Cryptotanshinone inhibits cytotoxin-associated gene A-associated development of gastric cancer and mucosal erosions
Zhang-Ming Chen,Jie Hu,Yuan-Min Xu,Wei He,Lei Meng,Ting Huang,Song-Cheng Ying,Zhe Jiang,A-Man Xu
Zhang-Ming Chen,Jie Hu,A-Man Xu,Department of General Surgery,Fourth Affiliated Hospital of Anhui Medical University,Hefei 230001,Anhui Province,China
Yuan-Min Xu,Lei Meng,Zhe Jiang,Department of General Surgery,First Affiliated Hospital of Anhui Medical University,Hefei 230022,Anhui Province,China
Wei He,Department of Surgery,East District of First Affiliated Hospital of Anhui Medical University(Feidong People's Hospital),Hefei 230001,Anhui Province,China
Ting Huang,Song-Cheng Ying,Department of Immunology,School of Basic Medical Sciences,Anhui Medical University,Hefei 230032,Anhui Province,China
Abstract BACKGROUND Approximately 90% of new cases of noncardiac gastric cancer(GC)are related to Helicobacter pylori(H.pylori),and cytotoxin-associated gene A(CagA)is one of the main pathogenic factors.Recent studies have shown that the pharmacological effects of cryptotanshinone(CTS)can be used to treat a variety of tumors.However,the effects of CTS on H.pylori,especially CagA+ strain-induced gastric mucosal lesions,on the development of GC is unknown.AIM To assess the role of CTS in CagA-induced proliferation and metastasis of GC cells,and determine if CagA+ H.pylori strains causes pathological changes in the gastric mucosa of mice.METHODS The effects of CTS on the proliferation of GC cells were assessed using the Cell Counting Kit-8(CCK-8)assay,and the abnormal growth,migration and invasion caused by CagA were detected by CCK-8 and transwell assays.After transfection with pSR-HA-CagA and treatment with CTS,proliferation and metastasis were evaluated by CCK-8 and transwell assays,respectively,and the expression of Src homology 2(SH2)domain-containing phosphatase 2(SHP2)and phosphorylated SHP2(p-SHP2)was detected using western blotting in AGS cells.The enzymelinked immunosorbent assay was used to determine the immunoglobulin G(IgG)level against CagA in patient serum.Mice were divided into four groups and administered H.pylori strains(CagA+ or CagA-)and CTS(or PBS)intragastrically,and establishment of the chronic infection model was verified using polymerase chain reaction and sequencing of isolated strains.Hematoxylin and eosin staining was used to assess mucosal erosion in the stomach and toxicity to the liver and kidney.RESULTS CTS inhibited the growth of GC cells in dose-and time-dependent manners.Overexpression of CagA promoted the growth,migration,and invasion of GC cells.Importantly,we demonstrated that CTS significantly inhibited the CagAinduced abnormal proliferation,migration,and invasion of GC cells.Moreover,the expression of p-SHP2 protein in tumor tissue was related to the expression of IgG against CagA in the serum of GC patients.Additionally,CTS suppressed the protein expression levels of both SHP2 and p-SHP2 in GC cells.CTS suppressed CagA+ H.pylori strain-induced mucosal erosion in the stomach of mice but had no obvious effects on the CagA-H.pylori strain group.CONCLUSION CTS inhibited CagA-induced proliferation and the epithelial-mesenchymal transition of GC cells in vitro,and CagA+ H.pylori strains caused mucosal erosions of the stomach in vivo by decreasing the protein expression of SHP2.
Key Words:Cytotoxin associated gene A;SHP2;Cryptotanshinone;Helicobacter pylori;Chronic infection model
INTRODUCTION
Gastric cancer(GC)is one of the most common malignant tumors in the digestive system,and new cases of noncardiac cancer are associated withHelicobacter pylori(H.pylori)infection[1].H.pyloriis a gram-negative microaerobic spiral bacterium that colonizes human gastric mucosa.The cytotoxin-associated gene A(CagA)protein is one of the main pathogenic factors and is encoded by the CagA gene on the cag pathogenicity island ofH.pylori[2].Epidemiological studies have shown thatH.pyloristrains with CagA(CagA+)are more virulent than strains without CagA(CagA-)and play an important role in the development of GC[3].H.pylorican directly inject CagA into gastric epithelial cells through the type IV secretion system[4,5].After entering the cytoplasm,CagA is localized on the cell membrane and is phosphorylated by various Src family kinases[6].Phosphorylated CagA(p-CagA)specifically binds to and activates SHP2 and induces morphological changes of gastric epidermal cells,including proliferation,elongation and cytoskeletal rearrangement,which is called the“hummingbird phenotype”[7-9].
SHP2 is encoded by theprotein tyrosine phosphatase non-receptor type 11gene and consists of two SH2 domains(N-SH2 and C-SH2)and a protein tyrosine phosphatase catalytic domain[10].SHP2 is widely considered an oncogene because it can enhance the malignancy of a wide variety of tumors[11,12].The mRNA and protein expression levels of SHP2 in GC tissues are significantly higher than those in normal gastric mucosa[13].Preventing the formation of CagA-SHP2 complexes could be an effective strategy in the treatment of lesions caused by CagA+H.pyloristrains.
Cryptotanshinone(CTS)is a natural compound extracted from the dried roots and rhizomes of Salvia miltiorrhiza Bunge[14].Based on its antiinflammatory,antioxidant,anticoagulant,and anticancer effects,CTS is used in the treatment of cardiovascular diseases and various cancers including lung and breast cancers[15-19].CTS induces reactive oxygen species-mediated apoptosis of cells and downregulates the expression levels of phosphorylated extracellular signal-regulated kinase,phosphorylated AKT,and phosphorylated signal transducer and activator of transcription 3(STAT3)in GC cells[19,20].CTS can irreversibly bind to the SHP2 protein and preferentially inhibit the activated form of SHP2[21].Given the important role of the SHP2 protein in the development of CagA-induced GC,we determined whether CTS inhibited the CagAinduced proliferation,migration and invasion of GC cells.
