Neoantigen vaccine:An emerging immunotherapy for hepatocellular carcinoma
Pu Chen,Qiong-Xuan Fang,Dong-Bo Chen,Hong-Song Chen
Pu Chen,Qiong-Xuan Fang,Dong-Bo Chen,Hong-Song Chen,Peking University Hepatology Institute,Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Disease,Peking University People’s Hospital,Beijing 100044,China
Abstract Tumor-specific neoantigens,which are expressed on tumor cells,can induce an effective antitumor cytotoxic T-cell response and mediate tumor regression.Among tumor immunotherapies,neoantigen vaccines are in early human clinical trials and have demonstrated substantial efficiency.Compared with more neoantigens in melanoma,the paucity and inefficient identification of effective neoantigens in hepatocellular carcinoma(HCC)remain enormous challenges in effectively treating this malignancy.In this review,we highlight the current development of HCC neoantigens in its generation,screening,and identification.We also discuss the possibility that there are more effective neoantigens in hepatitis B virus(HBV)-related HCC than in non-HBV-related HCC.In addition,since HCC is an immunosuppressive tumor,strategies that reverse immunosuppression and enhance the immune response should be considered for the practical exploitation of HCC neoantigens.In summary,this review offers some strategies to solve existing problems in HCC neoantigen research and provide further insights for immunotherapy.
Key Words:Hepatocellular carcinoma;Neoantigen;Hepatitis B virus;Screening and identification;Immunosuppression;Immunotherapy
INTRODUCTION
Hepatocellular carcinoma(HCC)is the most common type of primary malignant liver cancer.It is also one of the most common malignant tumors with high mortality in the world.Due to the influence of risk factors such as viral hepatitis,alcoholism,and obesity,the incidence rate of HCC continues to rise.Despite progress in treatments for HCC,such as surgery,percutaneous intratumoral injection of absolute ethanol,radiofrequency ablation,hepatic artery embolization,liver transplantation,and targeted therapy,the prognosis remains poor because of the high recurrence rate and high metastasis rate[1,2].
In recent years,immunotherapy holds great promise to patients with HCC.Immune checkpoint inhibitor(ICI)therapy[3]and adoptive cell therapy,represented by antiprogrammed cell death 1(PD-1)antibody therapy and chimeric antigen receptor T cell(CAR-T)therapy[4],have made a major contribution to HCC immunotherapy.However,there remain limitations in these immunotherapies.Anti-PD-1 antibody therapy induces severe side effects in most patients and the beneficiary group is limited;CAR-T therapy has low effectiveness in solid tumors.These shortcomings limited wide clinical application of HCC immunotherapy,and new therapeutic strategies are needed to remedy these obstacles.The study of HCC neoantigens and personalized neoantigen vaccines is a promising direction.Here,we review the current research related to HCC neoantigens.Specifically,we discuss the methods for screening and identifying HCC neoantigens and strategies for exploiting HCC neoantigens in immunotherapy.
HCC NEOANTIGENS
Neoantigens are novel peptides that are generated by special events,such as gene mutation,alternative splicing,and virus integration in tumor cells.They not only have good tumor specificity but also a high degree of immunogenicity.Therefore,neoantigens can be well recognized and attacked by the immune system in humans[5-8].
The neoantigens that have been identified are mainly produced by gene mutations.Most mutations are single-base mutations and produce single neoantigens whose lengths range from 7 to 11 amino acids[9,10].However,there are also some special cases.For example,mutation of C to T in thePGM5gene replaces the histidine residue with tyrosine.As such,two 9-peptide neoantigens are generated under the action of the proteasome[11](Figure 1).Tumor that have higher tumor mutation burden(TMB)can produce more neoantigens as a result of mutations than those with lower TMB.A study showed that melanoma has the highest TMB among 30 different types of tumors,and it is generally believed that melanoma has the most mutation-induced neoantigens.The TMB of HCC ranks 12th,and the median TMB in HCC is approximately 2.0 mutations/megabase[12].A study found some mutation-induced neoantigens in 5 cases of melanoma but failed in 16 cases of HCC with the same workflow[13].The above results suggest that the process used for detection of mutation-induced neoantigens in melanoma may not be ideal for HCC mutationinduced neoantigens.And it is necessary to optimize this workflow according to the characteristics of HCC when we use it in HCC.We have an in-depth discussion in this review.
