Carboxyl-Functionalized Graphene for Highly Efficient H2-Evolution Activity of TiO2 Photocatalyst
Ping Wang , Haitao Li , Yanjie Cao , Huogen Yu ,2,*
1 School of Chemistry, Chemical Engineering and Life Sciences, Wuhan University of Technology, Wuhan 430070, China.2 State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China.
Abstract: The use of semiconductor photocatalysts (CdS, g-C3N4, TiO2, etc.)to generate hydrogen (H2) is a prospective strategy that can convert solar energy into hydrogen energy, thereby meeting future energy demands. Among the numerous photocatalysts, TiO2 has attracted significant attention because of its suitable reduction potential and excellent chemical stability. However, the photoexcited electrons and holes of TiO2 are easily quenched, leading to limited photocatalytic performance. Furthermore, graphene has been used as an effective electron cocatalyst in the accelerated transport of photoinduced electrons to enhance the H2-production performance of TiO2, owing to its excellent conductivity and high charge carrier mobility. For an efficient graphene-based photocatalyst, the rapid transfer of photogenerated electrons is extremely important along with an effectual interfacial H2-production reaction on the graphene surface. Therefore, it is necessary to further optimize the graphene microstructures (functionalized graphene) to improve the H2-production performance of graphene-based TiO2 photocatalysts. The introduction of H2-evolution active sites onto the graphene surface is an effective strategy for the functionalization of graphene. Compared with the noncovalent functionalization of graphene (such as loading Pt, MoSx, and CoSx on the graphene surface), its covalent functionalization can provide a strong interaction between graphene and organic molecules in the form of H2-evolution active sites that are produced by chemical reactions. In this study, carboxyl-functionalized graphene (rGO-COOH) was successfully modified via ring-opening and esterification reactions on the TiO2 surface by using an ultrasound-assisted self-assembly method to prepare a high-activity TiO2/rGO-COOH photocatalyst. The Fourier transform infrared (FTIR) spectra, X-ray photoelectron spectroscopy (XPS), and thermogravimetric (TG) curves revealed the successful covalent functionalization of GO to rGOCOOH by significantly enhanced ―COOH groups in FTIR and increased peak area of carboxyl groups in XPS. A series of characterizations, including X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), XPS, and UV-Vis adsorption spectra, were performed to demonstrate the successful synthesis of TiO2/rGO-COOH photocatalysts. The experimental data for the hydrogen-evolution rate showed that the TiO2/rGO-COOH displayed an extremely high hydrogen-generation activity (254.2 μmol·h−1·g−1), which was 2.06- and 4.48-fold higher than those of TiO2/GO and TiO2, respectively. The enhanced photocatalytic activity of TiO2/rGO-COOH is ascribed to the carboxyl groups of carboxyl-functionalized graphene, which act as effective hydrogen-generation active sites and enrich hydrogen ions owing to their excellent nucleophilicity that facilitates the interfacial hydrogen production reaction of TiO2.This study provides novel insights into the development of high-activity graphene-supported photocatalysts in the hydrogen-generation field.
Key Words: Carboxyl-functionalized graphene; Carboxyl groups; TiO2; Photocatalytic H2 evolution
1 Introduction
Photocatalytic H2production utilizing semiconductor photocatalytic material is a greatly prospective way toward sustainable and clean energy sources over future energy demands1–8. The well-known TiO2is regarded as a prospective photocatalytic material owing to its chemical stability and nontoxicity9–14. Nonetheless, pure TiO2suffers from the speedy quenching of photoexcited carriers to exhibit a finite hydrogenproduction performance15–18. To surmount the deficiency and further boost the hydrogen-generation efficiency of TiO2,graphene as an effective electron cocatalyst has been used in the rapid transport of photoinduced electrons owing to its excellent conductivity and high mobility of charge carriers19–22.However, for an efficient graphene-based photocatalyst, besides the speedy electron transport, it is strongly required the effective H2-production active sites on graphene which can enrich H+from the solution23–25. Therefore, the seeking of effective strategies to introduce more interfacial hydrogen-ion absorption sites on graphene for the improved hydrogen-production efficiency of graphene modified-TiO2remains an important goal.