MATERIALS AND METHODS
Patients
Fifty-one patients diagnosed with GC underwent surgical resection from June 2015 to January 2016 in the First Affiliated Hospital of Anhui Medical University(Hefei,China).The sex,age,and pathological characteristics(tumor location,differentiation,tumor size and tumor-node-metastasis[TNM]stage)of the patients were collected for this study.Tumor stage was determined according to the TNM staging system(American Joint Committee on Cancer,8thedition).All patients signed informed consent forms,and this study protocol was approved by the First Affiliated Hospital of Anhui Medical University Ethics Committee.
Enzyme-linked immunosorbent assay
The Human CagA immunoglobulin G(IgG)enzyme-linked immunosorbent assay kit(Cat.# CSB-EQ027541HU;Cusabio,Houston,TX,United States)was used to measure the expression levels of IgG against CagA protein in the serum of GC patients.Samples and all reagents were prepared according to the manufacturer’s protocols.One hundred microliters of negative control,positive control or diluted samples were added to the wells of the coated assay plate,and blank wells without any solution were established at the same time.After incubating at 37 °C for 30 min,each well was washed three times with washing buffer,and all buffer in the well was discarded.Then,100 μL horseradish peroxidase conjugate was added to each well and incubated at 37 °C for 30 min.After each well was washed five times,90 μL tumor mutational burden substrate was added to the wells and incubated at 37 °C in the dark for 20 min.Fifty microliters of stop solution were added to each well,and the absorbance at 450 nm was measured using a universal microplate reader(BioTek,Winooski,VT,United States).
Cell culture and transfection
The human GC cell line AGS was obtained from the Cell Bank of the Chinese Academy of Sciences,and MGC-803 was a kind gift from Dr.Ping Wu(Anhui Medical University).AGS cells were cultured in RPMI 1640 medium(Gibco,Life Technologies,Carlsbad,CA,United States),and MGC-803 cells were cultured in DMEM(Gibco,Life Technologies,Carlsbad,CA,United States)supplemented with 10% fetal bovine serum(FBS)(TianHang,Hangzhou,China),1% penicillin and streptomycin(Beyotime,Shanghai,China).All cells were incubated in a humidified atmosphere containing 5%CO2at 37 °C.Cells in the exponential growth phase were plated on 12-well plates.When the cells in the 12-well plates reached 80%-90% confluence,1.25 μg/well of p-SRα plasmid or p-SRα-HA-CagA was transfected into the AGS cells using Lipofectamine 3000 reagent(Invitrogen,Carlsbad,CA,United States)according to the manufacturer’s protocol.Transfected cells were collected for subsequent experiments.
16. Peeped through the keyhole: Note that the prince s curiosity has vastly different results from the woman in Bluebeard. She is almost murdered for her curiosity while the prince finds his future wife.Return to place in story.
Preparation of CTS solution
CTS(molecular weight:296.35,purity ≥ 98%;Figure 1A)was purchased from Sigma-Aldrich(St.Louis,MO,United States).CTS was dissolved in dimethyl sulfoxide(DMSO,Sigma-Aldrich)to prepare a 10 mmol/L stock solution and stored at-20 °C.Before the experiment,the stock solution was diluted to the required experimental concentration with DMSO.The final concentration of DMSO in the testing solution was < 0.1%(v/v)to prevent its cytotoxicity from affecting the experimental results.
Western blotting
Total protein was extracted from cells or tissues using radioimmunoprecipitation assay lysis buffer containing 1 μmol/L/mL phenylmethanesulfonyl fluoride and phosphatase inhibitor.After measuring the protein concentration with the BCA Protein Assay Kit,an equal amount of protein extract(30 μg)was separated on an 8%or 10% SDS-PAGE gel and then transferred to a polyvinylidene fluoride membrane.The membrane was blocked in TBS buffer with 0.1% Tween-20(TBS-T)and 5%(w/v)nonfat milk for 2 h at room temperature.After blocking,the membrane was washed three times with TBS-T and then incubated with the primary antibodies at 4 °C overnight.Then the membrane was incubated with goat anti-mouse IgG secondary antibody or goat anti-rabbit IgG secondary antibody for 1 h at room temperature.Finally,the proteins were detected using an enhanced chemiluminescence system(Tanon,Shanghai,China)and analyzed with a digital imaging system(Tanon).Among the primary antibodies used,anti-p-SHP-2(Tyr580,#3703)and anti-SHP2(#3752)were purchased from Cell Signaling Technology(Danvers,MA,United States),anti-HA tag(#TA100012)was purchased from OriGene Technologies(Rockville,MD,United States),anti-β-Actin(#TA-09)was purchased from ZSBio(Beijing,China),and all secondary antibodies(#ZB-2301,#ZB-2305)were purchased from ZSBio.
Cell Counting Kit-8 assay
GC cells were seeded into 96-well plates at approximately 2 × 103cellsperwell.The experimental groups were treated with different concentrations of CTS for the appropriate time.Since CTS was dissolved in DMSO,the control group was treated with the same amount of DMSO to exclude the effect of DMSO cytotoxicity from the experimental results.Five replicate wells were set up for each group.After drug treatment,the original medium was discarded,and culture medium containing 10%Cell Counting Kit-8(CCK-8)reagent(Beyotime,Shanghai,China)was added to each well and incubated at 37 °C for 2-4 h.Finally,the absorbance was measured at 450 nm using a universal microplate reader(BioTek).
