Environmental influence on transparent exopolymer particles and the associated carbon distribution across northern South China Sea
M. Shahanul ISLAM , Jun SUN , , Haijiao LIU , Guicheng ZHANG
1 College of Food Engineering and Biotechnology, Tianjin University of Science and Technology University, Tianjin 300457,China
2 Research Centre for Indian Ocean Ecosystem, Tianjin University of Science and Technology, Tianjin 300457, China
3 Tianjin Key Laboratory of Marine Resources and Chemistry, Tianjin University of Science and Technology, Tianjin 300457,China
Abstract Microgels are plankton-derived transparent exopolymer particles (TEP) and have a significant impact on global marine carbon cycle. We investigated the influence of biogeochemical variables on the pattern of TEP abundance and its associated carbon (TEP C) distribution through a vertical transect of the northern South China Sea (nSCS) during summer, 2014. The average TEP concentration was 58.32±30.56 μg Xeq./L. Vertically, it was higher in the subsurface water column and lower at 200-m water depths. As chlorophyll a (chl a), TEP, and TEP C were highly concentrated at the bottom of the study transect,mainly on the continental shelf bottom and slope regions. Among biotic factors, cyanobacteria, especially Trichodesmium thiebautii showed significant positive correspondences with TEP through studied water columns in nSCS. In addition, TEP showed a positive correlation with chl- a distribution and clustered closely with diatom as well. It indicates a combined contribution of them on TEP sourcing accordingly.Nutrient concentrations were also high due to estuarine diluted water from Zhujiang (Pearl) River in the season that may intrigue those scenarios. Significant positive correlation ( P <0.05) among biotic and abiotic parameters also supported the statement. Furthermore, mentionable contribution of TEP-derived TEP C was found after comparing the particulate organic carbon data, which may signify the importance of TEP in local carbon cycle in the nSCS.
Keyword: South China Sea; transparent exopolymer particles (TEP); carbon with TEP; monsoon
1 INTRODUCTION
The South China Sea (SCS), especially its north(nSCS) is one of the productive zones among coastal seas of China (Liu et al., 2016). It is decorated with a number of dynamic features, which act as controlling factors on its ecosystem (Sun et al., 2012).Geodynamics of SCS showed the presence of rifted basin of continental shelf near these estuaries (Sibuet et al., 2016). Complex geophysical interactions driven by SCS summer monsoon (SCSSM) were observed in here which aff ect regional gyre dilution and temperature (Choi et al., 2016) near the Kuroshio(Tian and Wang, 2010). Additionally, ENSO wind driven SCS weather was observed during summer,controlled by western Pacific currents and wind circulations (Mao and Chan, 2005; Ding et al., 2018).It is also loaded by sediment and organic carbon (Jiao et al., 2018) through Modaomen, Lingdingyang, and Huangmaohai sub-estuaries. Among them,Lingdingyang or Zhujiang (Pearl) River estuary contribute most i.e. 0.1%-0.2% of total organic carbon carried by river in the world (Ni et al., 2008).Phytoplankton played an important role in this ecosystem i.e. carbon exchange, primary productivity,and so on (Sun et al., 2012). Its concentration,distribution, and associated particle transports were predicted to be highly influenced by these environmental factors and geophysical circulations as well (Xie et al., 2003; Yang et al., 2013; Huangfu et al., 2018).
Transparent exopolymer particles (TEP) are found as microgels through all aquatic systems in various sizes (Passow, 2002). They are formed by certain colloidal polysaccharide secretion from phytoplankton and bacteria (Alldredge et al., 1993). Theoretically,they are consumed and utilized by nekton (Miller et al., 2013), micronekton, zooplankton (Mari et al.,2017), and bacteria during its sinking process (Passow,2002). Their formations influence vertical mass fluxes, restrict optical properties, and drive the basic carbon cycling in ocean (Mari et al., 2017).Additionally, primary and secondary production may have an indirect eff ect on TEP concentration, which may regulate global climate changes eff ectively (Wurl et al., 2011). On this note, interactions of TEP with environmental controlling factors need to be addressed more to understand their biogenic importance deeply.
Present study is focused on TEP distribution at nSCS and its probable contribution in carbon concentration across this transect. Notably,concentration of TEP has closer relation with the abundance ofits primary source, phytoplankton(Passow et al., 1994) and showed positive correspondence with chlorophylla(chla) on a global scale (Passow, 2002). Alternatively, coagulation of dissolve organic matter (DOM) by abiotic process is also a source of the TEP (Chin et al., 1998). It generates colloidal organic matter, especially at surface of ocean (Passow and Alldredge, 1994;Passow, 2000). Besides, it was reported that TEP increased from the bottom of the mixed layer depth(MLD) to the top of the subsurface chlorophyll maximum (SCM) based on active photosynthesis(Kodama et al., 2014). Though nSCS is a eutrophic zone with all these factors (Huangfu et al., 2018), no studies were done on TEP distributions and its contributions. Therefore, it will be worthy to investigate the influence of environmental factors on TEP at nSCS. Furthermore, production of TEP also influenced by water turbulence, laminar shear and Brownian motion (Passow, 2000; Burd and Jackson,2009), which are notable features of nSCS (Choi et al., 2016).
Positive floating mechanism of TEP can drive it to accumulate at the sea surface microlayer (Wurl et al.,2009) and provide gelatinous composition to this interfacial film between atmosphere and ocean surface(Wurl and Holmes, 2008). Measuring vertical distribution of TEP has been done in diff erent seas(Bar-Zeev et al., 2009; Ortega-Retuerta et al., 2010;Bar-Zeev et al., 2011) and oceans (Wurl et al., 2011).In estuaries, TEP sedimentation measurement near East China Sea (Changjiang (Yangtze) River estuary)and TEP aggregation near SCS (Zhujiang River estuary) were examined previously (Sun et al., 2010;Guo and Sun, 2018). However, there is no suffi cient reports on deep sea profiling of TEP and its distribution, especially at the nSCS. Comparison of TEP between on-shelf and the open SCS areas were insuffi cient too. Therefore, the present study was conducted on the vertical TEP profiling and its associated carbon to determine correspondences among physical (temperature and salinity), chemical(NO3ˉ, NO2ˉ, P, and Si), and biological parameters accordingly (phytoplankton abundance and chla).Based on satellite data, this study will also try to understand the relation of TEP with particulate organic carbon (POC) for redirecting future research on TEP at nSCS.
2 MATERIAL AND METHOD
2.1 Sampling area
Stations were designed in straight-lined patterns for having a clear picture of TEP abundance through the sea-shelf and the open SCS area (Fig.1a). Parts of this area are occupied by continental shelf rifted basins (Sibuet et al., 2016). The sampling depths (0-200 m) were determined based on bottom depths and varied between 34-3 846 m. However, water sample was taken from diff erent layers of 11 stations during summer (August 20-September 12, 2014). The cruise was conducted from 22°N/114°E to 18°N/116°E.Station A9 was located near Zhujiang River estuary,Stns. J1-J5 were positioned on continental shelf and Stns. K3-SEATS were situated at open seas (Fig.1b).All stations were categorized further based on local geological positions and features accordingly.
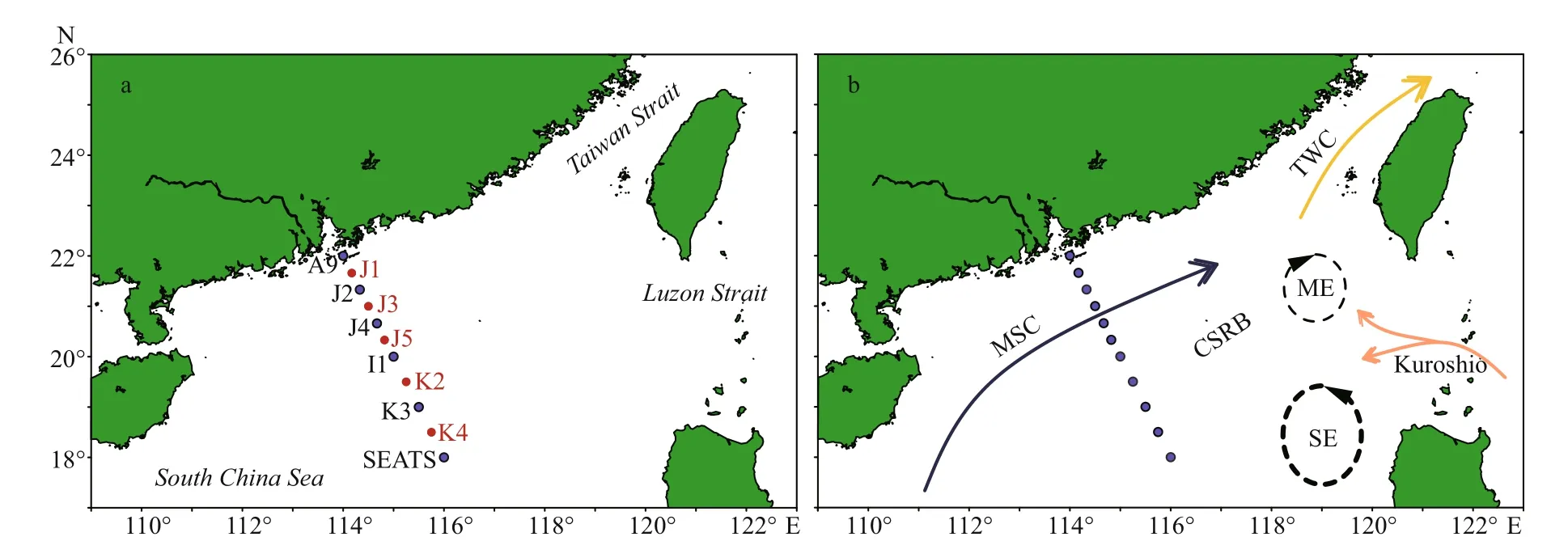
Fig.1 The study stations (a) and the currents during August (b) in the study area
2.2 Sample collection
Multiple rosette sampler (with a Seabird SBE 17 plus CTD sensors) were used to collect water sample from diff erent depths of each stations. Deeper bottom has multiple depth samples as required. Samples were collected separately to determine phytoplankton abundance, chla, TEP, and nutrients. Phytoplankton was collected in 1-L sampling bottle with 1%formaldehyde (final concentration) for further microscopic identification and analysis. Seawater samples were filtered through 25-mm GF/F membranes (Whatman Inc., Florham Park, NJ, USA)and stored at -20 °C (Less than 25 days) for chl-ameasurement. Seawater was collected in 100-mL bottles from all sampling depths of each station and stored at -20 °C for nutrients concentrations.
