Recent Advances in Surface-Modified g-C3N4-Based Photocatalysts for H2 Production and CO2 Reduction
Yunfeng Li , Min Zhang , Liang Zhou , Sijia Yang , Zhansheng Wu , Yuhua Ma
1 College of Environmental and Chemical Engineering, Xi’an Polytechnic University, Xi’an 710000, China.
2 College of Chemistry and Chemical Engineering, Xinjiang Normal University, Urumqi 830054, China.
Abstract: Solar energy is the largest renewable energy source in the world and the primary energy source of wind energy, tidal energy, biomass energy, and fossil fuel. Photocatalysis technology is a sunlight-driven chemical reaction process on the surface of photocatalysts that can generate H2 from water,decompose organic contaminants, and reduce CO2 into organic fuels. As a metal-free polymeric material, graphite-like carbon nitride (g-C3N4) has attracted significant attention because of its special band structure, easy fabrication, and low costs. However, some bottlenecks still limit its photocatalytic performance.To date, numerous strategies have been employed to optimize the photoelectric properties of g-C3N4, such as element doping, functional group modification, and construction of heterojunctions. Remarkably, these modification strategies are strongly associated with the surface behavior of g-C3N4, which plays a key role in efficient photocatalytic performance. In this review, we endeavor to provide a comprehensive summary of g-C3N4-based photocatalysts prepared through typical surface modification strategies (surface functionalization and construction of heterojunctions) and elaborate their special light-excitation and response mechanism,photo-generated carrier transfer route, and surface catalytic reaction in detail under visible-light irradiation. Moreover, the potential applications of the surface-modified g-C3N4-based photocatalysts for photocatalytic H2 generation and reduction of CO2 into fuels are summarized. Finally, based on the current research, the key challenges that should be further studied and overcome are highlighted. The following are the objectives that future studies need to focus on: (1) Although considerable effort has been made to develop a surface modification strategy for g-C3N4, its photocatalytic efficiency is still too low to meet industrial application standards. The currently obtained solar-to-hydrogen (STH) conversion efficiency of g-C3N4 for H2 generation is approximately 2%, which is considerably lower than the commercial standards of 10%. Thus,the regulation of the surface/textural properties and electronic band structure of g-C3N4 should be further elucidated to improve its photocatalytic performance. (2) Significant challenges remain in the design and construction of g-C3N4-based S-scheme heterojunction photocatalysts by facile, low-cost, and reliable methods. To overcome the limitations of conventional heterojunctions thoroughly, a promising S-scheme heterojunction photocatalytic system was recently reported.The study further clarifies the charge transfer route and mechanism during the catalytic process. Thus, the rational design and synthesis of g-C3N4-based S-scheme heterojunctions will attract extensive scientific interest in the next few years in this field. (3) First-principle calculation is an effective strategy to study the optical, electrical, magnetic, and other physicochemical properties of surface strategy modified g-C3N4, providing important information to reveal the charge transfer path and intrinsic catalytic mechanism. As a result, density functional theory (DFT) computation will be paid increasing attention and widely applied in surface-modified g-C3N4-based photocatalysts.
Key Words: Photocatalysis; H2 generation; CO2 reduction; Surface modification; Heterojunction
1 Introduction
The renewable energy technologies should be developed to address the global warming caused by carbon dioxide from fossil fuel consumption and to build a sustainable society1–5. Solar energy, as the largest renewable energy of the world, is the primary energy source of wind energy, tidal energy, biomass energy and fossil fuel6–10. Photocatalysis technology is a sunlight-driven chemical reaction process on the surface of photocatalysts that can generate H2from water11–14, decompose organic contaminants15–17, photocatalytic sterilization18–20and reduce CO2into organic fuels21–24. In recent years, the graphite carbon nitride (g-C3N4) has emerged as a “star” material in photocatalysis field due to its special band structure and easy fabrication25–28. g-C3N4with a narrow bandgap of 2.7 eV, could be excited by visible light irradiation that shows a large potential value for the solar energy utilization and conversion29–32.However, there are still some bottlenecks that limit the photocatalytic activities of g-C3N4, such as rapid recombination of photo-induced charges, limited active sites for surface catalytic reactions and low specific surface area,etc33–35.
The photocatalytic reaction of g-C3N4involves three steps,including (1) photo-generation of carriers excited by visible light, (2) charge carriers transfer to catalyst surface, (3) electrons and holes initiate chemical reactions (Fig. 1). In addition, the photo-induced charges may recombine due to the effect of coulombic force36–38. In order to obtain excellent photocatalytic activity, the photocatalyst should have a wide light response range, rapid carriers transfer rate, and high redox ability. So far,many methods have been tried to optimize the photocatalytic activities of g-C3N4, such as doping elements39–41, functional group modification42–44and constructing heterojunctions45–47,etc. Remarkably, these modification strategies are strongly associated with the surface behavior of g-C3N4that plays a major role in efficient photocatalytic performance. On the one hand, all the oxidation or reduction reactions involved in photocatalytic process occur at the surface of photocatalysts. On the other hand,the surface behavior of photocatalysts will influence the charge carriers separation and migration. In addition, some special surface modifications, such as elements doping, surface defect engineering and functional group grafting, can also optimize the band structure of g-C3N4to greatly enhance its light utilization.Thus, surface modifications over g-C3N4have attracted much attention owing to its significant advantages in improving photocatalytic performance48.
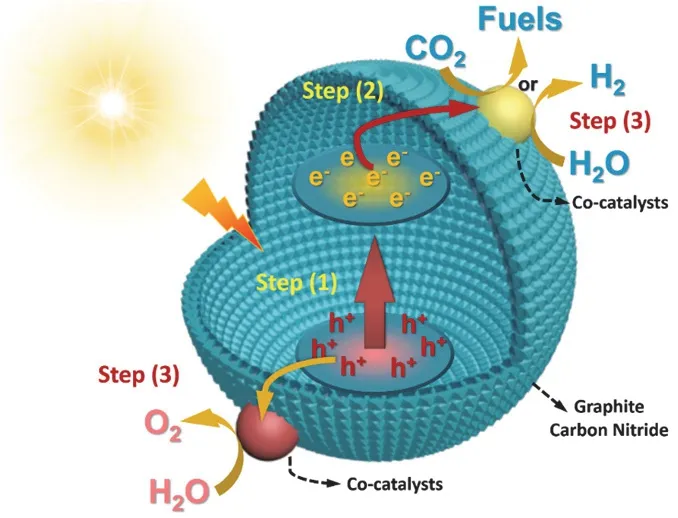
Fig. 1 The photocatalytic reaction steps of g-C3N4 driven by visible light irradiation.