The functionalized graphene is one of effective strategies to graft interfacial catalytic sites on graphene owing to the introduction of useful adsorption sites or anchoring points26–28.Instead of the noncovalent functionalization, covalent functionalization of graphene can form a strong interaction between each of the four groups (carboxyl groups,ketone/aldehyde groups, hydroxyl groups and epoxides) on the graphene surface and organic compound by chemical reactions(nucleophilic ring-opening, acylation, diazotization,isocyanate/esterification, and cycloaddition reactions,etc.)29.Interestingly, the covalently carboxyl-functionalized graphene has been widely reported to increase the number of carboxyl groups and further graft more interfacial active sites for comprehensive application fields30–33. For example,Muhammadet al.34reported that carboxyl-functionalized graphene provided the enhanced active sites as the covalent attachment of the amine functionalized CdSe QDs to obtain a high sensitivity of the immunosensor. Liuet al.35investigated the carboxyl functional group of carboxyl-functionalized graphene could function as a catalytic site to effectively capture polyaniline through the interaction between carboxyl and amino groups for supercapacitor application to reach a high capacitance. Narayanet al.36described that the carboxylfunctionalized graphene with suitable level of structure defects(16.2%) served as an effectual electrode for high-performance supercapacitor due to higher electroactive surface area, less aggregation, wettability and hydrophilicity. Additionally, some researches also indicated that the covalently carboxylfunctionalized graphene can be applied in biological medicine,electrochemical energy storage and water treatment37–41.Considering that carboxyl groups can effectively enrich H+due to their excellent nucleophilicity, it is expected that carboxylfunctionalized graphene as hydrogen-adsorption catalytic sites can facilitate the photocatalytic H2-generation rate of TiO2.
In this paper, covalently carboxyl-functionalized graphene(rGO-COOH) by a simple and mild two-step organic reaction(ring-opening reaction and esterification reaction) was successfully modified onto TiO2for enhancing the photocatalytic H2-evolution rate of TiO2by ultrasound assisted self-assembly. The prepared TiO2/rGO-COOH presented an apparently promoted photocatalytic hydrogen-production rate in comparison with the TiO2and TiO2/GO. Moreover, the photocatalytic mechanism of TiO2/rGO-COOH had been explored, that is, the carboxyl groups of covalently carboxylfunctionalized graphene can effectually adsorb hydrogen ions to accelerate interfacial hydrogen evolution reaction of TiO2. This work may provide novel ideas to design high-efficiency functionalized graphene-supported photocatalytic materials.
2 Experimental and computational section
Graphene oxide (GO) was obtainedviathe modified Hummer’s method in accordance with our prior study42. The titanium dioxide (TiO2(P25), 99.5%), hydrobromic acid (HBr,40%), ethanedioic acid (HOOCCOOH, 99.5%) and ethanol solution (99.7%) were of analytical grade and provided through Shanghai Chemical Reagent Ltd.
2.1 Preparation of covalently carboxylfunctionalized graphene (rGO-COOH)
Covalently carboxyl-functionalized graphene was prepared by a two-step organic reaction (ring-opening reaction and esterification reaction). In brief, 50 mL of hydrobromic acid(HBr) was added into 300 mL of GO solution (2.5 mg·mL−1).After stirred for 12 h, 15.0 g of oxalic acid (HOOCCOOH) was mixed with the abovementioned solution and stirred for four hours. Finally, the dispersion filtered and dried at 60 °C in vacuum for 24 h. The obtained sample was referred to rGOCOOH.
2.2 Preparation of TiO2/rGO-COOH
The rGO-COOH-supported TiO2photocatalysts were synthesized by ultrasound assisted self-assembly. Briefly, TiO2(0.5 g) was immersed into the certain content of rGO-COOH solution (0.5 mg·mL−1). After stirred for two hours, the products were acquired by filtration and drying at 60 °C (12 h). The amounts of rGO-COOH were adjusted to be 0.5%, 1.0% and 2.0% (w), and the acquired photocatalysts named as TiO2/rGOCOOH (0.5%), TiO2/rGO-COOH (1%), and TiO2/rGO-COOH(2%), respectively. In contrast, the GO-supported TiO2photocatalyst (TiO2/GO) also prepared through a parallel process to TiO2/rGO-COOH (1%) of GO as a substitute for rGOCOOH.
2.3 Characterization
The XPS (X-ray photoelectron spectroscopy) spectra (Thermo Fisher EscaLab 250Xi XPS, U.K.), the FTIR (Fourier transform infrared spectra) (Nicolet6700, Thermo Electron Scientific Instruments, USA), and the TG (thermogravimetric) curves(SDT Q600 instrument, TA, USA) were used to confirm the convert from GO to rGO-COOH. The powder XRD (X-ray diffraction) patterns (Rotation Anode High Power X-ray Diffractormeter, Japan), the FESEM (field emission scanning electron microscope) (JSM-7500, JEOL, Japan), the TEM(transmission electron microscope) (JEM-2100F, JEOL, Japan)images, and the ultraviolet-visible absorption spectra (UV-2450,Shimadzu, Japan) of the obtained samples were analyzed.