2.3 Measuring biotic and abiotic parameters
Following a colorimetric method (Passow and Alldredge, 1995), colloidal TEP measurements were done after making xanthan gum curve by absorption measurement (Eq.1). A mount of 50-mL (expressed asVfin equation 1) seawater was filtered (6 or 12 replicates) at low and constant vacuum (150 mm of Hg) onto polycarbonate filters (0.4-μm pore-size) and staining particles on the filter for ~2 s with 500 μL of a 0.02% aqueous solution of Alcian blue (8GX) in 0.06% acetic acid (pH 2.5). After being stained, filters were rinsed once with distilled water to remove excess dye. Rinsing will not wash off dye bound to substrates. Filters were then transferred into 25-mL beakers with 6 mL of 80% sulfuric acid and soaked for 2 h. The beakers were gently agitated 3-5 times over this period. Maximum absorption of the solution(termed asE787) lies at 787 nm using l-cm cuvette against distilled water (recorded absorption tabulated asB787) as the reference.
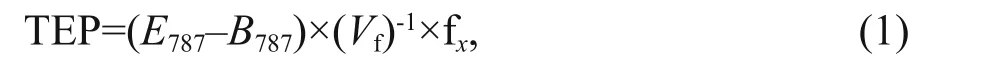
where, fxis a constant and fx=9.83; calculated as Passow and Alldredge (1995). Phytoplankton samples(1 L, preserved with 1% formaldehyde) were analyzed by using modified Utermohl methods followed by Sun et al. (2002). Samples (25 mL) were placed in Utermohl chamber (settled 24 h) under inverted microscope for identification and counting. According to Welschmeyer (1994), chlawas measured using fluorescence method in laboratory after soaking in 90% acetone. With standard calibration, a Turner-Designs TrilogyTMfluorometer was used for chl-adetermination. CTD sensors provided necessary data of temperature and salinity while sampling from diff erent depths in the study area. Nutrients (NO3ˉ,NO2ˉ, P, and Si) were examined using an Auto Analyzer 3 (Bran+Luebbe) (SEAL, Germany) based on continuous flow injection analysis (Liu et al., 2011, 2016).
2.4 Statistical analysis
Data were categorized into two horizontal segments i.e. on-shelf (Stns. A9-J4) and the open SCS area (Stns.J5-SEATS). Vertical divisions of transect were also done in MLD (0-50 m), SCM (50-100 m), and BSCM(100-200 m) zones accordingly (Fig.2a). Satellite data for POC was produced by SatCO2(Data package name:SIO_Merged_Merged_20140801TO20140831_L3B_CMS_2KM_POC_HE2018_2020_05_17_08_01_48_High) for understanding probable carbon contribution of TEP. To investigate the detail correspondence among biotic and abiotic parameters along studied stations,multivariate analysis was performed using Multi Biplots software (Vicente Villardón, 2015). Neutral(yellow hue), positive (red hue), and negative (green hue) correlation was showed in Bicluster analysis.Associated carbon content or TEPμg CC(unit, μg C/L)was measured (Eq.2) by following equation (Engel and Passow, 2001):
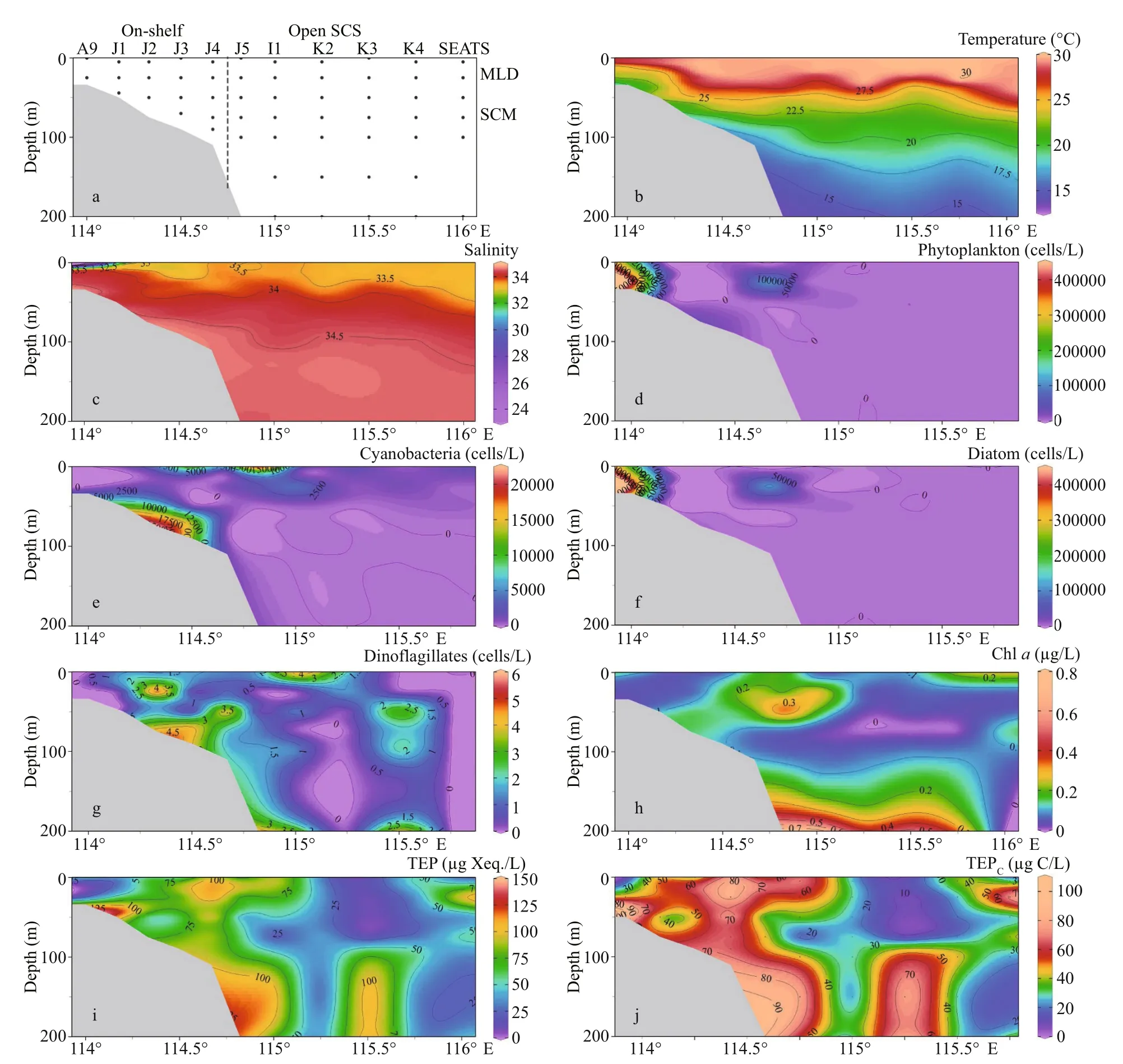
Fig.2 Transect patterns (a) and its environmental parameters, i.e. temperature (b) and salinity (c), phytoplankton (d),cyanobacteria (e), diatom (f), dinoflagellates (g), chlorophyll a (h), concentration of TEP (i), and TEP C (j)
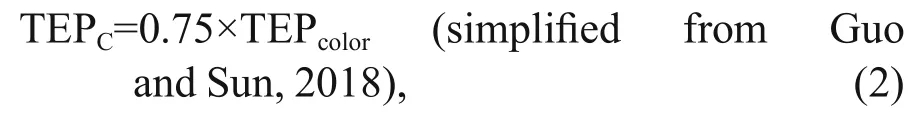
where TEPcoloris the concentration of TEP with the unit of μg Xeq./L.