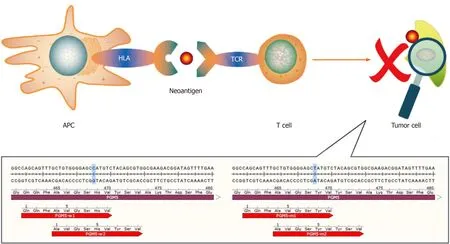
Figure 1 The human PGM5 gene,as an example.Antigen-presenting cells present neoantigens to T cells through human leukocyte antigen molecules,and the T cell receptor recognizes neoantigens.This recognition activates T cells to eliminate neoantigen-specific tumor cells.The bottom part of the figure shows mutations of the PGM5 gene that produce two neoantigens.APC:Antigen-presenting cell;HLA:Human leukocyte antigen;TCR:T cell receptor.
Hepatitis B virus(HBV)infection is the main cause of liver cirrhosis and HCC.HBVrelated HCC accounts for the majority of HCC cases in the world,especially in Asians[14,15].Therefore,the neoantigen spectrum of HCC in Asians may be quite different from that in other populations.Due to long-term viral infection that may have an impact on tumor mutation patterns,we conducted a preliminary analysis of the HBVrelated HCC genome using data from The Cancer Genome Atlas(TCGA)database(https://www.cancer.gov/tcga)and found that the mutated genes in this cohort were significantly different from those in a non-HBV-related HCC cohort(Figure 2).A total of 1973 mutation-induced peptides were identified in 159 cases of HBV-related HCC,including some mutation-induced neoantigens[16].It gives us a clue that there could be more effective mutation-induced neoantigens in HBV-related HCC than in non-HBV-related HCC.
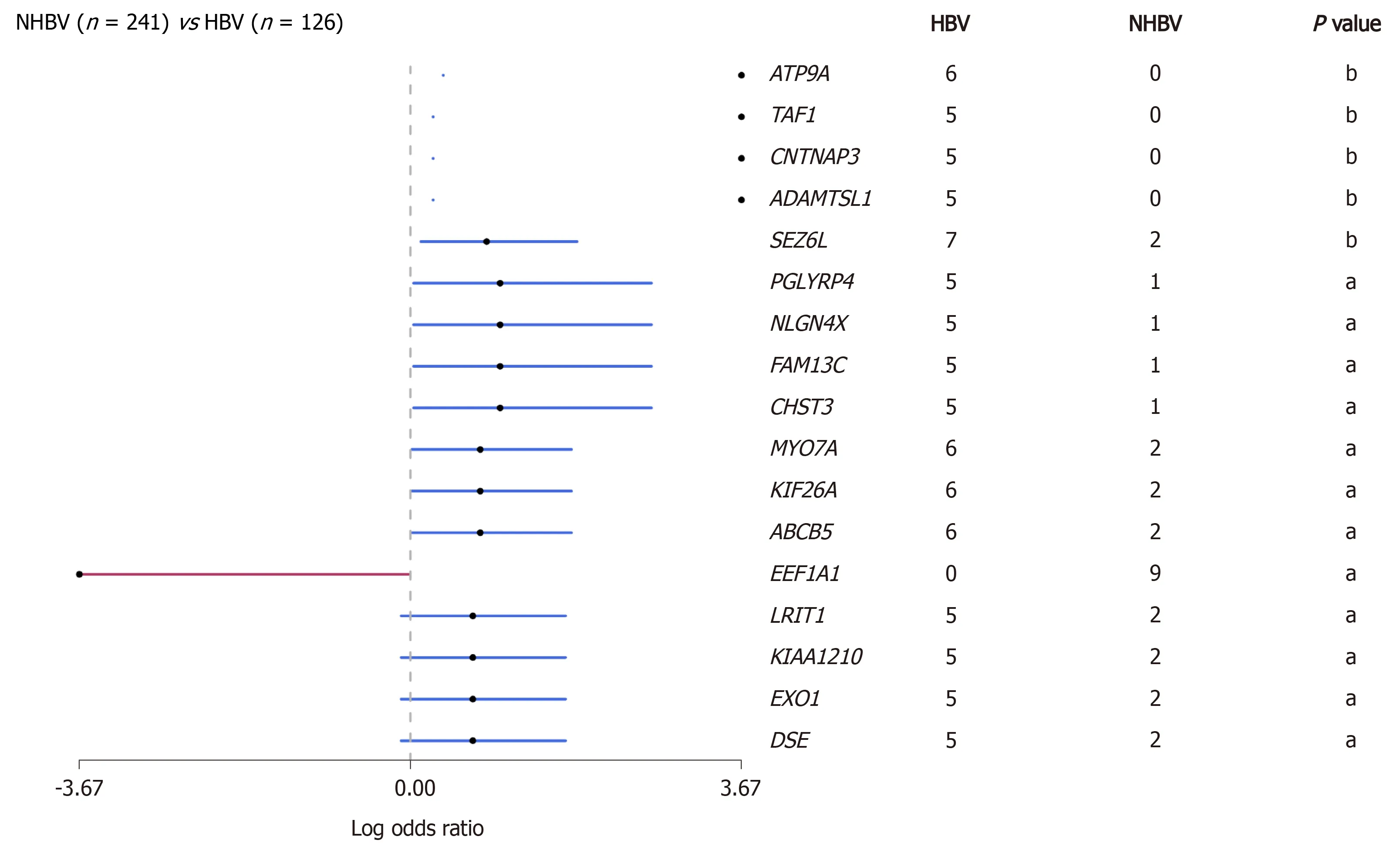
Figure 2 Comparison of gene mutations between hepatitis B virus-reIated hepatoceIIuIar carcinoma and non-hepatitis B virus-reIated hepatoceIIuIar carcinoma in data from the The Cancer Genome AtIas database(aP < 0.05;bP < 0.01).The red line represents genes in nonhepatitis B virus(HBV)-related hepatocellular carcinoma(HCC)cohort,and the blue line represents genes in HBV-related HCC cohort.HBV:Hepatitis B virus.