According to the different characteristics of above surface behavior, the surface modification over g-C3N4can be summarized from two perspectives: (1) surface functionalization including functional group modification, nonmetal element modification, metal element modification and surface defect modification; (2) constructing heterojunction leads to the composite system with heterostructure, such as step-scheme (Sscheme) heterojunction, type-II heterojunction and direct Zscheme heterojunction,etc. The classification of typical surface modification of g-C3N4is summarized in Fig. 2. The rational development and design of novel surface strategy is considered to be a significant route to create the excellent g-C3N4-based photocatalysts for the application in environment and energy fields.
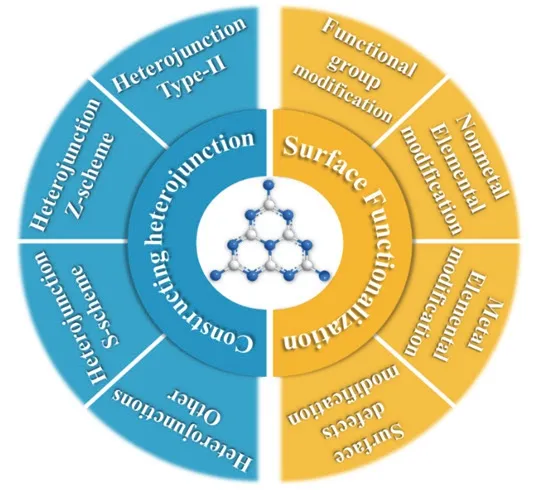
Fig. 2 The classification of typical surface modification for g-C3N4 photocatalyst.
In this review, we try to give a comprehensive summary of g-C3N4-based photocatalysts prepared through the typical surface modification strategies, and introduce their special light excitation and response mechanism, photo-generated carriers transfer route and surface catalytic reaction in detail under visible light irradiation. Moreover, the potential applications of surface modified g-C3N4-based photocatalysts in photocatalytic H2generation and reduction CO2into fuels have been also introduced. Finally, according to the present research, the key problems to be further studied and solved are put forward.
2 Basic principles of g-C3N4 photocatalysis process
Up to now, the g-C3N4-based photocatalysts have been widely
studied in contaminant degradation, sterilization, H2evolution and CO2reduction,etc. Due to the relatively low potential of conduction band (CB) for g-C3N4, the photo-generated electrons on its CB show a strong reduction ability to initiate the surface reduction reactions. Based on this band structure characteristic,g-C3N4has proven to be a promising photocatalysts in photocatalytic H2generation and CO2reduction.
2.1 Basic principles of photocatalysis H2 production
The use of sunlight for overall water splitting is considered to be a major chemical challenge. According to the thermodynamic requirements, the CB potential of photocatalyst must be more negative than the potential of H+/H2, while the valence band(VB) potential must be more positive than the potential of O2/H2O6. For example, the CB position of g-C3N4usually locates at −1.1 eV, thus it is more negative than the hydrogen production potential. However, the low efficiency of g-C3N4for overall water splitting is mainly due to the complicated oxygen evolution reaction involving four electrons, as well as the fast recombination of photo-induced charges. Notably, the H2generation by g-C3N4-based photocatalysts (half reaction of H2O splitting) possesses a greater advantage than overall H2O splitting owing to the relatively high quantum efficiency.
2.2 Basic principles of photocatalysis CO2 reduction
The increasing CO2content in the atmosphere is an important factor to generate the greenhouse effect to make global warming.As we all know, CO2is mainly produced by the combustion of fossil fuel in industrial development. In addition, fossil fuels are still the major energy source over the coming decades.Therefore, it is urgent to develop some feasible emission reduction strategies to reduce the CO2in the environment.Inspired by natural photosynthesis, which uses CO2and H2O to produce hydrocarbons and oxygen by utilizing solar energy, they can generate energy and reduce CO2in the atmosphere at the same time. When the photocatalysts are excited by radiation light, the generated charge carriers will migrate to the surface of photocatalyst to interact with the CO2molecules adsorbed at the surface. Especially, the photocatalytic CO2reduction reaction contains the CO2reduction and H2O oxidation involving C―O bond breaking and C―H bond creating. Thus, the photoreduction of CO2is not a single-electron reaction process,instead, it is a proton-induced multi-electrons reaction containing the following process8:
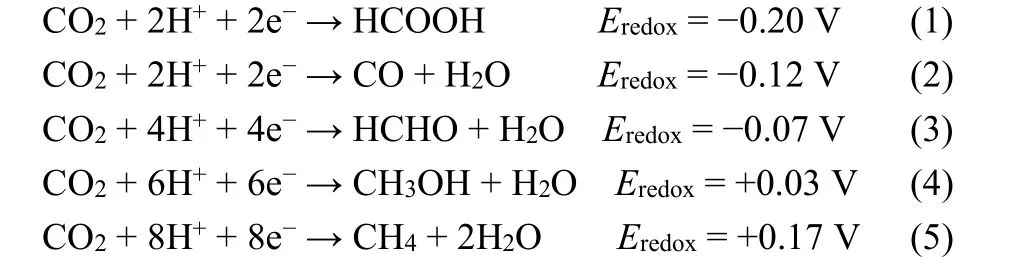
Because of the high bond energy, all these half-reactions are difficult to trigger. In addition, the linear CO2molecule shows a high stability, which also increases the difficulty of these reactions. Although a lot of studies have been done to improve the CO2conversion rate of g-C3N4, there are still great challenges.
3 Typical surface modification strategies of g-C3N4 photocatalyst
Surface engineering is a basic method to improve the photocatalytic performance of g-C3N4. Considering the diversity and complexity of surface strategies, a reasonable classification is the prerequisite to summarize and discuss the g-C3N4photocatalyst used for photocatalytic H2evolution and CO2reduction. According to the different characteristics of above surface behavior, the surface modification over g-C3N4can be summarized from two perspectives: surface functionalization and constructing heterojunction48.
Surface functionalization is the strategy that optimizes and regulates the basic structure units of g-C3N4through introducing the metal/nonmetal elements, organic functional group or only tune the melon units of g-C3N4without introducing other external components. Specifically, doping modification of g-C3N4can be defined as a process of introducing external elements into melon units of g-C3N4to optimize its optical, electrical,magnetic and other physicochemical properties. Doping modification of g-C3N4can be divided into nonmetal doping and metal doping. Functional group modification is considered to be a molecular doping process to optimize the inherent conjugate system, optical and electrical properties, as well as the band structure of g-C3N4. Due to the characteristics of conjugate structure, the physicochemical properties of g-C3N4could be effectively improved through the modification of the special aromatic groups or organic units by using the copolymerization reaction. Defect modification is another typical strategy to adjust the surface parameter of g-C3N4through some special surface treatment methods without introducing other external components. Defect modification can not only enrich surface catalytic active sites, but also change the electronic band structure of g-C3N4.