Linear regression, Pearson correlation, and covariance were performed by Microsoft Excel 2016 software. Dominance index (Eq.3) was used to describe phytoplankton dominant species under this equation:

where,Nis the total cell abundance of all species,niis total cell of speciesiand ƒis the count of occurrence of speciesiin all sample (Guo et al., 2014). Carbon biomass was calculated by following Eqs.4 & 5 (Yang et al., 2016). They were found suitable with current datasets and relatable relationships. They were as follows:
whereCrepresents the amount of carbon (μg). Carbon biomass was computed using 0.011 μg C/trichome as the conversion factor for genusTrichodesmium(Carpenter, 1983).
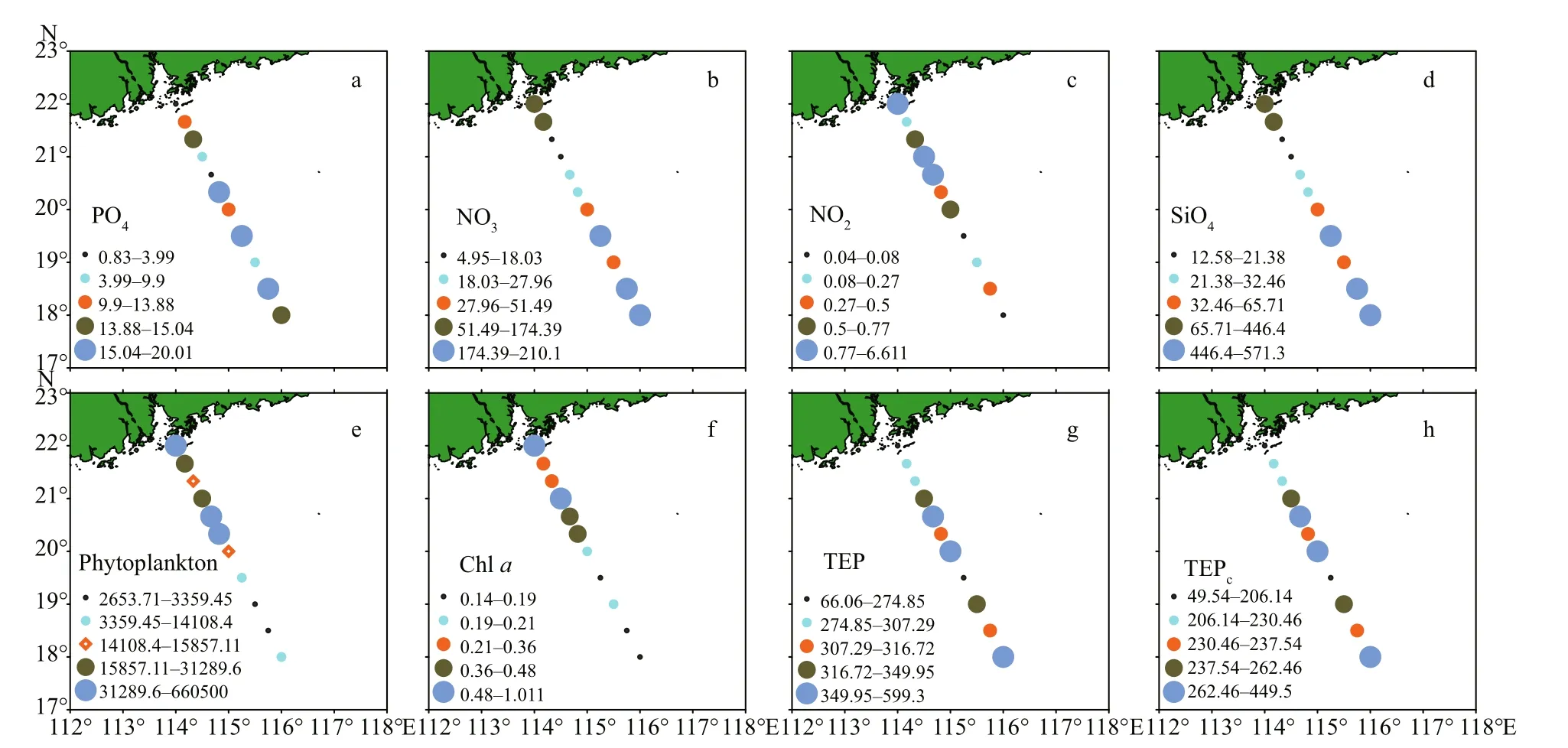
Fig.3 Average concentrations of various parameters at all stations in the study area (surface view by software Surfer 12)
Canonical correspondence analysis (CCA) were done by Canoco software (version 4.14) among biotic and abiotic parameters of nSCS after performing Detrended Correspondence Analysis (DCA) with respected eigen values. (Ter-Braak and Šmilauer,2002). Golden Software’s Surfer program (https://support.goldensoftware.com/hc/en-us/categories/ 115000653807-Surfer) was used for integration and visualization of all recorded and examined parameters. Vertical concentrations of diff erent parameters were shown by Ocean Data View (ODV) 4.7.6 software (https://odv.awi.de/en/).T-Sdiagrams were mentioned to understand the correlations of biotic parameters and TEP with associated water masses.
3 RESULT
3.1 Hydrobiology of SCS transect
Seasonal characteristics of nSCS summer was observed during present investigation. Applying horizontal segmentations, high temperature (Fig.2b)and high salinity (Fig.2c) were found in the open SCS than on-shelf stations during summer. Vertically,temperature (ranged 14.25-29.95 °C) was high in surface area (29.34±0.89 °C). Low salinity was found at the surface of on-shelf stations (Fig.2a), especially near the estuarine stn. A9 (23.62). High concentration of PO4were found at the open SCS near the mid transect (Fig.3a). Average PO4and SiO4concentration were 1.3 and 7.02 μmol/L, respectively. Furthermore,average nitrite (Fig.3b) and SiO4(Fig.3d) were comparatively low at Stns. J3 and J4 near MSC(Fig.1b). Average NO2and NO3were 0.21 and 5.33 μmol/L, accordingly (Fig.3b & c). Vertically,surface of estuarine stations were highly concentrated with nutrients except PO4(Fig.4). It was found high at the bottom of the open SCS (Fig.4d) followed by nitrite (Fig.4a) and SiO4(Fig.4c), especially at Stns. K2 and K4. For better understanding, POC data was extracted by SatCO2 software from satellite data collections. According to the satellite data, POC concentration was found high (100 mg/m3) near estuarine station and low (50 mg/m3) in open nSCS(Supplementary Fig.S1).
3.2 Phytoplankton composition and biomass
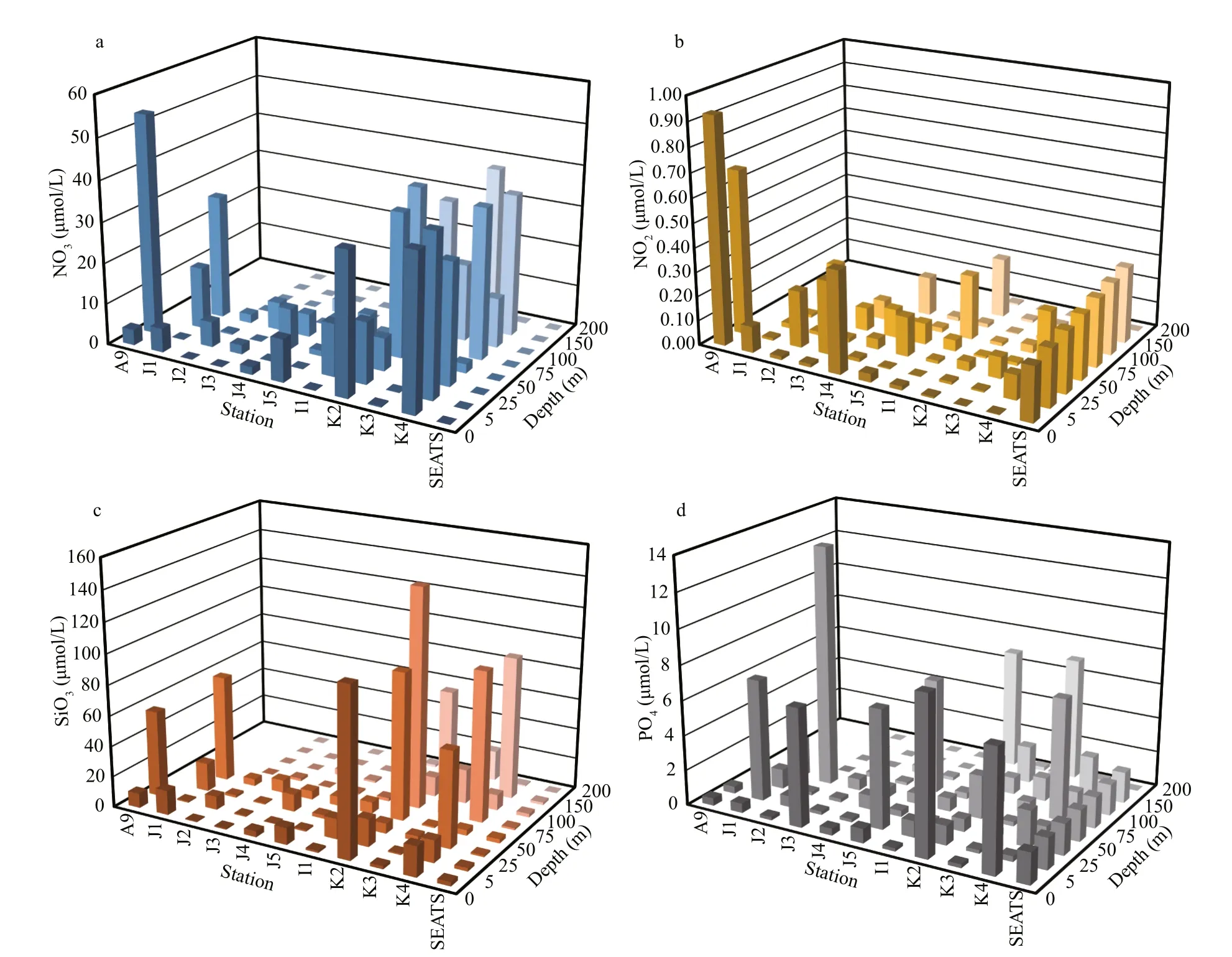
Fig.4 Average nutrients concentrations at diff erent depths across SCS
Phytoplankton assemblages (Fig.2d) were mainly composed of 3 groups i.e. cyanobacteria (Fig.2e),diatoms (Fig.2f), and dinoflagellates (Fig.2g).Average abundances of phytoplankton were 16 318 cells/L of which approximately 90% was contributed from diatoms (average 14 652 cells/L)and about 10% were cyanobacteria. Average concentration of dinoflagellates was very low at the study area (1.08 cells/L). Dominant phytoplankton species wereTrichodesmiumthiebautii,Skeletonemacostatum(Greville) Cleve,Chaetocerosbrevis,Thalassionemanitzschioides(Grunow)Mereschkowsky,Pseudo-nitzschiadelicatissima(Cleve) Heiden,Pseudo-nitzschiapungens(Grunow et Cleve) Hasle,Chaetoceroscompressus,C.lorenzianusGrunow, andC.pelagicusCleve. It was observed that average concentration of phytoplankton (Fig.3e), especially cyanobacteria(average 1 659 cells/L) was high at on-shelf stations(Fig.2e). Chlorophyllawas also followed similar horizontal patterns of phytoplankton as well.Vertically, it was high at the SCM of Stns. J4 and J5(average 0.13 μg/L) and in deep water layer of Stns. I1-K4 (Fig.2h). Horizontally, it was found higher at stations of the shelf area (Fig.3f).