In addition,there are many events of alternative splicing and virus integration in HCC.These events are also likely to produce neoantigens,but they have not been fully studied.There are more alternative splicing events in tumorsvsnormal tissue.Among them,retained intron(RI)is the most worthy of attention.The neoantigens produced from the specific RI in the tumor may have great immunogenicity[17-19].One of the most noteworthy virus integration is HBV integration.By inserting a promoter into the noncoding region,HBV can transcribe not only its own genes but also part of the noncoding region sequence,resulting in the production of viral peptides and the peptides derived from noncoding region.This integration of HBV is an early driver event and remains extremely stable during HCC progression[20].Therefore,if this mechanism can produce neoantigens,it will be a very good target for HCC immunotherapy.In addition,adeno-associated virus 2(AAV2)also can integrate into the genome in HCC.Moreover,the positions of virus integration are relatively located in the same sites.For example,both HBV integration and AAV2 integration have been found in theCCNA2gene in HCC,and all the integration sites were located in the intron region between the second exon and the third exon of the gene[21,22].Therefore,through systematic analysis of these integration events,we may be able to find some neoantigens that exist in the early dominant clones and remain relatively stable throughout virus-related HCC progression.
Unfortunately,the research on HCC neoantigens is mostly limited to a single sample of the HCC tumor now.In comparison,the sampling of circulating tumor cells(CTCs)in human peripheral blood is more convenient.A large amount of biological information such as gene mutations can be obtained from CTCs[23].The detection of genes related to HCC in CTCs or circulating tumor DNA is also helpful for obtaining the neoantigen spectrum of inoperable advanced HCC or unresectable metastatic HCC.It is also beneficial for obtaining the neoantigen spectrum of potential recurrent and metastatic lesions(earlier than could be achieved by imaging)[24-27].In addition,there may be some tumor cells in the ascites of patients with HCC.Whether the HCC neoantigen spectrum can be determined from the ascites is also worth studying.