Constructing heterojunction is the strategy that employs g-C3N4as a kind of visible-light photocatalyst and another semiconductor with a suitable electronic band structure (or a cocatalyst) are loaded at the surface of g-C3N4to construct g-C3N4-based heterojunction photocatalytic system. Combining g-C3N4with other photocatalytic materials (or metals) to construct heterojunction shows the great advantages in raising the separation efficiency of photo-generated carriers and promoting the reduction and oxidation sites separation in space for improved photocatalytic H2evolution and CO2reduction.According to the characteristic of charges transfer route and photocatalytic mechanism, the typical g-C3N4-based heterojunctions have been divided into type-II heterojunctions,direct Z-scheme heterojunctions, S-scheme heterojunctions, and other heterojunctions,etc(Fig. 3).
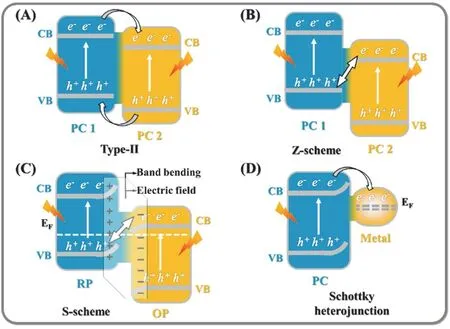
Fig. 3 The charges transfer route of typical g-C3N4-based heterojunction photocatalysts.
4 Recent advances of surface modified g-C3N4-based photocatalysts
The rational development and design of novel surface strategy is a promising route to create more efficient g-C3N4-based visible-light-driven photocatalytic materials for environmental and energy applications due to its significant advantages in separating photo-generated charges, improving sunlight utilization and promoting surface catalytic reaction, thus attracting much attention in recent years, especially in the fields of photocatalytic H2generation and CO2reduction.
4.1 Surface functionalization
Surface functionalization can improve photocatalytic performance of g-C3N4from regulation and optimization its basic structure units (molecular level), which shows an obvious difference compared with the strategy of constructing heterojunction. Surface functionalization mainly involves functional group modification, nonmetal element modification,metal element modification and surface defect modification.
4.1.1 Functional group modification
Regulating the molecular structure of g-C3N4to expand its light response and reduce recombination of photo-induced charges is an effective method to enhance the photocatalytic performance of g-C3N449–52. Considering the organic properties of g-C3N4conjugate structure, tuning molecular composition by copolymerization to prepare the g-C3N4-based photocatalysts shows a high feasibility. Notably, the diversity of organic reaction can provide various ways of design supermolecules using nitrogen-rich precursors and co-monomers to modify g-C3N4. The surface properties, texture and electronic structure of g-C3N4can be optimized by introduction of the special functional groups into g-C3N4conjugate system through copolymerization53–55.
Grafting aromatic compounds into melon networks will change the electronic distribution of g-C3N4system, thus optimizing its intrinsic electronic/optical properties56–59. For example, a general and effective Schiff base chemical method for constructing of aromatic grafted g-C3N4-based copolymer was reported by Shiet al. According to this method, various aromatic hydrocarbons could be grafted into the melon units. All the as-prepared g-C3N4shows an outstanding photocatalytic H2generation rate under visible light. In addition, the co-monomers used in this study are low cost with small adding amount, which may be one of the most promising strategies to prepare the g-C3N4-based photocatalysts60. Zhenget al.61introduced the benzene ring into g-C3N4networks to adjust the local symmetry without altering its long-order structure. Theoretical calculation indicates that introduction of benzene ring can optimize the electronic structure and accelerate the charges transfer of g-C3N4. The prepared optimum sample shows a 10.8 times higher H2generation rate than that of bare g-C3N4with the quantum efficiency of 11.3% at 400 nm.
Besides grafting the aromatics into the carbon nitride networks, sulfur doping terminal-methylated g-C3N4nanosheet(SMCN) with an adjustable bandgap was fabricated by using thioacetamide as the co-monomer of urea (Fig. 4). Density functional theory (DFT) indicates that doping S atom into the methyl modified melon networks could induce the VB near Fermi level to form the special midgap state that results in a great reduction of bandgap (~0.7 eV). The as-synthesized samples show an excellent photocatalytic H2generation rate62.
4.1.2 Nonmetal element modification
Doping of g-C3N4is a process of introducing external elements into melon units to optimize its optical, electrical,magnetic and other physicochemical properties. In the field of photocatalysis, bandgap engineering through doping nonmetals,or the co-doping with multiple elements plays a key role in optimizing the light absorption and electronic band structure of g-C3N4. Up to now, many studies on the nonmetal elements doping such as C63,64, S65,66, O67,68, P69,70, B71,72and halogen element have been reported73,74.
It is well known that modifying the electronic band structure of g-C3N4is an efficient method to enhance the light absorption and charges transfer75. Thus, Liuet al.71introduced boron (B)into the “empty position” between the melon frameworks through the interaction with two-coordinated nitrogen atom.DFT studies indicate that the photo-generation of charges in B doping g-C3N4from N (2px, 2py) to B (2px, 2py) with the same orbital direction is much easier than N (2px, 2py) to C 2pzin pure g-C3N4leading to a fast carriers migration. In addition, B atoms doping could absorb more CO and acts as the reaction sites for CH4generation. The obtained optimum photocatalyst shows a higher selectivity of CH4with about 32 times higher efficiency compared to that of bare g-C3N4.
In addition, the phosphorus doping g-C3N4nanoflakes(PCNNFs) were synthesized by employing phytic acid biomass as phosphorus source and urea as g-C3N4precursor. Especially,PCNNFs possess a narrowed bandgap from VB to the midgap electronic states resulting in a broadened light absorption up to 800 nm (Fig. 5). The fragmented nanoflakes structure of PCNNFs also reduces the charges transfer distance from the internal of catalyst to its surface. Owing to the effective phosphorus doping, as well as the special two-dimensional structure, PCNNFs show an outstanding photocatalytic H2evolution rate of 15.92 mmol·h−1·g−1with apparent quantum efficiency of 6.74% at 420 nm69. In addition, some DFT calculations for the influence of nonmetal doping on the molecular structure, photoelectric performance and photocatalytic activity of g-C3N4was also investigated and studied76,77.