Through the on-shelf transect, dominant phytoplankton species wereNitzschiasp.,S.costatum(Greville) Cleve,T.nitzschioides(Grunow)Mereschkowsky,P.delicatissima(Cleve) Heiden,T.thiebautii, andT.erythraeumEhrenberg ex Gomont. The open SCS stations possessedT.nitzschioides,BacteriastrumcomosumJ. Pavillard,C.messanenseCastracane,C.atlanticusvar.skeletonHustedt,C.psedodichaetaIkari,T.thiebautii, andT.erythraeumas dominant species. Estimations of phytoplankton carbon biomass (Phyto-C)demonstrated higher cyanobacteria (Fig.5b),especiallyT.thiebautiithrough on-shelf segment(Fig.5d). Phytoplankton, mostly all dominant diatoms were higher at Stn. A9 (Fig.5a), especiallyS.costatum(Fig.5e) andC.brevis(Fig.5f). Dinoflagellates were less abundant through all stations generally (Fig.5c).T.nitzschioides(Fig.5g) andP.pungens(Fig.5i) were less diverse with high biomass at on-shelf stations.High abundances with low biomass ofC.compressus(Fig.5j),C.lorenzianus(Fig.5k), andC.pelagicus(Fig.5l) were found through whole transect.
T-Sdiagram segmented the on-shelf data into two water masses (Fig.6a), i.e., high temperature high salinity (HTHS) and low temperature high salinity(LTHS). The open SCS demonstrated two water masses (Fig.6g), i.e., high temperature low salinity(HTLS) and low temperature high salinity (LTHS).Phytoplankton concentration was higher at LTHS(Fig.6b) of on-shelf area and at HTLS of the open SCS (Fig.6h), followed by diatom (Fig.6c & i) and cyanobacteria as well (Fig.6e & k). On the other hand,dinoflagellates were distributed less but pretty evenly(Fig.6d & j) through both zones (on shelf and the open SCS).
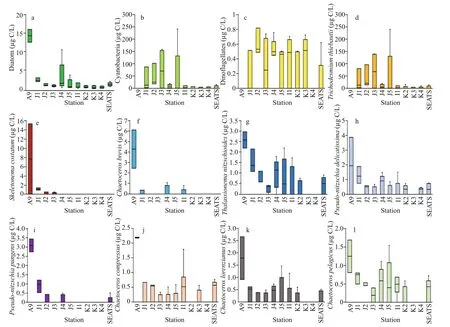
Fig.5 Carbon biomass of average diatom (a), cyanobacteria (b) and dinoflagellates (c) with average biomass of dominant phytoplankton species, i.e., T richodesmium thiebautii (d), Skeletonema costatum (e), Chaetoceros brevis(f), Thalassionema nitzschioides (g), Pseudo- nitzschia delicatissima (h), Pseudo- nitzschia pungens (i), Chaetoceros compressus (j), Chaetoceros lorenzianus (k), and Chaetoceros pelagicus (l)
3.3 Concentration of TEP across study transect
Average TEP concentration (Fig.2i) was not constant after applying layered segmentation on study transect (58.32±30.56 μg Xeq./L). Horizontally, high TEP was found at the mid transect (Stns. J4 & I1) and at the end of open SCS in average (Fig.3g). The highest and the lowest recorded TEP was 140.77 and 9.44 μg Xeq./L, respectively. Average vertical concentration of TEP was higher in BSCM (64.99±36.69 μg Xeq./L) than MLD (62.43±32.15 μg Xeq./L)and SCM (50.67±25.40 μg Xeq./L). Additionally,TEPCshowed similar gradients as TEP accordingly(Figs.2j & 3h). The highest TEPCwas 105.57 μg C/L and the lowest was recorded 7.07 μg C/L in SCM(50-100 m) near open SCS (Fig.1b).T-Sdistribution of TEP (Fig.6f & l) followed the distribution patterns of dinoflagellates (Fig.6d & j) across both zones (on shelf and the open SCS).
3.4 Correlation of TEP with environmental parameters
TEP showed significant positive correlation with cyanobacteria and chla(P<0.05) at both horizontal segments through Pearson analysis (Table 1).Positively high coeffi cients of PO4with cyanobacteria and dinoflagellates were found at the open SCS as well (Table 1). Following these, CCA also demonstrated close correspondences of TEP with dinoflagellates (Fig.7a) and cyanobacteria (Fig.7d),especially withT.thiebautii(Fig.7b & c). It was also positively correlated with TEP through all water layers. Additionally, CCA also revealed close correspondences ofC.pelagicuswith concentration of TEP at on-shelf stations (Fig.7b) andC.compressusat the open SCS (Fig.7c). CCA showed close relation of depths with chlathrough all vertical water layers(Fig.7d-f). Furthermore, close clustering in group 6 of heatmap (Fig.8) and linear regression demonstrated significant (R2=0.4) correlations of average TEP with the carbon biomass of cyanobacteria positively(Fig.9a) and with selected nutrients (SiO4, NO3)negatively (Fig.9b & c). Supporting these, TEP clustered closely with diatom (Group 1) and cyanobacteria, especially withT.thiebautii(Group 2)after considering their carbon biomass reading as variables during cluster analysis (Fig.10).