Cell migration and invasion assays
Transwell chambers were used for cell migration assays,and cell invasion assays were performed using matrigel-coated transwell cells.Transwell chambers were purchased from Corning Inc.(New York,NY,United States),and BD matrigel was purchased from BD Biosciences(San Jose,CA,United States).A total of 1 × 105transfected cells were treated with different drug concentrations in medium containing 1% FBS and were added to the upper chamber,and 600 μL medium containing 10% FBS was added to the lower chamber.Cells were used for the migration assay after incubation at 37 °C for 8 h or for the invasion assay after 12 h of incubation.The remaining cells on the upper surface of the chamber were wiped off.After fixation in 4% paraformaldehyde for 20 min,the migrated or invaded cells were incubated in crystal violet solution for 15 min.The stained cells were photographed under an optical microscope,and five fields were randomly selected for cell counting.
H.pylori cultivation
The mouse-colonizing strain Pre-Mouse Sydney Strain 1(PMSS1 wild-type)and the mutant CagA knockout strain(PMSS1 ΔCagA)were kind gifts from Prof.Lv Nonghua(First Affiliated Hospital of Nanchang University,Nanchang,China).AllH.pyloristrains were cultured in agar plates containing Columbia blood agar powder(3.9%),fresh sterile defibrated sheep blood(7%),vancomycin(10 mg/L),amphotericin B(10 mg/L),polymyxin B(2500 U/L),and trimethoprim(5 mg/L)under microaerobic conditions(produced by using a microaerobe air bag from Japanese Mitsubishi Company)at 37 °C for 72 h.The PMSS1 ΔCagA strain was grown on agar plates supplemented with chloramphenicol(200 mg/L).TheH.pyloristrains were authenticated by polymerase chain reaction using CagA-and 16S RNA-specific primers as well as 16S RNA sequences(General Biology Company,Baoshan,China).The primers were as follows:CagA,F:GGA GCC AAG CAC GAT TGG AAC G,R:CTT GAC TCA ATG CTC GTT GTG A;16S RNA,F:GCT AAG AGA TCA GCC TAT GTC C,R:CAA TCA GCG TCA GTA ATG TTC.
Animal model
The protocol for the animal experiment was approved by the Animal Use and Care Committee of Anhui Medical University,and all surgical procedures and care administered to the mice were in accordance with institutional guidelines.C57BL/6 female mice at 3 wk of age were randomly divided into four groups(n= 8pergroup)and kept for another week in a specific pathogen-free environment.The mice were administeredH.pyloristrains(PMSS1 wild-type and PMSS1 ΔCagA strains)intragastrically twice per week for 4 wk.After 4 wk ofH.pyloricolonization,the mice were administered CTS(10 mg/kg)or PBS intragastrically once a week for 8 wk.
Statistical analyses
All experiments were repeated at least three times.Differences between two groups were compared by the Student’st-test using GraphPad Prism 8.0(GraphPad Software,Inc.,San Diego,CA,United States).The correlation between the expression of p-SHP2 and other factors was analyzed by the chi-square test using SPSS 16.0(IBM,Chicago,IL,United States).P< 0.05 was considered statistically significant.
RESULTS