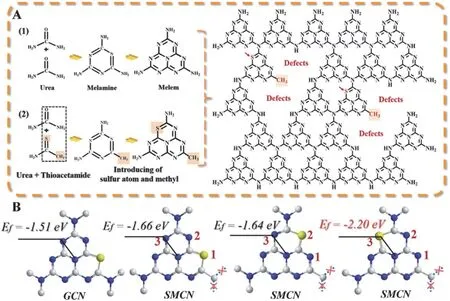
Fig. 4 (A) Illustration for fabrication of the S doping methyl-modified g-C3N4. (B) The formation energy (Ef) of substitute possible N atoms by S atoms in g-C3N4 networks.
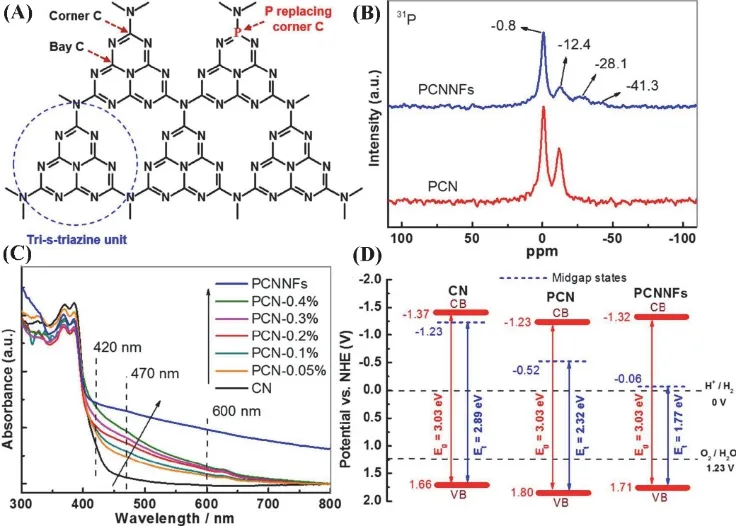
Fig. 5 (A) Illustration of g-C3N4 networks with P replacing corner C. (B) 31P MAS NMR spectra of the samples.(C, D) Electronic band structure of the prepared samples.
4.1.3 Metal element modification
In contrast to nonmetal doping, doping metals such as Cu78,Mo79, Pd80, Mn81, Eu82, Fe83and alkali metals84, has also been widely studied to optimize the photoelectric performance of g-C3N4by efficiently expanding light response, narrowing bandgap, promoting carriers transfer to increase the photocatalytic activity. In fact, the three-coordinated nitrogen atoms connecting the melon units usually exist in the molecular structure of g-C3N4. The formed nitrogen pots containing six nitrogen lone pair electrons are considered to be the suitable space for accommodating the metal ionsviaan ion coordination process in the melon networks.
For example, Jianget al.80prepared palladium doping g-C3N4(Pd-g-C3N4) through the strong coordination of palladium atoms with the pyridinic nitrogen atoms of six-fold cavities in g-C3N4.The as-prepared Pd-g-C3N4photocatalysts show a much higher H2generation rate than that of pure g-C3N4. The highest H2generation rate of Pd-g-C3N4is about 15.3 times that of pure g-C3N4. The enhancement of H2generation performance is attributed to both the optimization of charges excitation mode and the promotion of H2generation kinetics induced by palladium.
As we know, the photocatalytic CO2reduction performance is greatly inhibited by the limited light response range and high recombination rate of photo-induced charges. It is an effective method to increase the efficiency of CO2photo-reduction by doping alkali metals into g-C3N4framework to engineer its electronic properties. Xuet al.85reported an interesting study on K doping g-C3N4(KCN) being employed for photocatalytic CO2reduction. DFT calculations and experimental results indicate that K doping is interlayer doping, optimizing the electronic band structure of g-C3N4. KCNs show an extended light response range and rapid photo-induced charges transfer. As a result, the optimum sample exhibits an outstanding CO2reduction performance with CO formation rate of 8.7 umol·g−1·h−1, which is 25 times that of pure g-C3N4.
4.1.4 Surface defects modification
Defect engineering is an interesting strategy for optimizing the photocatalytic efficiency of semiconductor materials. It is well known that defect engineering on g-C3N4can effectively enhance the charges separation, optimize the band structure and expand the light response86–89. Thus, various surface defects of g-C3N4, such as carbon vacancies90, nitrogen vacancies91,cyanamide defects92and structural edge defects93have been widely investigated in recent years for improving the photocatalytic performance of g-C3N4in CO2reduction and H2production.
Recently, Tanget al.92successfully prepared the cyanamide defects modified g-C3N4through using thiourea as precursor with the assistance of potassium chloride. Potassium isothiocyanate can bein situformedviathiourea isomerization and they could react with the amino groups (―NH2and =NH)in melon units to generate the two-type cyanamide defects. The separation efficiency of photo-induced charges is greatly enhanced. Moreover, the cyanamide defects could also inhibit the generation of in-plane hydrogen bonds between the melon units, leading to the special porous structure with numerous active sites for photocatalytic H2evolution.
In addition, Zhanget al.94designed a new strategy to prepare the g-C3N4that possesses the rich porous structure,heterostructure defects with sulfur atom doping through treating g-C3N4in the existence of both CH3CN and H2S. The original g-C3N4nanosheets are etched to generate nanopore under this special gaseous environment. Moreover, the melon units are destroyed to form the C―S bond, cyano groups and atomic S due to the incomplete conversion of H2S. The prepared photocatalysts show an excellent band structure, extended light absorption and fast carriers transport (Fig. 6), which results in tremendously enhanced photocatalytic performance of H2O splitting.
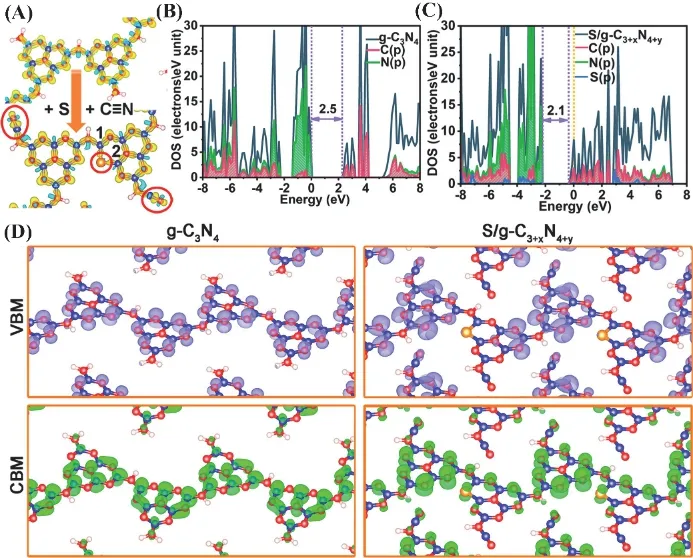
Fig. 6 (A) Charge density difference of the prepared samples.(B–D) Electronic band structure of the samples obtained from DFT calculation.