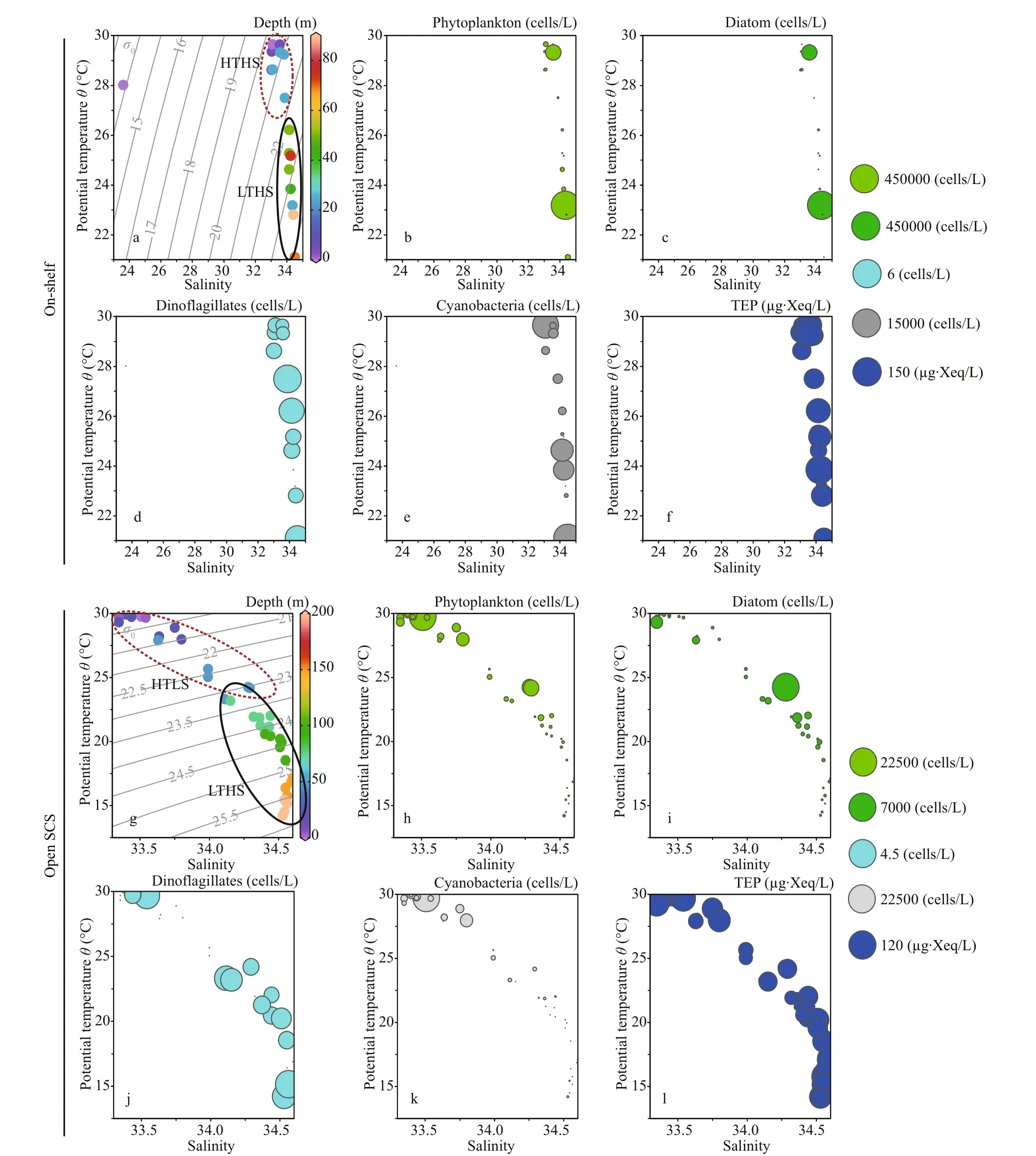
Fig.6 T-S plots with depths (a, g) and assemblages of phytoplankton (b, h), diatom (c, i), dinoflagellates (d, j), cyanobacteria(e, k), and TEP concentration (f, l) of on-shelf (a, b, c, d, e, and f) and the open SCS segments (g, h, i, j, k, and l)
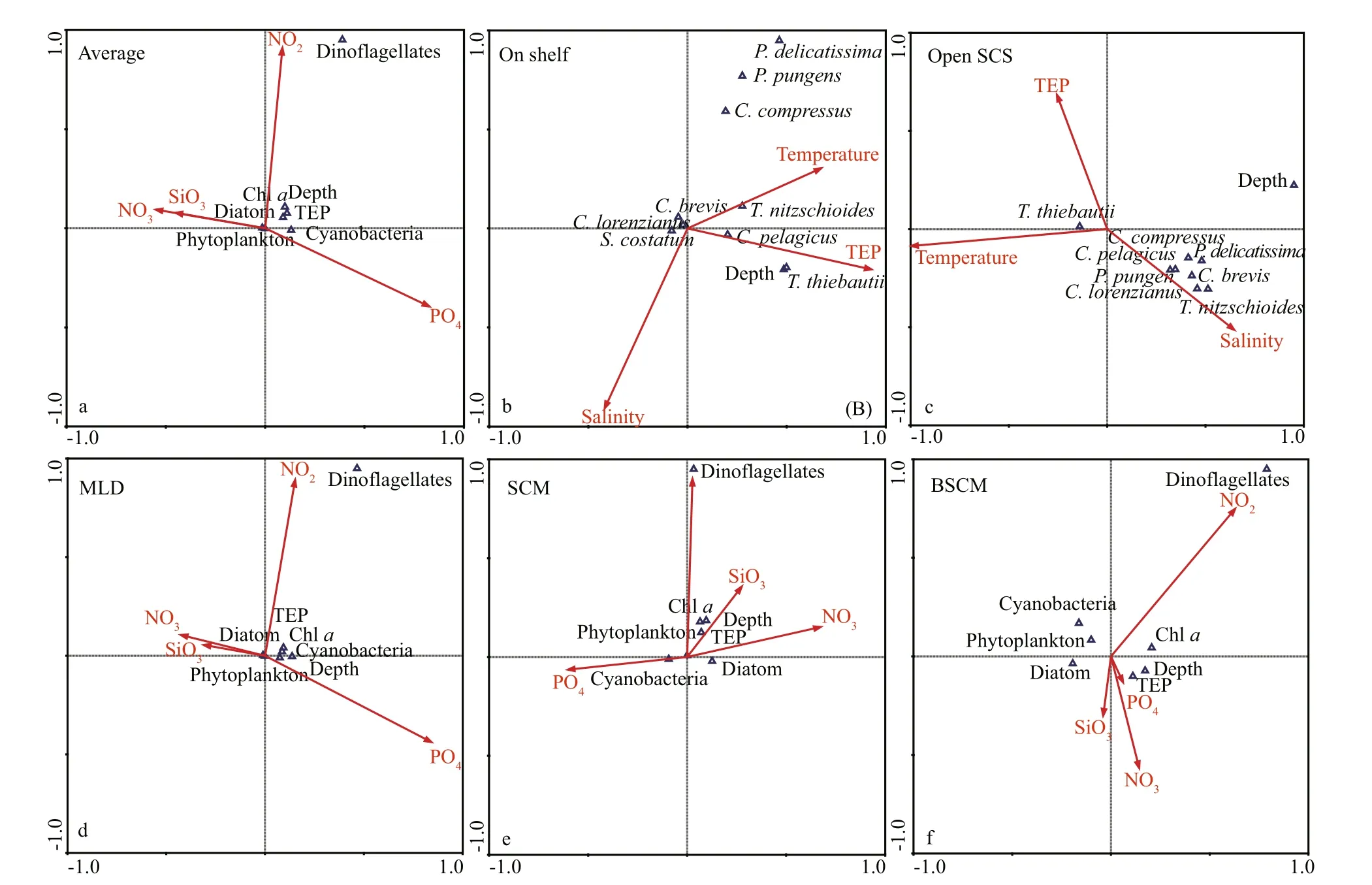
Fig.7 Canonical correspondence analysis (CCA) among biotic and abiotic variables from sampling stations
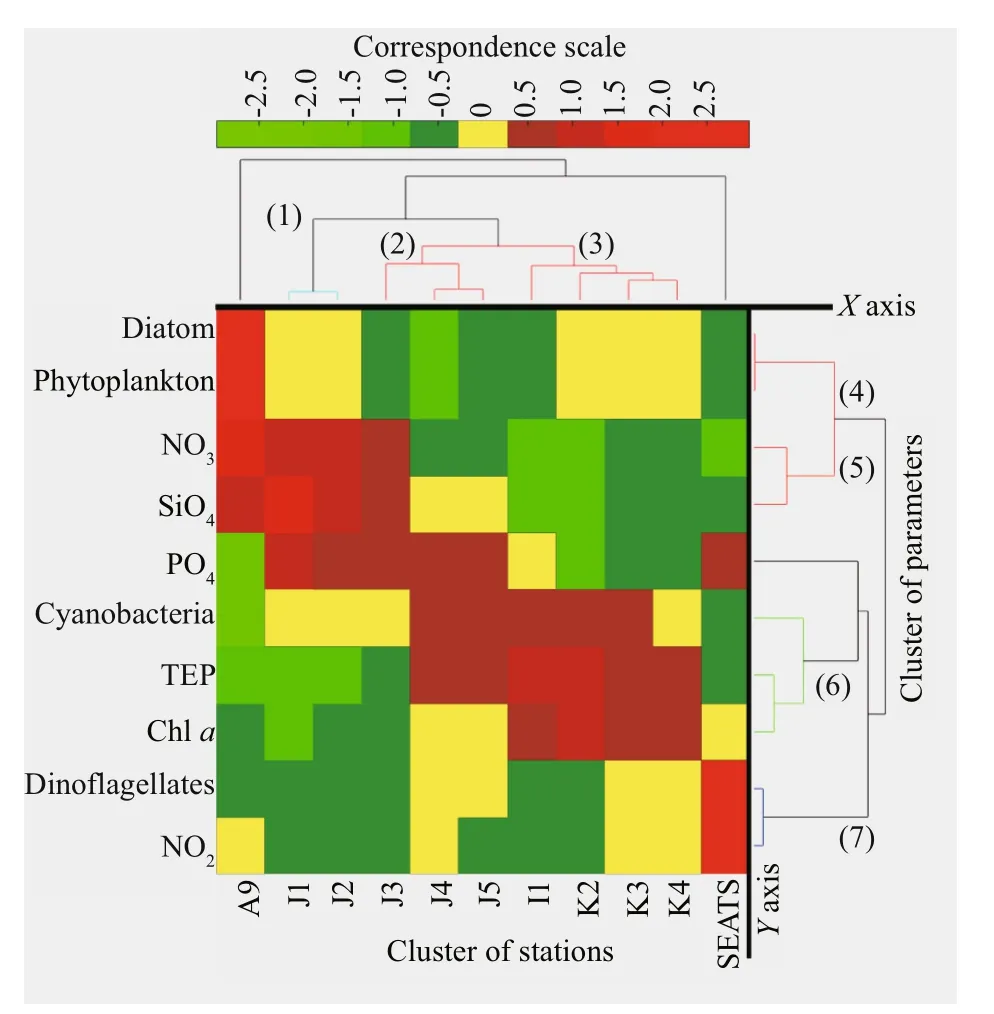
Fig.8 Bi-Cluster of sampling stations ( Y axis) and various parameters ( X axis) of study area with 7 cluster groups
Bi-Cluster (heat map) analysis expressed close positive (Red hue) and negative (Green hue) relations by considering most of the variables under a correspondence scale from 2.5 to -2.5 (Fig.8). Diatom dominated phytoplankton was revealed at estuarine station (A9) by close clustering (Group 4). Close cluster of cyanobacteria and TEP were mostly seen at on-shelf stations i.e. J2, J3, and J4 (Group 6). On the other hand, TEP showed average positive correlation with chla(YAxis), especially at the open SCS stations i.e. I1 and K3 (Fig.8; red hue). Additionally, most of positive nutrient clusters (NO3, SiO2, and PO4) were also found here in open SCS (Stns. K2, K4). In summary, bi-cluster analysis indicated that cyanobacteria (in on-shelf stations) and nutrients influenced chla(at open SCS) were liable of TEP distributions in those horizontal sections accordingly.