CTS suppresses the proliferation of GC cells
Previous studies have reported that CTS can inhibit prolifer ation and induce apoptosis of GC cells[20].First,we verified that CTS inhibits the growth of GC cells.Figure 1A shows the chemical structure of CTS.As shown in Figure 1B,CTS inhibited the proliferation of MGC-803 cells in a dose-dependent manner.Moreover,CTS also suppressed the growth of GC cells in a time-dependent manner,especially at 72 h(Figure 1C).
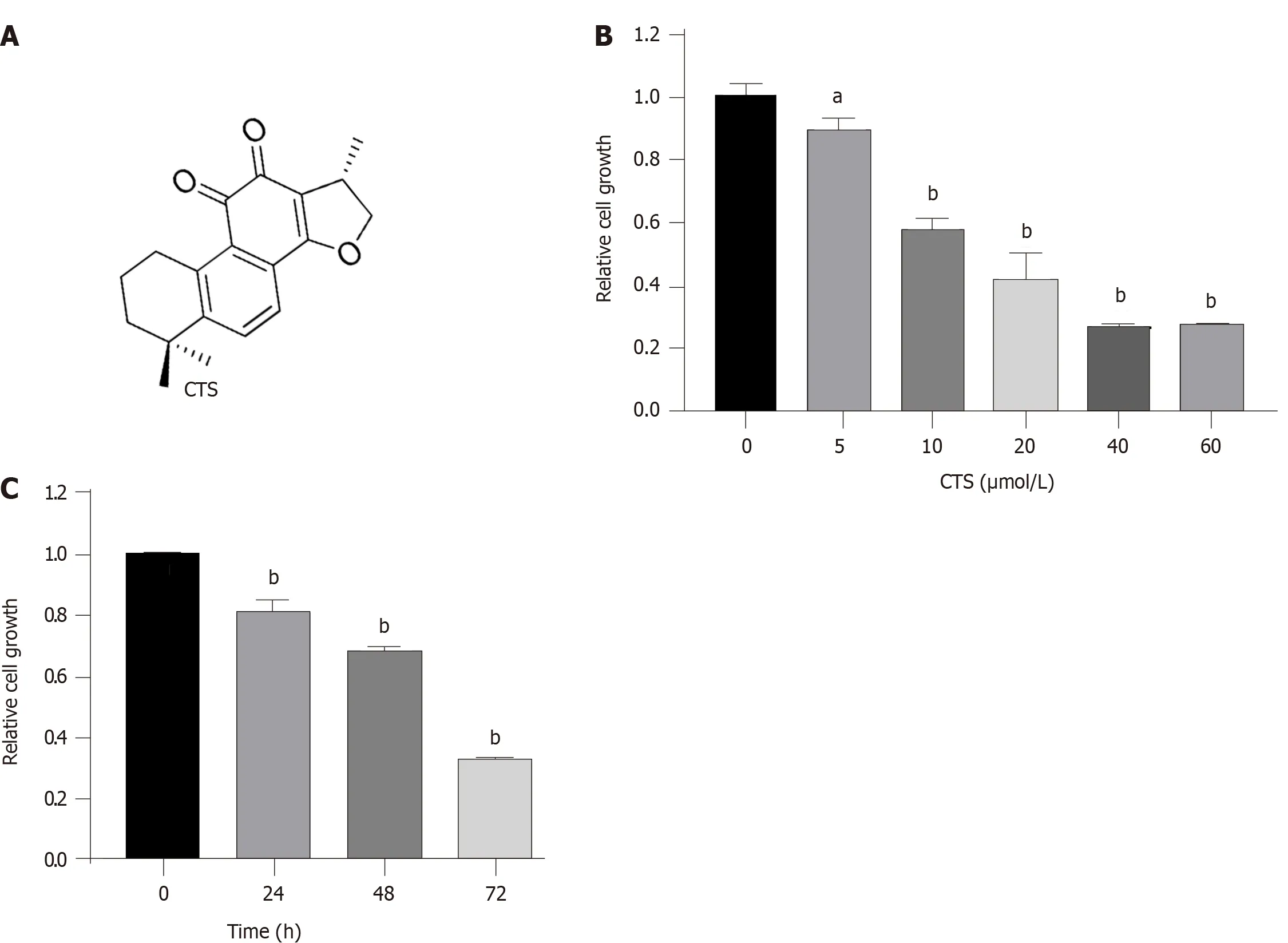
Figure 1 Cryptotanshinone suppresses the proliferation of gastric cancer cells.A:The chemical structure of cryptotanshinone(CTS);B:MGC-803 cells were treated with the indicated doses of CTS for 48 h,and cell growth was detected by the Cell Counting Kit-8(CCK-8)assay;C:MGC-803 cells were treated with 10 μmol/L CTS for 0-72 h,and cell growth was detected by the CCK-8 assay.Data are expressed as the mean ± SD from triplicate experiments.aP < 0.05;bP <0.01.
CagA promotes the proliferation,migration,and invasion of GC cells
Next,we evaluated the role of CagA in the proliferation,migration,and invasion of GC cells.AGS cells were transfected with the pSRα-HA-CagA plasmid for 48 h,and western blot analysis showed that CagA was expressed successfully,whereas it was not expressed in p-SRα-transfected cells(Figure 2A).The proliferation ability of AGS cells in the CagA group was significantly enhanced compared the control group(Figure 2B).Moreover,as shown in Figure 2C-F,the migration and invasion capabilities of CagA-expressing AGS cells were significantly improved compared to control cells.Therefore,the results suggested that CagA promoted the proliferation,migration,and invasion of GC cells.
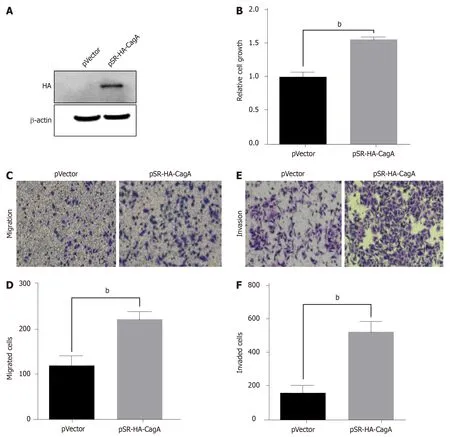
Figure 2 Cytotoxin-associated gene A induces abnormal proliferation and metastasis of gastric cancer cells.A:AGS cells were transfected with pSR-HA-cytotoxin-associated gene A(CagA)or an empty vector plasmid for 48 h.The expression of CagA protein was assessed by western blotting;B:Cell growth was detected by the Cell Counting Kit-8(CCK-8)assay;C and E:AGS cells with or without CagA expression were seeded into the upper transwell chamber.The cells were stained and photographed to evaluate migration(C)and invasion(E);D and F:The histograms showed the numbers of migrated(D)and invaded(F)cells.Cells were counted from five randomly selected microscope fields.Data are expressed as the mean ± SD from triplicate experiments.bP < 0.01.
CTS inhibits the CagA-induced proliferation of AGS cells
We further investigated whether CTS inhibits the development of CagA-related GC.First,we compared the effects of different doses of CTS on the proliferation of GC cells to determine the optimal concentration range of CTS.CTS at ≤ 1.25 μmol/L had no effect on the proliferation or viability of AGS cells(Figure 3A).The proliferation ability of AGS cells expressing CagA was significantly improved compared with the control cells(Figure 3B).Moreover,CTS inhibited the CagA-induced growth of AGS cells in a dose-dependent manner(Figure 3B).