4.2 Heterojunction
For photocatalytic H2production and CO2reduction, as we all know that it is impossible for the single semiconductor photocatalyst to exhibit a broad light response range, high carriers separation rate and strong redox ability simultaneously.Thus, only rational fabrication of g-C3N4-based heterostructure photocatalysts with other semiconductors (or metals) is expected to solve these issues above95–98. Thus, constructing g-C3N4-based heterojunction photocatalytic systems, such as type-II heterojunction, Z-scheme heterojunction, S-scheme heterojunction, P-N heterojunction and Schottky heterojunction have been prepared and widely studied in photocatalytic H2production and CO2reduction.
4.2.1 Type-II heterojunction
Through establishing a unique contact interface between the two kinds of photocatalytic materials that possess the special staggered band structure, the spatial separation of photo-excited charges could be achieved, which is known as the conventional type-II heterojunction. As we all know, the CB potential of g-C3N4is usually at −1.1 eV, thus it is commonly more negative than that of most other photocatalysts. Therefore, the photoinduced electrons can transfer from the CB of g-C3N4to the CB of other semiconductors. At the same time, the photo-excited holes will migrate in the opposite direction. The various g-C3N4-based type-II heterojunctions fabricated in recent years are shown in Table 199–116.
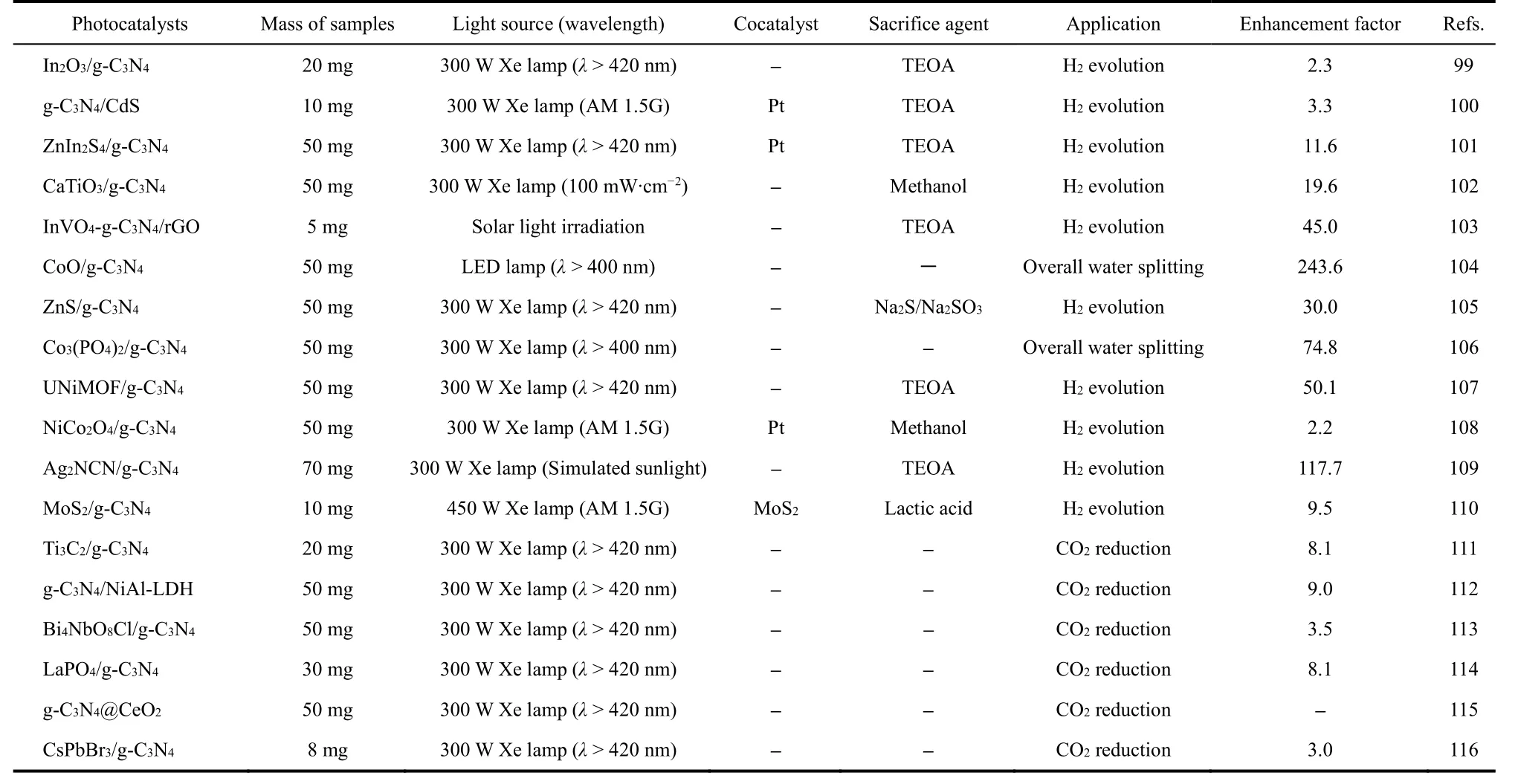
Table 1 The various g-C3N4-based type-II heterojunctions for photocatalytic H2 evolution and CO2 reduction reported in recent years.