A conceptual model was drawn based on results that will be illustrated with references in discussion section (Fig.11). The text position of each parametersshows highest density ofits abundances through the transect. Arrows show uptake of nutrients (green) and influences on TEP (yellow) accordingly. All these relations were built up based on previously described statistical correspondences. Blue dotted circle around monsoon summer currents, indicating a probable mixing impact zone of this throughflow. Slope runoffwith nutrients and freshwater (brackish water) from Zhujiang River is indicated by brown dotted arrow.Blue stain in background of Fig.11 indicates TEP concentrations according to Fig.2i for better understanding about sources of TEP with high aggregations though the study transects of SCS.
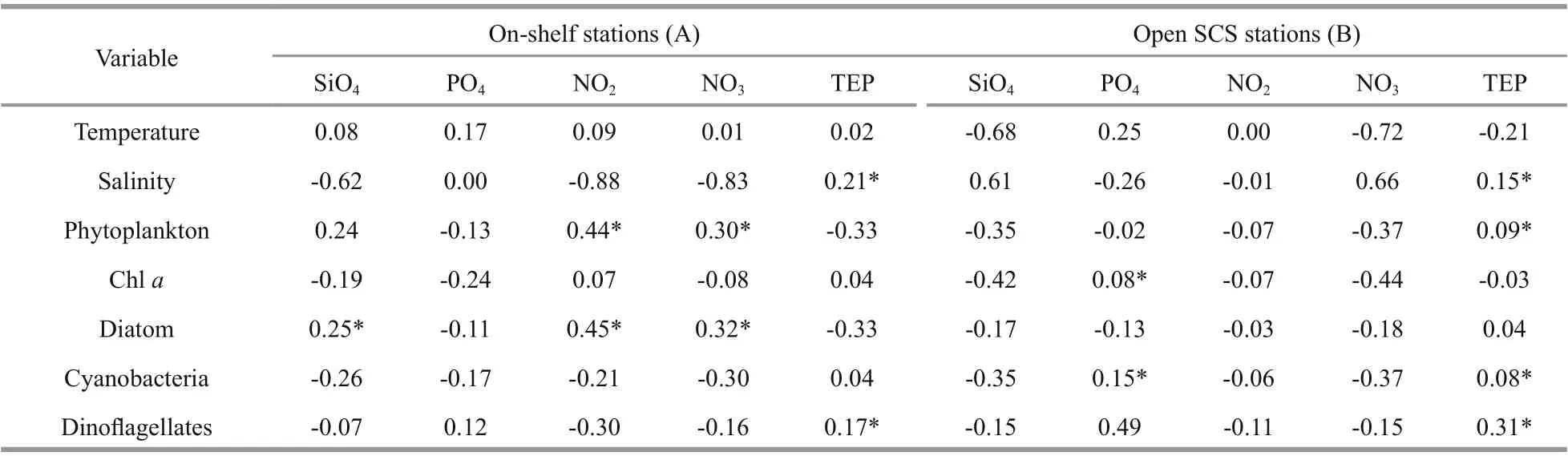
Table 1 Pearson correlation among biotic and abiotic parameters
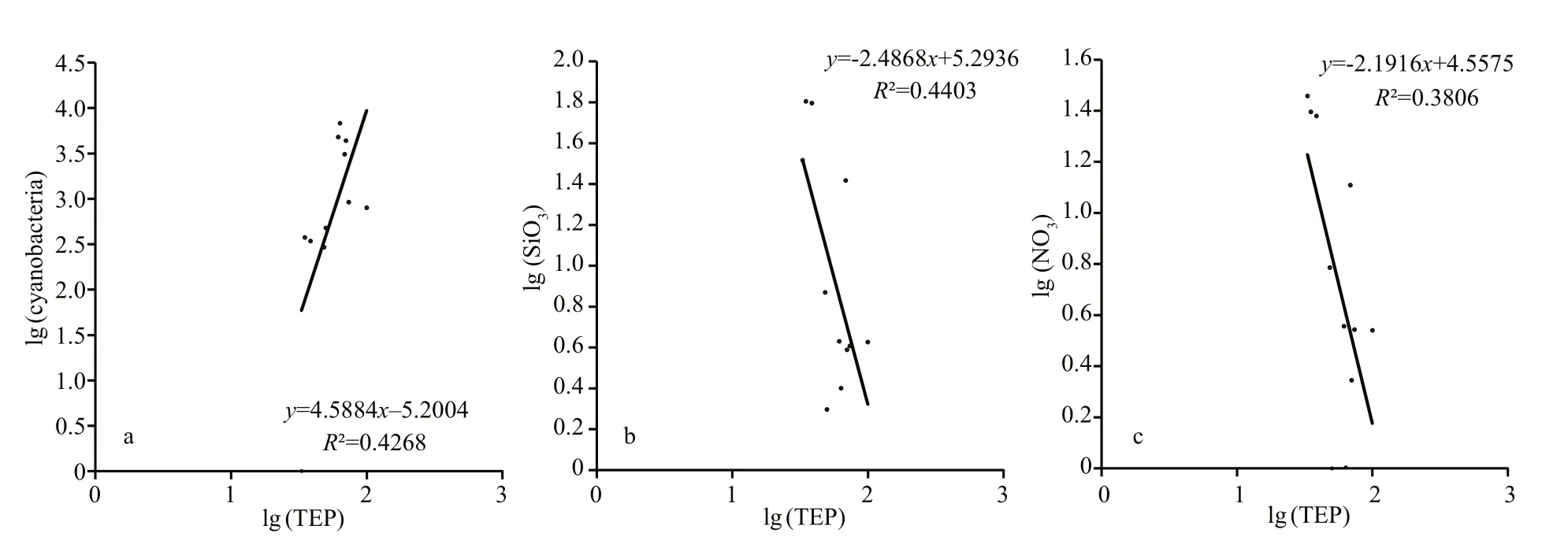
Fig.9 Linear regression of TEP concentration with cyanobacteria ( P=0.03) (a), SiO 4 ( P=0.03) (b), and nitrite ( P=0.03) (c)after 10-based log transformation
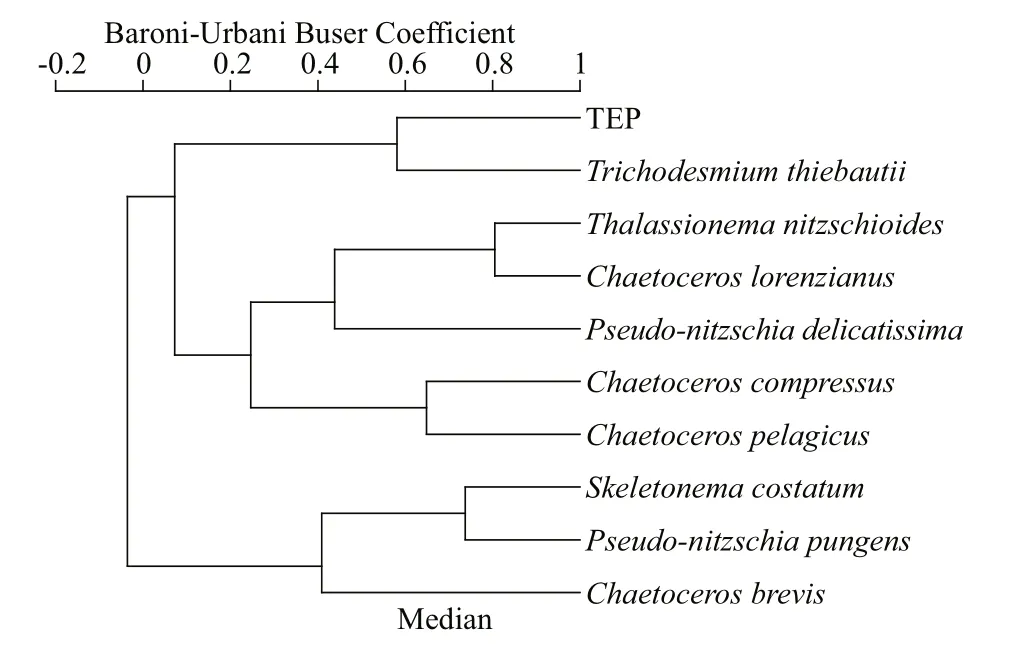
Fig.10 Cluster analysis of TEP with carbon biomass of dominant phytoplankton species
4 DISCUSSION
4.1 Hydrological influences at SCS
The largest marginal SCS possessed extreme seasonal variations round the year (Su, 2005; Hu et al., 2014). In summer, weather variations were derived by regional geophysical activities, i.e. flow of MSC and mixing of cyclonic SE through the study area (Hu et al., 2000; Liu et al., 2010; He et al.,2015). The past 32 years’ research showed that the intensity of the SCS summer-monsoon (SCSSM)was low while sea surface study temperature was high (Choi et al., 2016). On the other hand, its intensity is higher at the south part of SCS (Tian and Wang, 2010) with the influence of western Pacific weather and ENSO (Mao and Chan, 2005; Ding et al., 2018) followed by Kuroshio (Fig.1b). All these local phenomena may influence the distributions of TEP and other environmental parameters accordingly. Therefore, understanding local circulations at this transect is important.