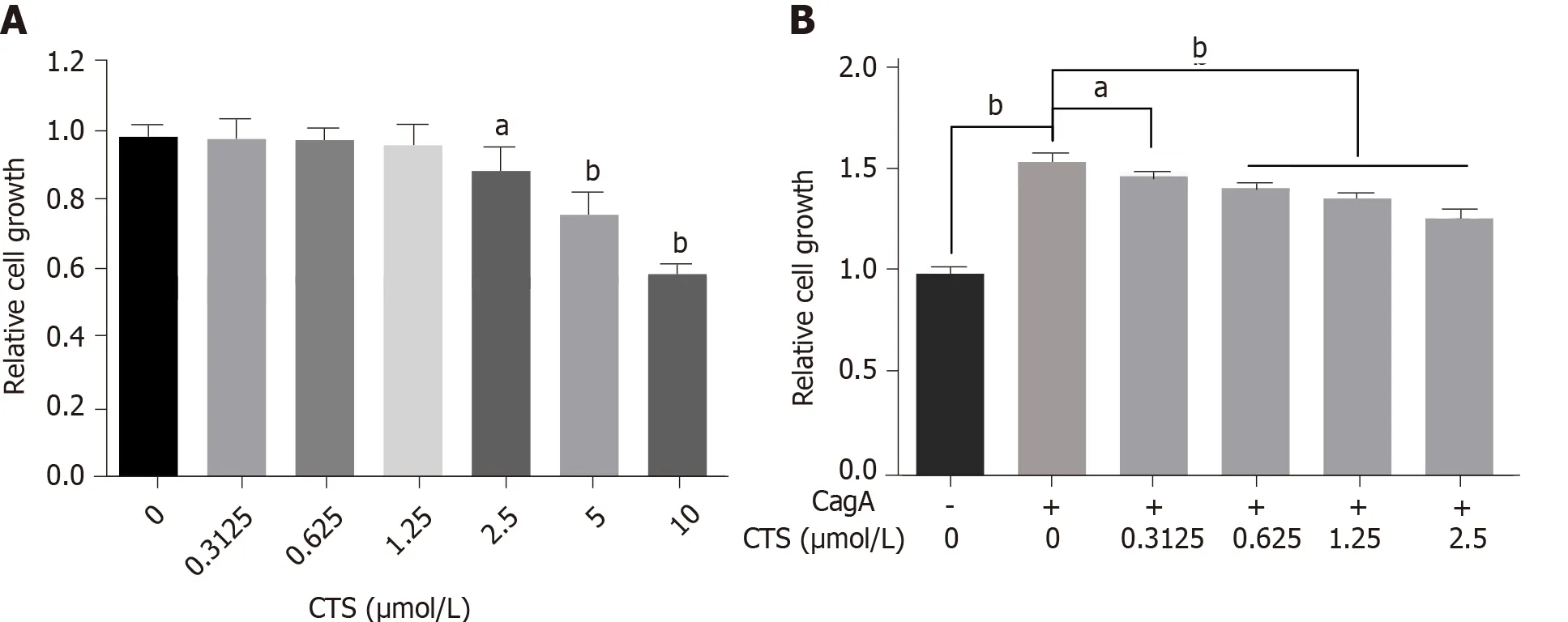
Figure 3 Cryptotanshinone inhibits the cytotoxin-associated gene A-induced proliferation of AGS cells.A:AGS cells were treated with cryptotanshinone(CTS)(0-10 μmol/L)for 24 h,and proliferation was detected using the Cell Counting Kit-8(CCK-8)assay.B:AGS cells with or without cytotoxinassociated gene A(CagA)expression were treated with the indicated doses of CTS(0-1.25 μmol/L)for 24 h,and cell growth was detected by the CCK-8 assay.Data are expressed as the mean ± standard deviation from triplicate experiments.aP < 0.05;bP < 0.01.
CTS inhibits CagA-induced enhancement of AGS cell migration and invasion
In addition to uncontrolled growth,migration and invasion are also malignant biological behaviors in GC.In this study,we also explored the effects of CagA on the migration and invasion of GC cells through transwell assays.As shown in Figure 4A and B,compared with control cells,the migration capabilities were significantly improved in AGS cells expressing the CagA protein.However,after treatment with CTS,the cell migration induced by CagA was significantly decreased(Figure 4A and B).Moreover,CTS also suppressed the CagA-induced invasion of AGS cells(Figure 4C and D).These results suggest that the application of CTS might be an efficient strategy for treating gastric mucosal lesions caused by CagA-positiveH.pyloristrains.

Figure 4 Cryptotanshinone inhibits the cytotoxin-associated gene A-induced migration and invasion of AGS cells.AGS cells were transfected with pSR-HA-cytotoxin-associated gene A(CagA)or an empty vector plasmid for 24 h and then treated with the indicated doses of cryptotanshinone(CTS)for 24 h.The cells were seeded into the upper transwell chamber.A and C:The invaded(A)and migrated(C)cells were stained and photographed;B and D:The histograms represent the numbers of invaded(B)and migrated(D)cells.Cells were counted from five randomly selected microscope fields.Data are expressed as the mean ±SD of triplicate experiments.bP < 0.01.
The protein expression level of p-SHP2 correlates with the level of IgG against CagA in the serum of GC patients
How CTS inhibits the development of CagA-related GC is not completely known.CTS preferentially inhibits the activated form of SHP2,and SHP2 is involved in the development of a variety of cancers[21].Activation of SHP2 is critical in CagA pathogenicity in GC.Therefore,we investigated whether SHP2 was related to CagArelated GC.The results showed that the protein expression of p-SHP2 had no significant correlation with GC patient age,sex,tumor location,or degree of differentiation(P> 0.05).However,the expression of p-SHP2 was closely related to tumor size,tumor invasion depth,lymph node metastases,and TNM stage(Table 1),suggesting that high expression of p-SHP2 protein played an important role in the development of GC.We further analyzed the relationship between the expression levels of IgG against CagA in GC patient serum and the expression of p-SHP2 in GC tissues.As shown in Table 1,expression of the activated form of the SHP2 protein was significantly correlated with that of IgG against CagA.These results suggest that SHP2 activation might be an important factor in CagA-associated development of GC.