4.2.2 Z-scheme heterojunction
The conventional type-II heterojunction seems to facilitate the charges transfer of photocatalysts, however, it still possesses some drawbacks. Through the oriented separation of the photogenerated charges, the electrons may enrich on the CB that possesses a less negative potential, while the holes will accumulate on the VB with a less positive potential, which greatly decreases the redox potential of the composite photocatalysts to induce catalytic reaction117–119. In addition,from the viewpoints of dynamics, the coulomb repulsion from the accumulative electrons in PC 2 may inhibit the continuous migration of electrons from PC 1. The similar phenomena could also be observed on the holes transfer (Fig. 3). Thus, the electrons and holes can not be separated efficiently according to the type-II charges transfer mechanism. In 1979, Bardet al.120reported the pioneering work of design and synthesis the Zscheme photocatalytic heterojunction. However, because the redox mediums are essential in this process, this Z-scheme system can be used only in the liquid phase. Therefore, it isextremely necessary to study the all solid state Z-scheme heterojunctions, and they will utilize solid materials as charge transfer mediums or even need not any mediums. Thus, Yuet al.121reported a new promising concept of heterojunction in 2013 without utilizing the charge mediums, that is defined as“direct Z-scheme transfer mechanism”. Owing to the great advantages of direct Z-scheme charge transfer mechanism, they have received extensive attention in recent years122–124.
Recently, Xuet al.125simultaneously combined the 2D structure and Z-scheme heterojunction to improve the photocatalytic efficiency of g-C3N4. As a result, the 2D/2D transition-metal-oxide/g-C3N4heterojunctions show a significant enhancement for photocatalytic H2generation compared to the pure g-C3N4. The quantum efficiency of the optimum Co3O4/g-C3N4heterojunction is about 53.6% atλ= 405 nm, and it is much higher than that of other g-C3N4-based heterojunction photocatalysts reported previously (Fig. 7).
In addition, the sheet-like ternary ZnO/ZnWO4/g-C3N4composite with double Z-scheme heterojunction was synthesized by Liet al.126. This double Z-scheme ZnO/g-C3N4/ZnWO4heterojunction not only further facilitates the separation and migration of photo-induced charges, but also endows the strong photo-reduction property (Fig. 8). As a result,this double Z-scheme heterojunction acquires a high-efficient conversion of CO2molecules into various solar fuels such as CO,CH4, CH3OH and CH3CH2OH, and the conversion rate of hydrocarbon fuel is highly up to 91.5%. The various g-C3N4-based Z-scheme heterojunctions for photocatalytic CO2reduction and H2production reported in the recent years are listed in the Table 26,8,117–119,123–126.
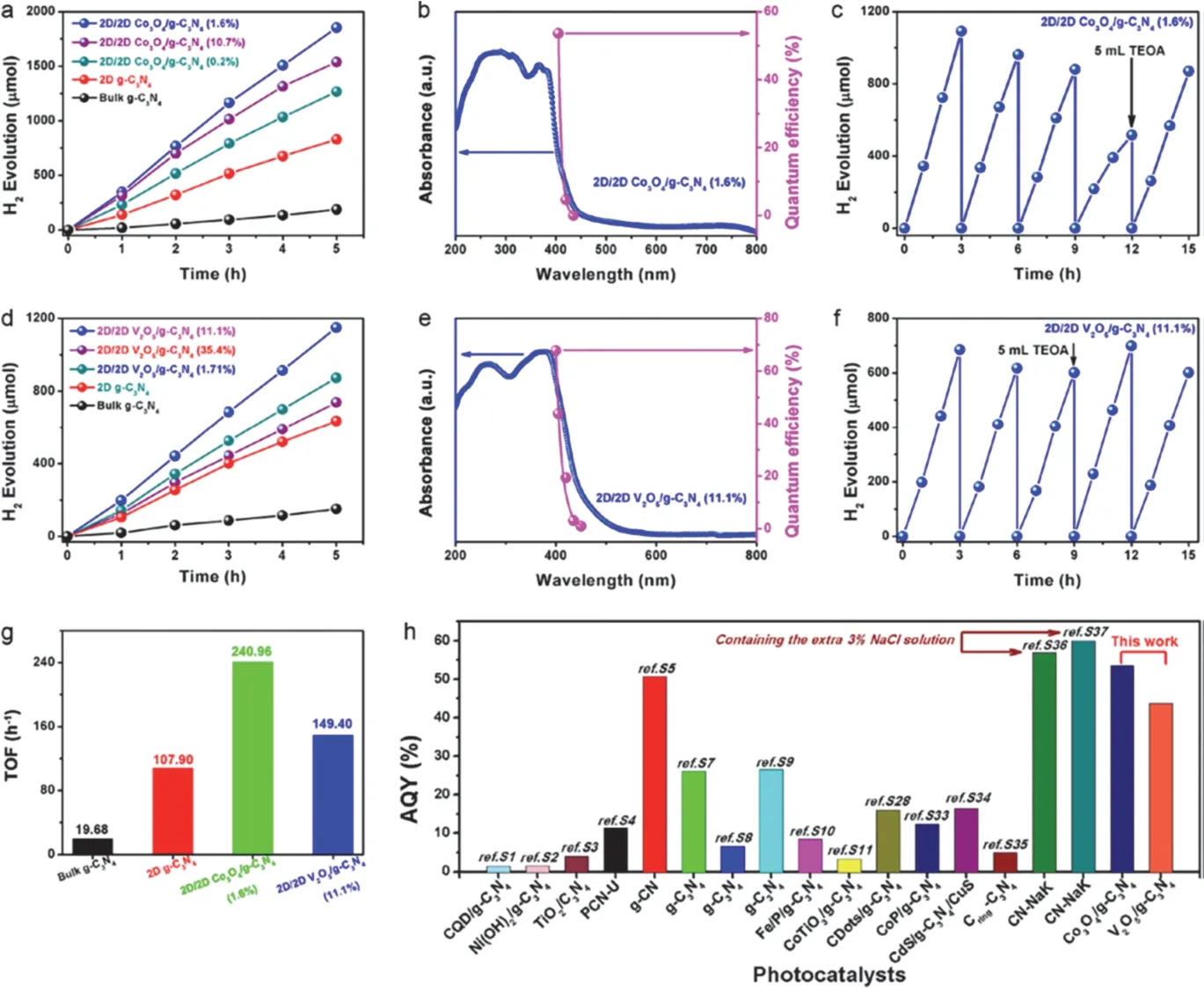
Fig. 7 Photocatalytic H2 evolution performance and apparent quantum efficiency of the prepared photocatalysts.
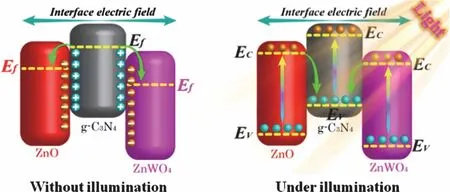
Fig. 8 Illustration of charges transfer route in the double ZnO/ZnWO4/g-C3N4 Z-scheme heterojunction.
