leads to excessive cell proliferation or apoptosis.Accordingly, cell division is critical to organisms, and protein kinase-mediated signal transduction is the primary means of cell cycle regulation. Cyclin (cdc)and cyclin-dependent kinase (cdk) are key kinases that regulate the cell cycle (Joubès et al., 2000).Cyclins comprise a heterogeneous family of proteins,the main feature of which is the ability to bind and activate members of the cyclin-dependent kinase(CDKs) family and participate in cell cycle regulation(John et al., 2001). We detected some related genes in the genome ofU.prolifera, including some cell cycle proteins. In general, the cell division activity ofU.proliferacells is vigorous, and rapid growth may result from high cell proliferation and diff erentiation activity.
Phosphorylation is the most important posttranslational modification (Yang, 2005; Hunter,2007; Temporini et al., 2008). Some protein phosphatase and protein kinase genes were identified in this study, and these genes constitute a switch system for phosphorylation and dephosphorylation(Table 4). It has been predicted that approximately 30% of the eukaryotic cell proteome is phosphorylated(Cohen, 1976). In fact, phosphorylation is involved in many key processes, such as intercellular communication, immune response, intracellular signal transduction, and epigenetic regulation, and this process plays an important role in the growth and environmental response ofU.prolifera.
The reason whyU.proliferais able to grow rapidly in a short period is closely related to its capacity to adapt to various hostile environments; that is, it possesses strong resistance to stress. Under lowtemperature stress, lipid molecules on the plasma membrane will change to adapt to a low-temperature environment (Chen et al., 2005). Under hightemperature conditions, plants have a heat shock response to maintain homeostasis, protecting cells and normal biological activity and thereby enhancing resistance to heat (Liu and Wang, 2006). When the active oxygen species produced exceeds the ability of the scavenging system, oxidative damage and evencell death ensue (Du et al., 2001; Saxena et al., 2016).Algae, similar to other plants, have evolved protective enzymes that scavenge free radicals and reactive oxygen (Sies, 1993), and antioxidant enzymes coordinate to maintain biological free radicals at low levels to prevent cell damage (Zelko et al., 2002;Zhang and Tian, 2007). The discovery of such stressresponsive genes inU.proliferaindicates that the alga has strong resistance to stress, and even under adverse growth conditions, this species can rely on the expression of these stress-resistant genes to eff ectively resist external unfavorable factors. This is consistent with the characteristic of strong survivability, supporting the premise thatU.proliferacan grow vigorously in nature.
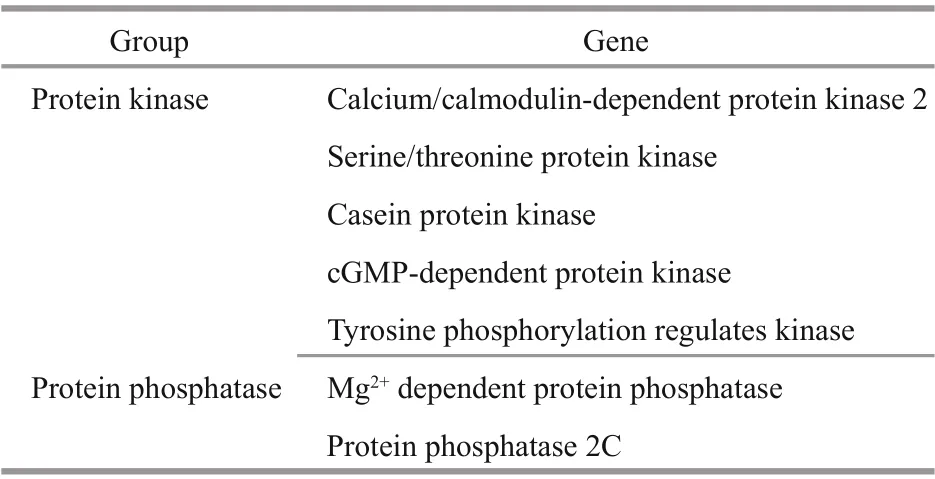
Table 4 Genes involved in phosphorylation according to the GO annotation
5 CONCLUSION
The green algaU.proliferais the main species responsible for green tides in the Yellow Sea. Here,we report an annotated draft genome ofU.prolifera,which is a candidate model alga ofUlva. Comparative analysis of our data with that ofU.mutabilisrevealed a close genetic relationship between the two species;however, some genomic diff erences exist between them, such as genome size and number of genes. To explore the evolutionary status ofU.prolifera, we constructed a phylogenetic tree using the genomes of green algae published in GenBank. Some genes involved in the cell cycle, phosphorylation, and stress resistance were found inU.prolifera, suggesting that its rapid growth is associated with these genes, which may explain the reason for green tides from a new perspective. These findings will help to elucidate the biology ofU.proliferaand will serve as a foundation for exploring the evolutionary history of other algae inUlva.
6 DATA AVAILABILITY STATEMENT
Sequence data that support the findings of this study have been deposited in the National Center for Biotechnology Information (NCBI) with the accession number SDUY00000000.1.
7 AUTHOR DECLARATION
The authors declared no conflict ofinterest. No conflicts, informed consent, human or animal rights applicable was concerned.
All authors have agreed to authorship and the submission of this manuscript for peer review. Yuan HE performed the experiments, analyzed the data,and drafted the manuscript; Songdong SHEN designed the experiments; Dachun YU, Yehua WANG, and Jiao YIN collected the samples; Zongling WANG analyzed the data; and Yuantu YE reviewed the manuscript.
 Journal of Oceanology and Limnology2021年4期
Journal of Oceanology and Limnology2021年4期
- Journal of Oceanology and Limnology的其它文章
- Numerical study of the seasonal salinity budget of the upper ocean in the Bay of Bengal in 2014*
- Study on evaluation standard of uncertainty of design wave height calculation model*
- A fast, edge-preserving, distance-regularized model with bilateral filtering for oil spill segmentation of SAR images*
- A Gaussian process regression-based sea surface temperature interpolation algorithm*
- Climatology and seasonal variability of satellite-derived chlorophyll a around the Shandong Peninsula*
- Sources of sediment in tidal flats off Zhejiang coast, southeast China*