2.4 Photoelectrochemical tests
The photoelectrochemical tests were measured with 0.5 mol·L−1of Na2SO4solution (CHI660E electrochemical workstation, China) in a conventional three-electrode configuration. The Ag/AgCl, Pt wire and the photocatalystcoated FTO act as reference, counter and working electrode,respectively. The working electrode was prepared on FTO glass according to our previous methods43,44. The LSV (linear sweep voltammetry) curve was detected with a bias range of −1.0 to−0.6 V (vsSHE) (scan rate: 10 mV·s−1) in the dark. Thei–tcurve was performed with a bias potential (+0.5 V) under a 365 nm LED lamp (80 mW·cm−2, 3 W) as light source. The corresponding electrochemical impedance spectroscopy (EIS)was conducted at a frequency range of 10−3to 106Hz at the opencircuit voltage with an ac amplitude of 10 mV.
2.5 Measurement of photocatalytic H2-evolution activity
The hydrogen-evolution rate of different samples was conducted in accordance with our prior approach45. Briefly, the photocatalysts (50 mg) were transferred into ethanol solution (80 mL, 25% (volume fraction)). Four 365 nm low-power UV-LEDs(3 W, Shenzhen LAMPLIC Science Co. Ltd.) acted as light sources to catalyze the photocatalytic reaction. The generated hydrogen (H2) was analyzed through a GC-2014C gas chromatograph (Shimadzu, Japan) equipped with a thermal conductivity detector and a 0.5 nm molecular sieve column. The apparent quantum efficiency (AQE) was calculated according to following equation:

3 Results and discussion
3.1 Preparation route of TiO2/rGO-COOH
The preparation strategy of TiO2/rGO-COOH photocatalysts is illustrated in Fig. 1A. First, the GO (Fig. 1A-a) can be well dissolved in the bottom water to form a uniform and stable yellow solution (Fig. 1D-a) due to the presence of a large amount of hydroxyl and carboxyl groups46. When the GO is sequentially added into HBr and HOOCCOOH solution at room temperature,the black well-dispersed rGO-COOH solution (Fig. 1B-b) is obtainedviaordinal ring-opening and esterification reaction. In this process, epoxide groups (―O―) of the GO converts into hydroxyl groups (―OH)viaa ring-opening reaction in the HBr(Fig. 1B-①)47and the above ―OH groups subsequently react with HOOCCOOH to form carboxylation groups on the grapheneviaan esterification reaction (Fig. 1B-②)47. To investigate the dispersion properties of GO and rGO-COOH,their corresponding photographs in water during different time are taken (Fig. 1C). When dispersed into water, both rGOCOOH (left) and GO (right) solutions display a good dispersion,which is attributed to the similar dissolve mutually theory of polar groups (such as ―OH and ―COOH) between graphene and water. After 3 days, the rGO-COOH and GO solutions without precipitation maintain homogeneous. Once lasted more than 7 days, the rGO-COOH exhibits limited stratification and little precipitation, while the GO is almost completely precipitated on the bottle bottom, clearly indicating that the rGOCOOH due to the increasing carboxyl groups shows stronger polarity and hydrophilicity than the GO36. This result verifies the transition from GO to rGO-COOH by ring-opening and esterification reaction.
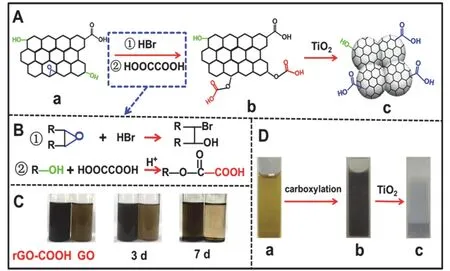
Fig. 1 (A) Graphical illustration for the synthesis of TiO2/rGOCOOH; (B) The reactions of carboxyl-functionalized graphene from GO to rGO-COOH; (C) Their dispersion photographs in water for different times; (D) The corresponding photographs of (a) GO, (b)rGO-COOH, and (c) TiO2/rGO-COOH.