4.2.3 S-scheme heterojunction
Based on the photosynthesis of plants, the artificial Z-scheme mechanism has been extensively utilized to reveal the photoexcited carriers migration process in photocatalytic reaction.Unfortunately, the current Z-scheme mechanism still shows some drawbacks to clarify the carriers migration and separation.For example, in traditional Z-scheme system, shuttle redox ion pairs, such as Fe3+/Fe2+and IO3−/I−, are indispensable to achieve charges separation. Thus, the traditional Z-scheme system can be used only in liquid phase, which greatly limits its application.Moreover, according to the traditional Z-scheme mechanism, the electron-hole pairs with a lower redox ability will be consumed with the assistance of shuttle redox ion pairs (Fig. 9A), leaving the charges with a higher redox ability to induce surface catalytic reaction. However, in fact, the electron-hole pairs with a higher redox ability will be easier to react with shuttle redox ion pairs in thermodynamics, as shown in Fig. 9B. The similar situation can also be observed in all solid state Z-scheme photocatalytic system (Fig. 9C,D).
In order to overcome the limitations of conventional heterojunctions thoroughly, Yuet al.127–129recently reported a promising S-scheme photocatalytic system, which consists of two kinds ofn-type semiconductor regarded as the oxidation photocatalyst (OP) and reduction photocatalyst (RP),respectively. The typical oxidation photocatalysts include WO3,TiO2, ZnO, Bi2MoO6, SnO2, CeO2and InVO4,etc. The typical reduction photocatalysts involve g-C3N4, CdS, MoS2, SnS2,MnS and In2O3,etc. The electrons will transfer from the Fermi level of RP to that of OP until the two systems attain equilibrium due to the difference of work function. This special electron transfer process can generate internal electric field and band bending, which accelerates the separation of photo-generated charges (Fig. 10). In macroscopic view, the carriers migration in the S-scheme photocatalytic system seems like a “step”, and it is similar to the “N” type in microscopic viewpoint130–133. Thus,design and preparation of novel g-C3N4-based S-scheme heterojunction photocatalysts has attracted more and more attention134–137.
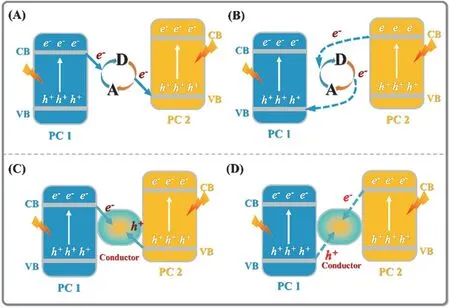
Fig. 9 Charges transfer route of (A, B) traditional Z-scheme heterojunction and (C, D) all solid state Z-scheme heterojunction.
Due to the high reduction ability of g-C3N4, a photocatalyst with a high oxidation ability is greatly beneficial for constructing the g-C3N4-based S-scheme photocatalytic system to maintain the strong redox ability. Notably, TiO2has received extensive attention owing to its high oxidation ability, low cost, and excellent structural stability. Thus, Yuet al.138designed and synthesized the 2D TiO2mesoporous nanosheets with several g-C3N4layers formedin situto fabricate a core-shell 2D/2D TiO2/g-C3N4S-scheme heterojunction. Then, the zerodimensional (0D) Ti3C2MXene quantum dots are successfully loaded on the surface of g-C3N4through electrostatic interactions (Fig. 11). The as-prepared S-scheme heterojunctions show an excellent CO2reduction performance.
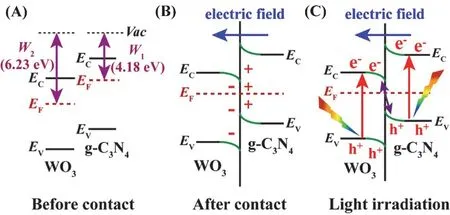
Fig. 10 The special S-scheme charges transfer route between WO3 and g-C3N4 nanosheets under visible light irradiation.
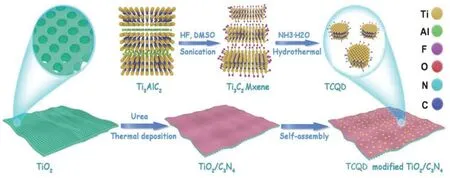
Fig. 11 Illustration for the preparation of ultrathin TCQD anchored TiO2/C3N4 S-scheme heterojunction.
As another metal oxide photocatalyst, tungsten oxide (WO3)with a high oxidation potential is considered as another ideal material to construct the S-scheme photocatalytic system with g-C3N4. Recently, Fanget al.139prepared the thin 2D/2D WO3/g-C3N4S-scheme heterojunction with carbon doping through employing anionic polyacrylamide as templet and carbon source. The S-scheme heterojunction, carbon doping as well as the WO3with oxygen vacancies lead to an efficient charge separation for photocatalytic reaction. The various g-C3N4-based S-scheme heterojunction photocatalysts reported in recent years are listed in the Table 2127,130,135–138.