SCREENING AND IDENTIFICATION OF HCC NEOANTIGENS
The most challenging process of neoantigen immunotherapy is the screening and identification of neoantigens.At present,there are three classical methods that can be used to screen and identify HCC neoantigens(Figure 3).
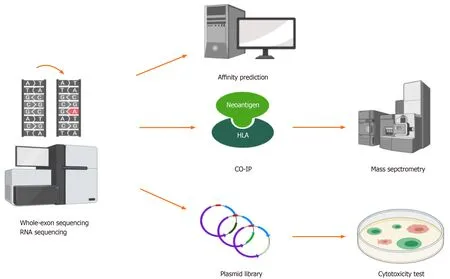
Figure 3 Whole-exon sequencing and RNA sequencing detect mutations in tumors,and then,different methods can be used to identify hepatocellular carcinoma neoantigens.(1)Human leukocyte antigen affinity prediction;(2)Coimmunoprecipitation followed by mass spectrometry;and(3)Construction of a plasmid library based on neoantigens followed by cytotoxicity tests in vitro.HLA:Human leukocyte antigen;CO-IP:Coimmunoprecipitation.
The first method is predicting neoantigens with a computer algorithm.For mutation-induced neoantigens,accurate prediction requires a large amount of data and many calculations,which mainly require the following aspects:(1)Accurate identification of all kinds of mutation sites from billions of bases;(2)Calculation of the frequency and expression level of mutation sites;(3)Accurate identification of patient alleles from thousands of human leukocyte antigen(HLA)alleles;and(4)Evaluation of the immunogenicity of the antigen peptides produced by each mutation site by bioinformatics and artificial intelligence(AI)algorithms.However,accurate prediction is difficult.A large number of tools and algorithms have been developed to predict the affinity between antigen peptides and HLA molecules,but their accuracy is still the main bottleneck of all kinds of methods.Khodadoustet al[28]predicted 108 candidate peptides in eight tumor patients by means of this approach.After verification experiments with peptide-HLA tetramer T-cell responses against predicted neoantigens,it was found that all of the predictions had failed.The accuracy of computer prediction of neoantigens needs to be improved.Since the quantity and quality of overall gene mutations as neoantigens in HCC are not high,the previously mentioned sources of neoantigens,that is,virus infection,virus integration,and alternative splicing,should be taken into account when making predictions.Deeply comparing the affinity of HLAs between candidate peptides and wild-type peptides,excluding the loss of HLA heterozygosity early,investigating the structure and properties of unique amino acid,and using AI tools to reconstruct and analyze the three-dimensional structure of HLA molecules,peptides,and T cell receptors,are better for screening and identifying HCC neoantigens than traditional prediction algorithms[29-33].
The second method is the combination of coimmunoprecipitation and mass spectrometry to screen and identify HCC neoantigens.There is experimental evidence supporting this combination at the peptide level,and its accuracy is higher than that of computer algorithms.The combination can be used to establish a high-affinity peptide database of high-frequency HLAs in human populations.The limitation of this method is that experiments are complex with a long period[34-36].
The two methods introduced above mainly depend on the affinity between candidate peptides and HLA molecules.However,not all candidate peptides with high affinity can induce a strong immune response.Therefore,functional experiments,in which neoantigens are screened and identified based on their susceptibility to killing by specific T cells,need to be performed.
Thus,the third method is constructing a library and performing cytotoxic experiments to screen and identify neoantigens.This method is superior at present.Luet al[37]cultured and activated tumor-infiltrating lymphocytes(TILs)from patients as effector cells and constructed target cells transfected tandem minigene libraries.Through detecting the secretion of interferon-γ(IFN-γ)in cytotoxic experiments,they successfully obtained two melanoma neoantigens[37].Since TILs from the HCC tissue samples could also be cultured and activated[38],this method is also feasible for the screening and identification of HCC neoantigens.However,there are some shortcomings of this method.On the one hand,it is inefficient to monitor the immune response by detecting the secretion of IFN-γ based on enzyme linked immunosorbent assay(ELISA),and the experimental steps are cumbersome.On the other hand,some nonnatural genes can be artificially generated by stringing genes together,which can produce systematic errors.Recently,Kulaet al[39]established a high-throughput platform called T-Scan to screen and identify antigens that can be specifically recognized by T cells.T-Scan uses lentiviral delivery of antigen libraries into cells for endogenous processing and presentation on HLA molecules.Target cells functionally recognized by T cells are isolated using a reporter for granzyme B activity,and the antigens mediating recognition are identified by next-generation sequencing[39].Such a platform can be used as a more optimized screening and identification tool for HCC neoantigens.