To further confirm that GO can be covalently carboxylfunctionalized, GO and rGO-COOH samples are characterized by FTIR spectra (Fig. 2A), XPS spectra (Fig. 2B) and TG curves(Fig. 2C). As shown in Fig. 2A-a, the bands in the GO of C―O stretching (1050 cm−1), phenolic C―O―H stretching (1050 cm−1), phenolic C―O―H stretching or epoxide C―O―C (1166 cm−1), alcoholic C―OH bending (1398 cm−1), C=C stretching(1589 cm−1), carboxylates or ketones C=O stretching (1721 cm−1) and ―OH bending (3386 cm−1) are detected48. In comparison with the GO sample, the rGO-COOH sample (Fig.2A-b) exhibits the obviously enhanced C=C, carboxylates C=O stretching, and alcoholic C―OH bending peaks (latter two mainly drive from ―COOH groups) with the decreased C―O―C and C―O peaks (mainly drive from epoxide groups and alkoxy groups, respectively). These FTIR results clearly prove that the hydroxyl groups of GO has partially become the carboxyl groups after the carboxyl-functionalization. In addition, the C 1score-level spectrum of GO (Fig. 2B-a) and rGO-COOH (Fig. 2B-b) samples also illustrates the presence of carbon functional groups, containing carbon-oxygen single bond(C―O―C or C―OH, ~286.5 eV), carboxylic acid groups(C(O)=O, ~288.5 eV), carbon-carbon single bond (C―C,~284.5 eV)49and carbon-oxygen double bond (C=O, ~287.2 eV). Significantly, after the carboxyl functionalization, all the peak area of carbon-oxygen bonds decreases and the peak area of carboxyl groups markedly increases to 11.96% in the rGOCOOH from 4.98% in the GO (Table 1), meaning the transition from GO to rGO-COOH. Moreover, TG curves (Fig. 2C) are used to analyze the thermal stability of GO and rGO-COOH.Both GO and rGO-COOH show obvious weight losses from 25 to 227 °C , which one mass loss derives from the hydroxyl groups of graphene and the adsorbed water in the range of 25–144 °C50, and the other mass loss is attributed to the thermal decomposition of the more stable oxygen-containing groups(such as carbon-oxygen bond, carbon-oxygen double bond, and carboxyl group) than hydroxyl groups at 144–227 °C. Compared with the mass loss of GO (ca. 22.3%), an obviously increased mass loss of the rGO-COOH (ca. 26.4%) can be found,suggesting that the content of carboxyl groups on graphenesignificantly increases after the carboxyl-functionalization.Consequently, the above results of FTIR, XPS and TG strongly confirm that the GO has been successfully carboxylfunctionalized to form rGO-COOH by the simple and mild twostep organic reaction.

Table 1 Tabulated XPS C peak area ratio for the samples.
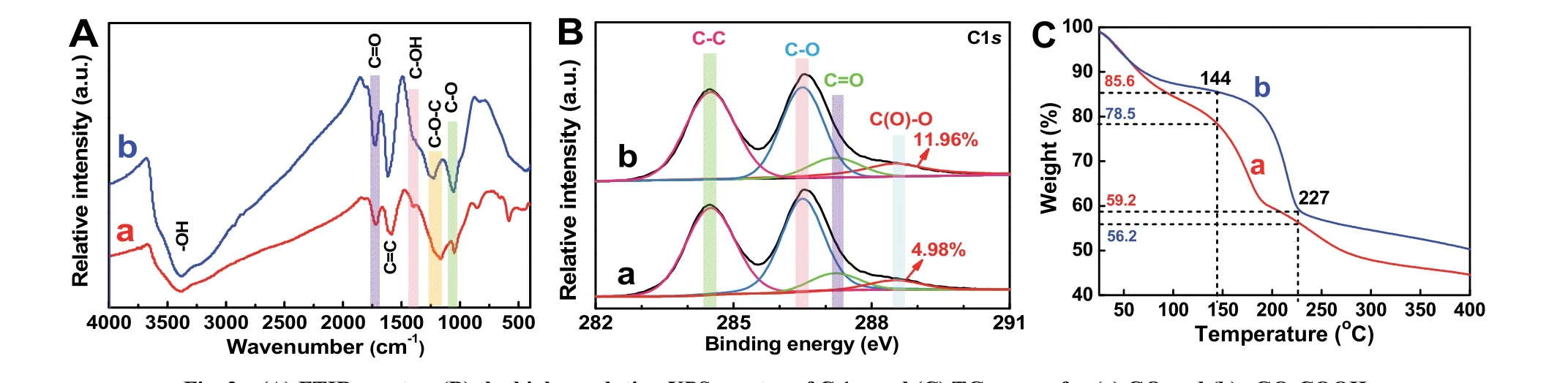
Fig. 2 (A) FTIR spectra, (B) the high-resolution XPS spectra of C 1s, and (C) TG curves for (a) GO and (b) rGO-COOH.