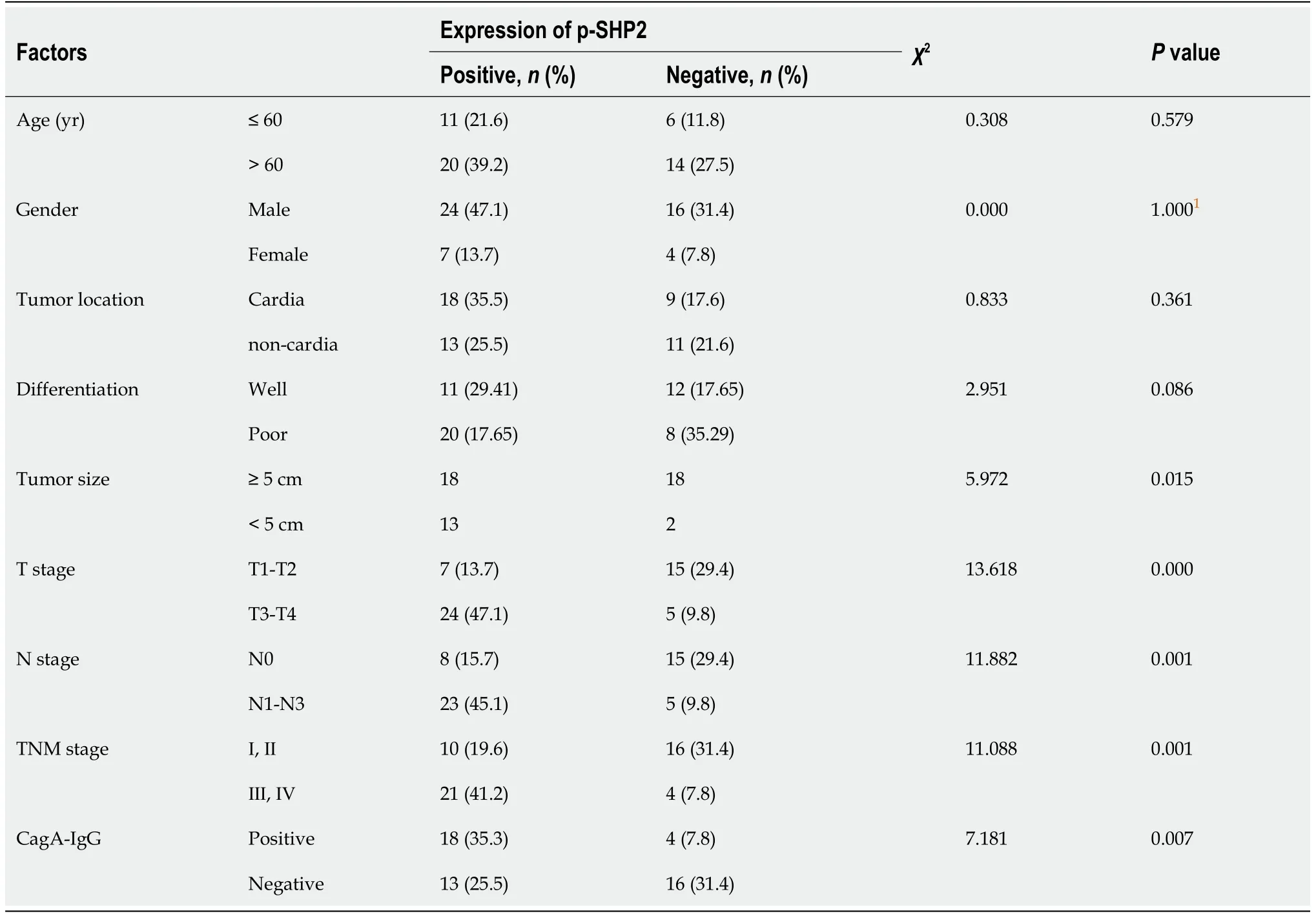
Table 1 CorreIation between expression of phosphoryIated Src homoIogy 2 domain-containing phosphatase 2 and other cIinicopathoIogic characteristics
CTS inhibits CagA-induced phosphorylation of SHP2 in GC cells
We further studied the changes in the SHP2 and p-SHP2 protein expression levels in AGS cells after CTS treatment.The expression level of p-SHP2 in AGS cells was significantly increased by overexpression of CagA(Figure 5A).Importantly,the CagAinduced expression of p-SHP2 was significantly inhibited by treatment with CTS(Figure 5A).Interestingly,CTS also suppressed the expression of total SHP2 protein in AGS cells(Figure 5A and B).Together,these results indicated that CTS inhibited CagA-induced phosphorylation of SHP2 by decreasing SHP2 expression in GC cells.
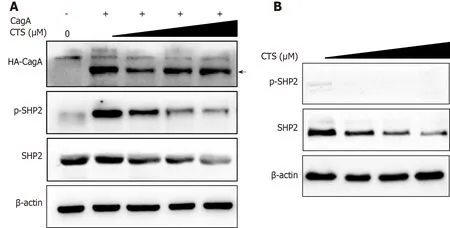
Figure 5 Cryptotanshinone suppresses the expression of Src homoIogy 2 domain-containing phosphatase 2(SHP2)and the cytotoxinassociated gene A-induced phosphoryIation of SHP2.A:AGS cells were transfected with pSR-HA-cytotoxin-associated gene A(CagA)or empty vector plasmid for 24 h and then treated with the indicated doses of cryptotanshinone(CTS)for 24 h;B:AGS cells were treated with the indicated doses of CTS for 24 h.Western blotting was performed to detect the expression of Src homology 2 domain-containing phosphatase 2(SHP2)and phosphorylated SHP2.β-actin was used as a loading control.