If HCC tumors can be induced to have more neoantigens,we could screen and identify more quality epitopes.Russoet al[40]found that targeted inhibition of epidermal growth factor receptor/B-raf could reduce gene repair and increase the mutation of genes and antigens in colorectal cancers[40].In the treatment of non-smallcell lung cancer,Formentiet al[41]found that radiotherapy could not only promote the activation of antitumor T cells but also partly induce the exposure of immunogenic mutations[41].In addition,another study showed that combination treatment with sialic acid and a histone deacetylase inhibitor in neuroblastoma could upregulate the expression of the GD2 antigen,which is the main target of neuroblastoma immunotherapy[42].Whether the above strategies that could improve antigen exposure and antigen expression can be used to increase the number and quality of HCC neoantigens is worthy of in-depth exploration.
APPLICATION OF HCC NEOANTIGENS AS TARGETS IN IMMUNOTHERAPY
Donget al[43]screened and identified some HCC neoantigens and evaluated the proportion and immune function of T cells recognizing them by peptide-HLA tetramer T-cell responses against predicted neoantigens and ELISAin vitro,which confirmed that HCC neoantigen-specific T cells have good killing functionin vitro[43].TP53is the most commonly mutated gene in HCC.Yanget al[4]found that patients with a TP53 neoantigen had longer overall survival,higher immune score,better prognosis,higher cytotoxic lymphocyte(CTL)infiltration,and higher cytolytic activity score than patients without.However,the prognosis of the patients was not correlated with TMB or neoantigen burden[44].Moreover,a study analyzed 115 cases from the TCGA database and found that somatic mutations and neoantigen burden were not associated with progression-free survival in HCC patients who did not receive immunotherapy.In patients with high expression of granzyme A,a direct correlation between the number and quality of neoantigens and survival is observed.Such evidence shows that neoantigens are useful only when the cytotoxic activity of TILs is high[45].The same results may be observed in cases of high expression of granzyme B and IFN-γ,which may be closely related to the fact that HCC is an immunosuppressive tumor[46].TILs in HCC often show an exhausted phenotype with low cytotoxic activity[47].An increasing number of genes related to TIL function exhaustion have been found,such asPD-1[48],programmed death-ligand 1[49],andCTLA4[50].Various targeted inhibitors aimed at these genes have been developed.Recently,nivolumab,which is a fully human immunoglobulin G4 anti-PD-1 monoclonal antibody,was approved by the Food and Drug Administration for liver cancer as a second line treatment after failure of sorafenib based on the data of the multi-cohort phase 1/2 trial CheckMate-040[51].Using combination treatment with interleukin-2(IL-2)[52]and other cytokines,TIL function exhaustion can be reversed.