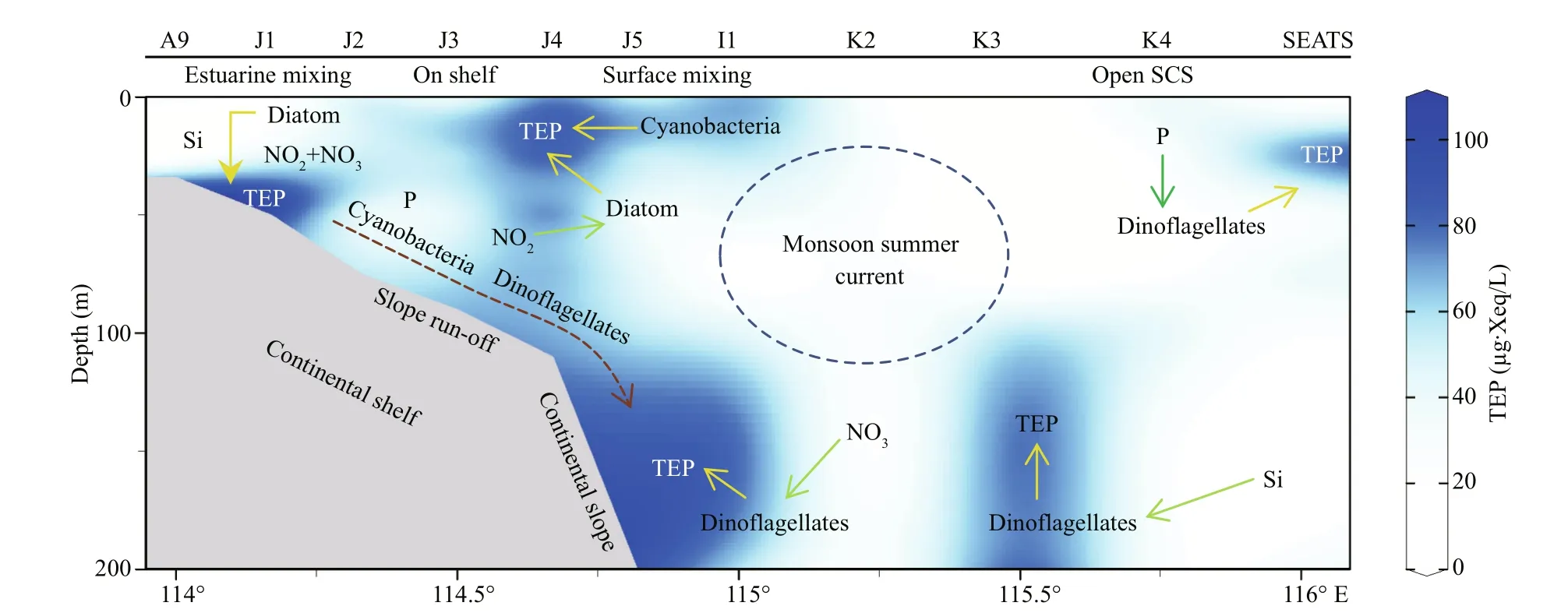
Fig.11 Conceptual model of TEP sourcing at SCS transect
During summer, formation of double gyre was seen(Xu et al., 2008) with an eastward MSC, minor eddy(ME) and summer eddy (SE) (Hu et al., 2000; Liu et al., 2010; He et al., 2015) through the study transect(Fig.1). These geophysical circulations actively played a role in particle distributions (i.e. TEP) and pumping the biological carbon export (Wurl et al.,2009, 2011). All these circulations can act as potential nutrients sources to local biodiversity (Wong et al.,2007). Current study observed low vertical concentration of biotic parameters near the zone (Stns.K4 and SEATS) of cyclonic SE (Fig.3). It may cause due to the eff ect of upwelling edge on phytoplankton(Xie, 2003; Yang et al., 2013) and wave mixing in MLD (Huangfu et al., 2018). On the other hand,vertical concentration of TEP, PO4, and chlawere found higher near MSC zone (Stns. J2-J5). Present study predicted that shelf upwelling of nutrient (PO4)may influence chla(phytoplankton) to produce TEP in this transect (Wong et al., 2007; Gan et al., 2009).Additionally, the concentration of all nutrients (Fig.3b-d) except PO4(P; Fig.3a) and phytoplankton groups(Fig.2) were higher at the MLD of continental shelf(Fig.1) near shore (Fig.2a). It was reported that zonal nutrient enrichments were liable behind these scenario(Xue et al., 2016). In short, nutrients circulations are controlled by geological phenomenon at SCS (Gan et al., 2009) which acted as controlling factors for biotic productions of SCS (Peng et al., 2006).
4.2 TEP correspondence in various environments
Diatom blooms (Mari, 1999) as well as Cyanobacteria i.e.Anabaenaflos-aquae(Surosz et al., 2006),Prochlorococcusmarinus(Iuculano et al.,2017), andSynechococcuselongatus(Thornton and Chen, 2017) are potential TEP source (Berman-Frank et al., 2007). Similar to the previous report (Xue et al.,2016), present study foundT.thiebautiias dominant cyanobacteria in both sections (on-shelf and the open SCS). This species also showed close relation with concentration of TEP in CCA (Fig.7b & c). Besides,TEP clustered with cyanobacteria (Fig.8, Group 6),especially withT.thiebautiiaccordingly (Fig.10).These may conclude thatT.thiebautiiinfluenced TEP concentration beside diatoms across nSCS (Passow,2002; Wurl et al., 2011). Significant linear regression of cyanobacteria with TEP (R2=0.42,F=2.86) also support these statements accordingly (Fig.9a).
Phytoplankton communities were aff ected by various zonal environmental parameters, especially nutrients during summer (Peng et al., 2006; Ma and Sun, 2014). Upwelling and eddies also had impact on phytoplankton growth (Chen et al., 2006) which may influence TEP production as well (Passow, 2002).Supporting this, bi-cluster analysis (heat map) showed that TEP made a close cluster with phytoplankton(Fig.8) rather than with nutrients. Additionally, both CCA and Pearson correlation showed significant correspondent between chlaand TEP as well(Table 1). However, it was an opposite statement of some open ocean reports (Wurl et al., 2011; Kodama et al., 2014).
Similarly, Hong et al. (1997) reported the highest range of concentration of TEP in Ross Sea due to high nutrient low productivity (HNLP) under icecaps(Table 2). In BSCM of nSCS (100-200 m), TEP clustered closely with dissolved nutrients (NO3, P,and Si) in CCA (Fig.7f) which was also consented by Wurl et al. (2011). High sinking rate to TEP may liable behind this scenario after coagulation from extracellular release (Mari et al., 2017). This marked the limitation of current study, which should be addressed in future research.