CTS suppresses CagA-induced pathological alterations in the stomach of mice
The above results indicated that CTS could be a potential drug to suppress the abnormal growth and epithelial-mesenchymal transition(EMT)caused by CagApositiveH.pyloristrains.To assess the effects of CTSin vivo,we established a mouse model with chronicH.pyloriinfection.Mice were divided into four groups and administeredH.pyloristrains(PMSS1 wild-type or ΔCagA)and CTS(or PBS)intragastrically according to the graphical representation in Figure 6A.Then the stomach was resected and sliced for hematoxylin and eosin staining,and the results showed that compared with the PMSS1 ΔCagA strain,the PMSS1 wild-type strain gave rise to more mucosal erosions in the stomach(Table 2 and Figure 6B).Furthermore,CTS had no influence on the occurrence rate of erosions induced by the PMSS1 ΔCagA strain but decreased the frequency of erosions caused by the PMSS1 wild-type strain(Table 2 and Figure 6B).As shown in Figure 6C,CTS had no toxic effects on the liver or kidney at 10 mg/kg.The PMSS1 wild-type and PMSS1 ΔCagA strains isolated from the mucosa of the resected stomach were authenticated(Figure 6D and E),which confirmed the successful establishment of a mouse model of chronicH.pyloriinfection.Thus,we concluded that CTS inhibited CagA-induced proliferation and EMT of GC cellsin vitroand the PMSS1 wild-type strain caused mucosal erosion of the stomachin vivoby decreasing the protein expression of SHP2(Figure 7).
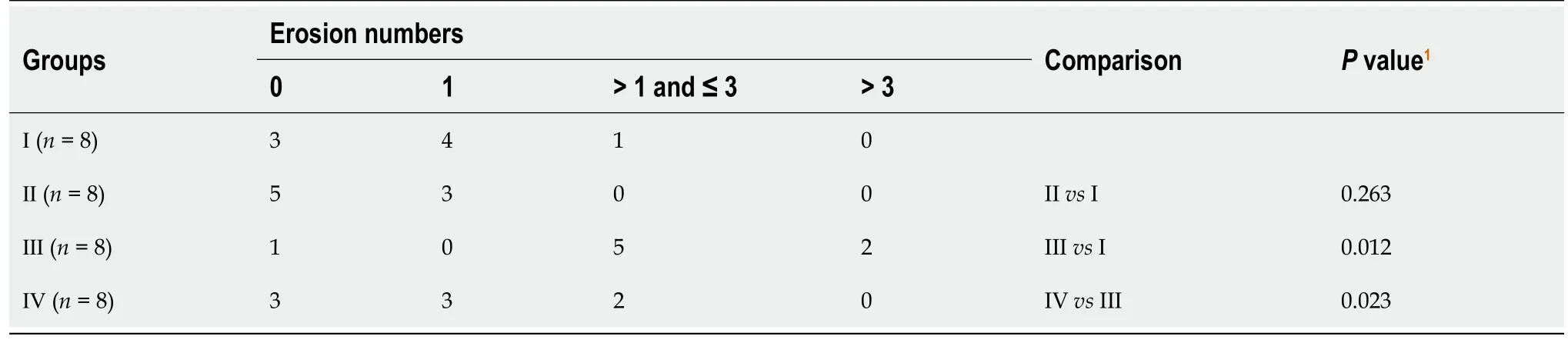
Table 2 Comparison of erosion numbers among four groups of Helicobacter pylori chronic infection
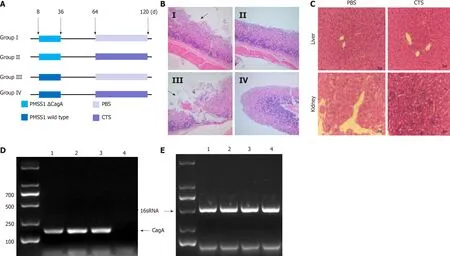
Figure 6 Cryptotanshinone inhibits cytotoxin-associated gene A-induced mucosaI Iesions in the stomach of mice.A:Mice were divided into four groups(n = 8 per group)and received the treatments indicated in the graphical representation;B:After the treatments,the mice were sacrificed,and the stomach was resected for hematoxylin and eosin(H&E)staining.The black arrow shows mucosal erosion;C:The mice were administered cryptotanshinone(CTS)(10 mg/kg)intragastrically for 1 mo,and the toxicity to the liver and kidney was assessed by H&E staining;D and E:Isolated Helicobacter pylori strains were authenticated through the polymerase chain reaction assay using specific primers.1:Pre-Mouse Sydney Strain 1(PMSS1)wild-type;2:SS1 strain-isolated;3:PMSS1 wild-type-isolated;4:PMSS1 Δcytotoxin-associated gene A(CagA)-isolated.

Figure 7 Mechanism by which cryptotanshinone reguIates Src homoIogy 2 domain-containing phosphatase 2 and cytotoxin-associated gene A-associated pathogenesis in gastric cancer.Cryptotanshinone(CTS)suppresses the expression of Src homology 2 domain-containing phosphatase 2(SHP2),which prevents cytotoxin-associated gene A(CagA)from phosphorylating SHP2.Therefore,CTS inhibits CagA-induced proliferation and the epithelialmesenchymal transition(EMT)in gastric cancer(GC)and mucosal erosion in the stomach of mice.
DISCUSSION
Due to the antiinflammatory and antitumor effects of Salvia miltiorrhiza,a variety of compounds have been extracted from the roots and rhizomes of Salvia miltiorrhiza,including tanshinone IIA,CTS,and dihydrotanshinone,that promote the apoptosis of GC cells[20,22,23].CTS and tanshinone IIA exert antitumor effects by downregulating the expression of STAT3 protein in GC cells[19,24].Tanshinone IIA regulates the growth and apoptosis of GC cellsin vivoand in vitro,and its regulatory effects onH.pylori-infected cells have also been reported[25,26].Our previous study showed that isocryptotanshinone,an analog of CTS,inhibited the growth of GC cells by inducing cell apoptosis and cell cycle arrest[27].In this study,CTS inhibited the growth of GC cells in a dose-and time-dependent manner.