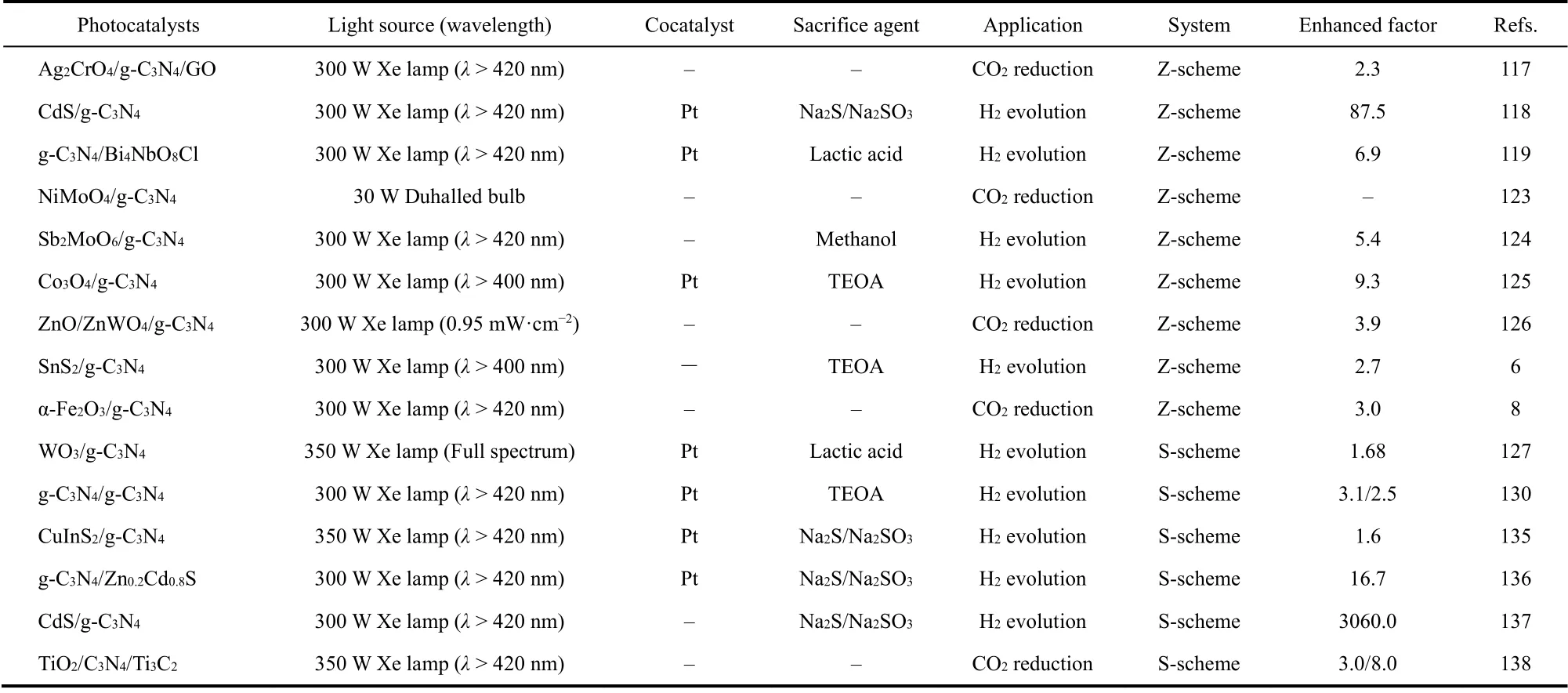
Table 2 The various g-C3N4-based Z-scheme and S-scheme heterojunctions for photocatalytic H2 evolution and CO2 reduction in recent years.
4.2.4 Other g-C3N4-based heterojunction systems
Besides the semiconductor-semiconductor heterogeneous photocatalytic system, the metals could also be used to fabricate the Schottky junction with g-C3N4to enhance its charge carriers migration140–142. Moreover, the metals or metallic compounds in g-C3N4-based Schottky heterojunction can provide the effective catalytic active sites, which further increases the photocatalytic performance of g-C3N4143–145. For example, the distinctive Cu/g-C3N4foam was successfully synthesized by Fanget al.146through combining template method and microwave method.The Schottky heterojunction effect between Cu-NPs and g-C3N4can accelerate the separation of photo-generated charges. The asprepared Cu/g-C3N4photocatalysts exhibit a remarkable photocatalytic CO2reduction performance with CO yield of 10.247 μmol·g−1·h−1. Moreover, Zhaoet al.147reported anin situpreparation route for g-C3N4/Ti3C2heterojunctionsviaone-step thermal treatment process. The formed Schottky heterojunction between g-C3N4and Ti3C2greatly suppresses the recombination of charge carriers, resulting in an increased photocatalytic H2evolution efficiency.
As we know, g-C3N4is a typical n-type semiconductor148–150and constructing of P-N heterojunction with ap-type semiconductor is also considered to be an efficient strategy to enhance the separation efficiency of photo-generated carriers.Notably, the Fermi level ofp-type semiconductor usually locates near its VB, while the Fermi level ofn-type semiconductor locates near the CB151–153. Thus, when they form the heterojunction, the electrons will transfer from the Fermi level ofn-type semiconductor to that ofp-type semiconductor until the two systems attain equilibrium. This special electron transfer process can generate an internal electric field, which promotes the photo-induced electrons transfer from the CB ofp-type semiconductor to that ofn-type semiconductor, and the photoinduced holes transfer in the opposite direction121. Up to now,many typicalp-type semiconductors, such as Bi5O7I154, Cu2O155,NiO156, BiOI157, Co3O4158, Ag2O159, α-AgAl0.4Ga0.6O2160have been employed to construct the P-N heterojunction with g-C3N4to achieve an enhanced photocatalytic activity.
5 Conclusions and future prospects
This review gives a comprehensive summary of g-C3N4-based photocatalysts prepared through the typical surface modification strategies and introduces their special light excitation and response mechanism, photo-generated carriers transfer route and surface catalytic reaction in detail. Moreover, the potential applications of surface modified g-C3N4-based photocatalysts in photocatalytic H2generation and reduction CO2into fuels are also introduced. Up to now, although the significant advances in surface strategy modified g-C3N4-based photocatalytic systems,their catalytic performance is still too low to meet the standard of industrial application. In order to solve this challenge, future study efforts are required in following aspects:
(1) Although much effort has been made to develop the surface modification strategy over g-C3N4, its photocatalytic efficiency is still too low to meet the standard of industrial application. For example, the currently obtained solar to hydrogen (STH) conversion efficiency of g-C3N4for H2generation is about 2%, which is much lower than the commercial standards of 10%. Thus, regulation of the surface/textural properties and electronic band structure for g-C3N4still should be further studied to greatly improve its photocatalytic performance.
(2) Significant challenges remain in design and construction of g-C3N4-based S-scheme heterojunction photocatalysts by facile, low cost and reliable methods. In order to solve the limitations of conventional heterojunctions thoroughly, Yuet al.recently reported a promising S-scheme heterojunction photocatalytic system that further clarifies the charges transfer route and mechanism during the catalytic process. Thus, the rational design and synthesis of g-C3N4-based S-scheme heterojunctions will attract extensive scientific interest in the next few years in this field.
(3) First principle calculation is an effective strategy to study the optical, electrical, magnetic and other physicochemical properties of surface strategy modified g-C3N4, such as element doping, defect modification and constructing heterojunction.The theoretical calculation could give us much important information to reveal the charges transfer path and intrinsic catalytic mechanism. As a result, DFT computation will be paid more and more attention and widely applied on surface strategy modified g-C3N4-based photocatalysts.
- 物理化学学报的其它文章
- Fluorinated TiO2 Hollow Photocatalysts for Photocatalytic Applications
- Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution
- 微波辅助快速制备2D/1D ZnIn2S4/TiO2 S 型异质结及其光催化制氢性能
- TiO2-Supported Single-Atom Catalysts for Photocatalytic Reactions
- Carboxyl-Functionalized Graphene for Highly Efficient H2-Evolution Activity of TiO2 Photocatalyst
- Review of Z-Scheme Heterojunctions for Photocatalytic Energy Conversion