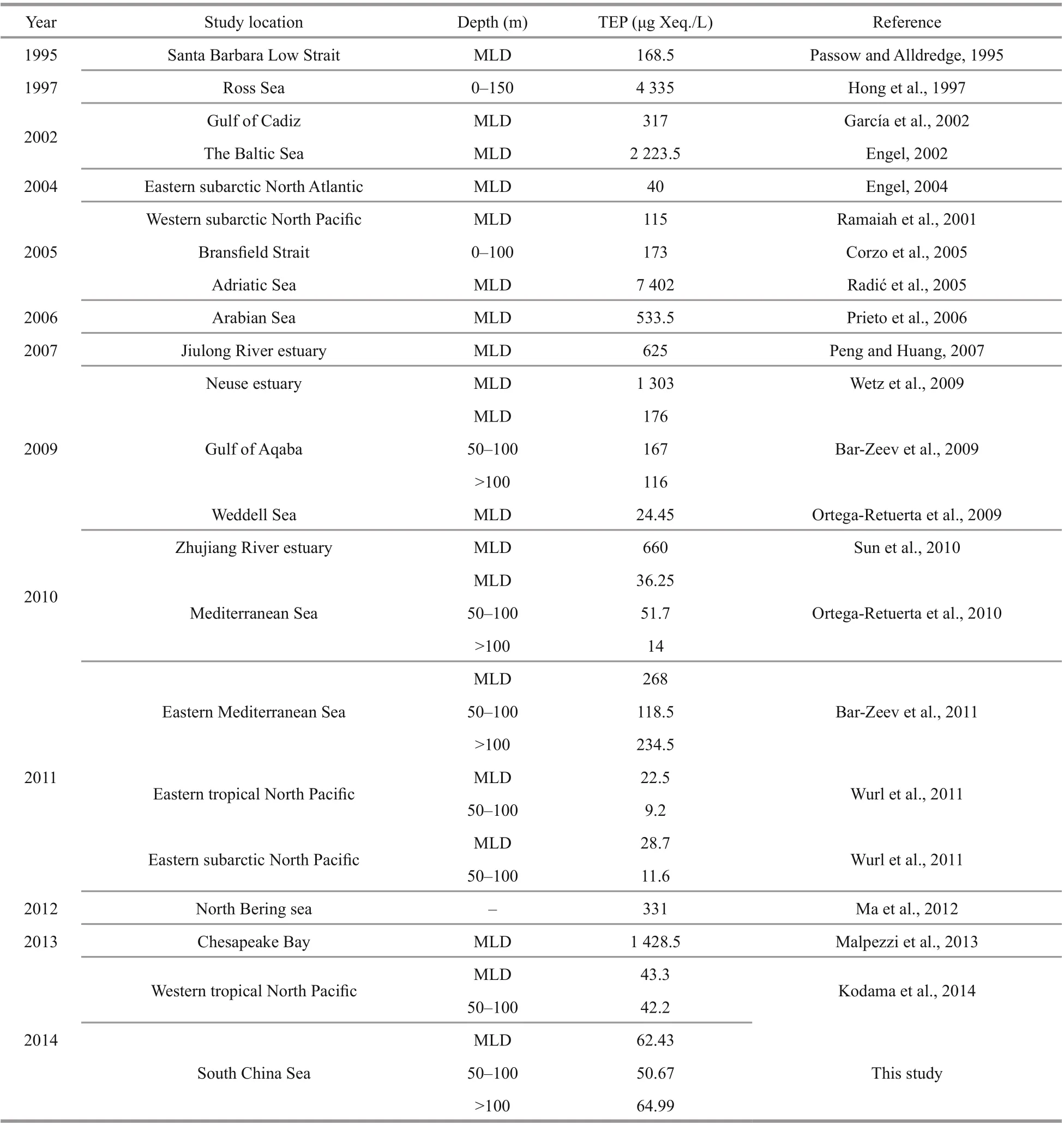
Table 2 Concentration of TEP ranges of various waters from diff erent studies
4.3 Comparative TEP distributions (Conceptual Model)
Geographically, the SCS is influenced by western Pacific currents (Tseng et al., 2016). Reports said that geophysical circulation had potential roles in abundance and variations of phytoplankton as well as particle distributions (Ortega-Retuerta et al., 2009;Liu et al., 2016). Present study found that MLD possessed high TEP (Fig.2i) and nitrates (Fig.3c) at on-shelf stations (Fig.11). Similarly, MLD of Neuse,Jiulong, and Zhujiang River estuaries as well as semiclosed Arabian Sea were also demonstrated moderately high TEP under coastal influences (Prieto et al., 2006; Peng and Huang, 2007; Wetz et al., 2009;Sun et al., 2010). MLD of close seas, i.e.Mediterranean, were also showed high TEP (Ortega-Retuerta et al., 2010; Bar-Zeev et al., 2011) than open ocean (Pacific) due to nutrients limitation on phytoplankton abundances by oligotrophic circulations (Wurl et al., 2011; Kodama et al., 2014).Reports (Table 2) said that the highest TEP concentration was found in Adriatic Sea (Radić et al.,2005). Terrestrial inputs were liable behind this scenario (Kodama et al., 2014; Shu et al., 2018).Similar influential factor from Zhujiang River (Ni et al., 2008; Sun et al., 2010) may cause high concentration of TEP (Fig.2i) at MLD of on-shelf stations accordingly (Fig.11).
Furthermore, high cyanobacteria were found in SCM of on-shelf stations (Fig.2e). It showed close correspondence with TEP in CCA (Fig.7e). Therefore,cyanobacteria may liable for the high concentration of TEP through this layer (Fig.11) which was supported by previous report as well (Surosz et al.,2006). High TEP was also found at I1 (Fig.3g),especially at 200 m (Fig.2i). This zone is decorated with slope runoff from Zhujiang River (Sun et al.,2010) and shelf upwelling as well (Xie et al., 2003;Yang et al., 2013). Present study also found high NOx(NO2+NO3) concentration at the bottom of on-shelf stations (Fig.4a & b), which may cause high chl-aconcentration in BSCM (Xue et al., 2016). Eventually,this scenario leads to high TEP aggregation across this zone (Passow, 2002; Wurl et al., 2011) in BSCM.
Beside those, current study found high phytoplankton abundances in HTHS and HTLS zone of whole transect (Fig.6). Additionally, high TEP was found in high diatom (Fig.6c & i) and high cyanobacteria (Fig.6e & k) dominated areas as well.They have potential influences on TEP formations in waterbody (Surosz et al., 2006; Thornton et al., 2016).It was reported that temperature showed significant positive correlations with phytoplankton groups during summer in nSCS (Xue et al., 2016). Therefore,temperature may also intrigue TEP sourcing indirectly.
4.4 Assumptions on carbon contribution
SCS is acting as CO2source with carbon exhaustion about 13.86-33.60 Tg C/a (Jiao et al., 2018). POC is a part of that cycle. Studies of POC was mostly focused on phytoplankton cell (Turner, 2002) and zooplankton fecal pellets rather than on TEPC(Turner,2015). Recent study found that TEPCis a dominant contributor in POC pool (Yamada et al., 2015). It was also suggested that results of TEP should consider in intensive POC study (Guo and Sun, 2018). This study calculated an average 58% contribution of TEPCin POC at the study transect and more than 70% at the open SCS area. It was closely ranged to open Atlantic Ocean (66%) in recent study (Zamanillo et al., 2019).However, it was higher than previous studies (Passow et al., 2001; Mari et al., 2017). High concentrations of phytoplankton and TEP were liable behind this scenario (Passow, 2002). Additionally, high Phyto-C,especially cyanobacteria (Fig.5b) can also act as a correspondent to POC pool (Bhaskar and Bhosle,2006; Ortega-Retuerta et al., 2009; de Vicente et al.,2010). Significant positive linearity of TEP with cyanobacteria supported these phenomena (Fig.9a).Moreover, high POC/TEPCratio was found in gulf stream (1.5) and slope water (1.4) of western north Atlantic (Jennings et al., 2017), which was similar to the ration at open SCS (1.4) in this study. It signifies intensive influences of TEPCon POC in term of open seawater.
Near nSCS coast, we observed 24.77% TEPCof POC after analyzing satellite data. It was much lower than previous investigation (30%) in the Santa Barbara Channel (Passow et al., 2001) and southern Spain reservoir (Mari et al., 2017). Various particle exporting coastal processes (sediment input, turbidity,mineral flow etc.) can influences these phenomena,which may reduce contribution of TEPCon POC across SCS shore (He et al., 2016). Besides, export of TEP had compatibility with phytoplankton cell sedimentation, which indicates the relationship of them and its importance in POC studies (Guo and Sun, 2018). Therefore, further research should be done on the sinking rate of TEP in SCS and its adjacent sea area after considering regional geophysical activities as well.
5 CONCLUSION
Various geophysical factors, i.e. SCSSM intensity,local current circulations and adjacent eddies’pumping and regional nutrient upwelling may influence particle distribution pattern (i.e., TEP) and partial carbon abundance (i.e., TEPC) at nSCS. A complex relation of TEP with biological factors and nutrients were also demonstrated by correlation analysis. A number of controlling factors acted separately in diff erent sections of nSCS for TEP concentration. In average, present study found significant positive correlation of TEP with diatom and cyanobacteria i.e.T.thiebautiithrough various multivariate analysis. It indicates potential influences of them on TEP sourcing. Besides, average TEPCwas higher at the deep in the open SCS than on-shelf area.It increases mentionable contribution of TEPCon POC at open water. Further TEP export studies can amplify more about these relations at nSCS.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
Authors express the gratitude to the captain and crews of the R/VShiYan1for their helps on sampling and on-deck operations, also to Haijiao LIU, Xue BING, and Yi SHU.
 Journal of Oceanology and Limnology2021年4期
Journal of Oceanology and Limnology2021年4期
- Journal of Oceanology and Limnology的其它文章
- Numerical study of the seasonal salinity budget of the upper ocean in the Bay of Bengal in 2014*
- Study on evaluation standard of uncertainty of design wave height calculation model*
- A fast, edge-preserving, distance-regularized model with bilateral filtering for oil spill segmentation of SAR images*
- A Gaussian process regression-based sea surface temperature interpolation algorithm*
- Climatology and seasonal variability of satellite-derived chlorophyll a around the Shandong Peninsula*
- Sources of sediment in tidal flats off Zhejiang coast, southeast China*