Infection withH.pyloriCagA-positive strains significantly increases the risk of the development of GC.Our results revealed that CagA protein enhanced the growth and metastatic ability of AGS cells(Figure 2).More importantly,CTS(0.3125,0.625 and 1.25 μmol/L)significantly inhibited the abnormal proliferation and metastasis of GC cells caused by CagA.In infection ofH.pylori,CagA protein plays an important regulatory role by binding to the SHP2 protein,which activates downstream signaling pathways.Previous studies have found high expression of SHP2 protein in GC tissues,and increased expression of the SHP2 protein is associated with poor prognosis of GC[13].Similarly,our results indicated that the expression of p-SHP2 was closely related to tumor size,tumor invasion depth,lymph node metastasis number,and clinical stage.CagA interacts with SHP2 and causes SHP2 activation[28].Moreover,our study further identified the positive correlation between the expression levels of IgG against CagA in patient serum and p-SHP2 protein in GC tissues.Thus,the activation of SHP2 by the CagA protein plays an important role in the development of GC,which could be a potential therapeutic target during infection withH.pylori.
Inhibiting CagA tyrosine phosphorylation,disrupting the CagA-SHP2 complex,and inhibiting SHP2 expression by small interfering RNA eliminated morphological changes caused by CagA in gastric epidermal cells[7].CTS can regulate the tyrosine phosphatase activity of SHP2[20].To further explore the inhibitory mechanisms of CTS on CagA-induced proliferation and metastasis of GC cells,we analyzed the protein expression levels of SHP2 and p-SHP2 in GC cells after CTS treatment.After CTS treatment,the expression levels of SHP2 and p-SHP2 in GC cells were decreased.However,CTS has no obvious effects on the expression of CagA proteins.Together,our results suggest that CTS might inhibit CagA-induced cell growth and metastasis by inhibiting the tyrosine phosphatase activity of SHP2.
CONCLUSION
In conclusion,this study demonstrated for the first time that CTS inhibited the CagAinduced abnormal proliferation and metastatic ability of GC cellsin vitro,and CagApositiveH.pyloristrains caused mucosal erosion of the stomachin vivoby decreasing the expression of the SHP2 protein.
ARTICLE HIGHLIGHTS
Research background
Cryptotanshinone(CTS)exhibits antitumor effects in a variety of tumors.Cytotoxinassociated gene A(CagA)is one of the main pathogenic factors inHelicobacter pylori(H.pylori)related gastric cancer(GC).However,the effects of CTS on CagA-induced development of GC and CagA+H.pyloristrains caused gastric mucosal lesions is unknown.
Research motivation
To identify the effect and mechanism of CTS on CagA associated GC.
Research objectives
We assessed the role of CTS in CagA induced proliferation and metastasis of GC cells,and in CagA+H.pyloristrains caused pathological changes of gastric mucosa of mice.
Research methods
The proliferation and metastasis of GC cells were evaluated by CCK-8 and Transwell assays,respectively,and the expression of SHP2 and phosphorylated SHP2 was detected using western blotting.Enzyme-linked immunosorbent assay was used to determine the immunoglobulin G(IgG)level against CagA in patient serum.Mice were divided into four groups and administeredH.pyloristrains(CagA+ or CagA-)and CTS(or PBS)intragastrically,and the establishment of the chronic infection model was verified using polymerase chain reaction and sequencing of isolated strains.Hematoxylin and eosin staining was used to assess mucosal erosion in the stomach and toxicity to the liver and kidney.
Research results
CTS significantly inhibited the CagA-induced abnormal proliferation,migration,and invasion of GC cells.Moreover,the expression of p-SHP2 protein in tumor tissue was related to the expression of IgG against CagA in the serum of GC patients.Additionally,CTS suppressed the protein expression levels of both SHP2 and p-SHP2 in GC cells.CTS suppressed CagA+H.pyloristrain-induced mucosal erosion in the stomach of mice but had no obvious effect on the CagA-H.pyloristrain group.
Research conclusions
CTS inhibited CagA-induced proliferation and epithelial-mesenchymal transition of GC cells in vitro,and CagA+H.pyloristrains caused mucosal erosion of the stomachin vivoby decreasing the expression of SHP2 protein.
Research perspectives
CTS might be an adjuvant agent in anti-H.pyloritherapy,decreasing the incidence ofH.pyloriassociated GC.
ACKNOWLEDGEMENTS
We wish to thank Ming Lu(Department of Immunology,School of Basic Medical Sciences,Anhui Medical University)and Dr.Dan-Dan Zang(Center for Scientific Research of Anhui Medical University)for their assistance in technical support.We also wish to thank Professor Si-Ying Wang(Department of Pathophysiology,Anhui Medical University)for providing the p-SRα and p-SRα-HA-CagA plasmids.
 World Journal of Gastrointestinal Oncology2021年7期
World Journal of Gastrointestinal Oncology2021年7期
- World Journal of Gastrointestinal Oncology的其它文章
- Laparoscopic liver resection for colorectal liver metastases — shortand long-term outcomes:A systematic review
- High expression of protein phosphatase 2 regulatory subunit B''alpha predicts poor outcome in hepatocellular carcinoma patients after liver transplantation
- Robotic resection of duodenal gastrointestinal stromal tumour:Preliminary experience from a single centre
- Clinical management for malignant afferent loop obstruction
- Neoantigen vaccine:An emerging immunotherapy for hepatocellular carcinoma
- Sporadic fundic gland polyps with dysplasia or carcinoma:Clinical and endoscopic characteristics