To further maximize the effect of neoantigen vaccines,researchers have developed a variety of new technologies.The latest research has shown that expressing neoantigens in chimeric HBV core antigens is a promising option that can selectively induce tumorspecific effector CD8+ T cell activation through DNA vaccination.In this study,the researchers constructed a Db/Sp244-252/R251H neoantigen epitope(changing an amino acid site)in the EndoB2-Sp protein and found that a single injection of the EndoB2-Sp expression vector into C57Bl/6j mice could effectively induce activation of IFN-γ+CD8+ T cells specifically targeting the neoantigen epitope(Db/Sp244-252/R251H).The expression of Db/Sp244-252/R251H within the core antigen of assembly deficient HBV induced a considerable number of CD8+ T cells specifically targeting Db/Sp244-252/R251H compared with the EndoB2-Sp vaccine[53].Zhanget al[54]used the low-toxicity cholesterol-modified antimicrobial peptide DP7(DP7-C),which has dual functions as a carrier and an immune adjuvant,to improve dendritic cell(DC)-based vaccine efficacy.It achieved promising antitumor effects in a mouse model.In addition,after stimulation with DP7-C,the antigen uptake efficiency of monocyte-derived DCs(MoDCs)in patients with advanced lung cancer increased from 14%-40% to 88%-98%,the antigen presentation efficiency increased from approximately 15% to approximately 65%,and the proportion of mature MoDCs increased from approximately 20% to approximately 60%[54].The antigen presentation strategy targeting mannose receptor on the surface of DC cells can also greatly increase the efficiency of antigen presentation,and clinical studies have shown that it has good safety and efficacy[55].In addition,Niet al[56]invented a bi-adjuvant neoantigen nanovaccine(banNV).In this formulation,two adjuvants were added to the original single neoantigen vaccine:The Toll-like receptor(TLR)7/8 agonist R848 and the TLR9 agonist CpG.The immunogenicity of the neoantigen was increased,and the acute systemic toxicity was reduced.In combination with anti-PD-1 therapy,banNV achieved a superior effect on colorectal cancer[56].We can also use the above new techniques to exploit application of neoantigens in HCC immunotherapy.
Therefore,on the one hand,efficiently screening and identifying quality neoantigens in HCC,evaluating the existing HCC-related antigens,and enhancing their quantity and quality by means of some strategies that can improve antigen exposure and antigen expression are goals for future studies.Such studies might provide a series of antigen targets with relatively high-level immunogenicity for accurate treatment of HCC.On the other hand,ICIs,some cytokines,such as IL-2,and a variety of new technologies should be applied to activate and expand TILs to generate sufficient numbers of powerful T cells,which will enable achievement of effective control of HCC in humans.This is a comprehensive strategy for the exploitation of neoantigens in HCC immunotherapy in the future.
According to ClinicalTraials.gov(https://clinicaltrials.gov/ct2/home),there are only five clinical studies related to HCC neoantigen vaccines,and no promising results have yet been generated(Table 1).With the rapid development of high-throughput sequencing and bioinformatics technologies,more efforts exploring neoantigens should be made to achieve effective treatment of HCC.A key requirement of such research is determining how to establish effective methods to screen and identify a large number of quality HCC neoantigens.To improve the accuracy of the screening and identification of HCC neoantigens,we should adopt more advanced HCC neoantigen prediction algorithms and more sensitive and less time-consuming methods of screening and identificationin vitro.A personalized method of screening and identification and a universal library of HCC neoantigens should be established,and a unique HCC neoantigen therapeutic strategy should be adopted to open a new avenue for HCC immunotherapy.

Table 1 Clinical trials of hepatocellular carcinoma neoantigen vaccines
CONCLUSION
There are a variety of events leading to the generation of HCC neoantigens.The study of HCC neoantigens should not only focus on mutation-induced neoantigens but also consider neoantigens generated by multiple paths and the characteristics of HCC.The screening and identification methods used for HCC neoantigens should be optimized considering the above factors.In addition,since HCC is an immunosuppressive tumor,strategies that reverse immunosuppression and enhance the immune response should be considered for the practical exploitation of HCC neoantigens.
ACKNOWLEDGEMENTS
We thank Xing-Wang Xie(Peking University People’s Hospital)and Wei-Jia Liao(Affiliated Hospital of Guilin Medical University)for helpful suggestions.
 World Journal of Gastrointestinal Oncology2021年7期
World Journal of Gastrointestinal Oncology2021年7期
- World Journal of Gastrointestinal Oncology的其它文章
- Laparoscopic liver resection for colorectal liver metastases — shortand long-term outcomes:A systematic review
- High expression of protein phosphatase 2 regulatory subunit B''alpha predicts poor outcome in hepatocellular carcinoma patients after liver transplantation
- Robotic resection of duodenal gastrointestinal stromal tumour:Preliminary experience from a single centre
- Cryptotanshinone inhibits cytotoxin-associated gene A-associated development of gastric cancer and mucosal erosions
- Clinical management for malignant afferent loop obstruction
- Sporadic fundic gland polyps with dysplasia or carcinoma:Clinical and endoscopic characteristics