To further prepare rGO-COOH modified-TiO2photocatalyst(TiO2/rGO-COOH) (Fig. 1A-c), the white TiO2powder is mixed with the above black rGO-COOH solution. Here, a bright gray TiO2/rGO-COOH (Fig. 1D-c) solid with colorless and clear supernatant liquid is produced, suggesting the rGO-COOH is completely integrated with TiO2. Due to their electrostatic interaction, the strong-coupling force between rGO-COOH and TiO2is constructed. Generally, in the neutral solution, graphene displays a positive charge surface51, while the well-known TiO2nanoparticles possess a negative charge surface52, resulting in the production of forceful electrostaticinteraction between the TiO2and rGO-COOH. Hence, the simple production of the TiO2/rGO-COOH photocatalyst is established on the primary formation rGO-COOH from GOviatwo-step organic reactions and the subsequent strong-coupling force between rGO-COOH and TiO2.
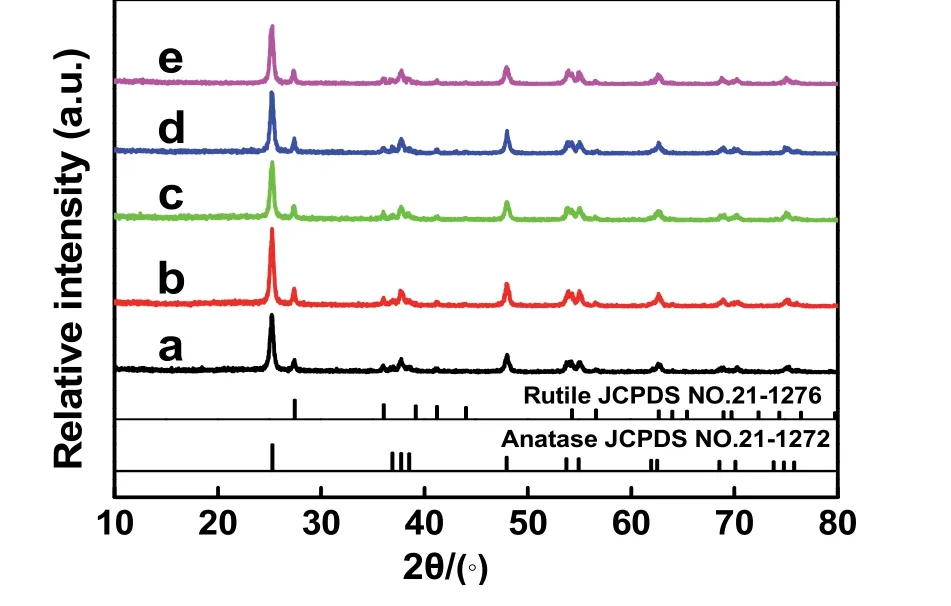
Fig. 3 XRD patterns of different samples: (a) TiO2, (b) TiO2/GO,(c) TiO2/rGO-COOH(0.5%), (d) TiO2/rGO-COOH(1%), and(e) TiO2/rGO-COOH(2%).
3.2 Microstructures of TiO2/rGO-COOH photocatalysts
The structures and morphologies of the TiO2/rGO-COOH are illustratedviathe subsequent different measurements. The XRD,FESEM and TEM spectra of the as-prepared photocatalysts are exhibited in Fig. 3 and Fig. 4, respectively. The TiO2sample(Fig. 3a and 4A) consists of uneven particles with a mean size ofca.30 nm and mixed phases of two different crystal phases(anatase and rutile)53,54. When modified by the GO, the crystal structure and morphology of the obtained TiO2/GO (Figs. 3b and 4B) is similar to that of the TiO2. After the modification of carboxyl-functionalized graphene, the TiO2/rGO-COOH (Figs.3c–e and 4C,D) exhibit the similar crystal phase and particle size to TiO2, indicating that the modifying carboxyl-functionalized graphene cannot affect the microstructure of TiO2. In particular,it is worth noting from TEM images that the rGO-COOH is tightly loaded on the surface of TiO2(Fig. 4E,F) due to the forceful interface between rGO-COOH and TiO2. Hence, the above results of XRD, FESEM and TEM spectra clearly prove that the rGO-COOH uniformly dispersed onto TiO2to synthesize TiO2/rGO-COOH samples.

Fig. 4 FESEM images of different samples: (A) TiO2, (B) TiO2/GO, (C) TiO2/rGO-COOH (1%), and(D) TiO2/rGO-COOH (2%); HRTEM images of TiO2/rGO-COOH (1%) (E and F).
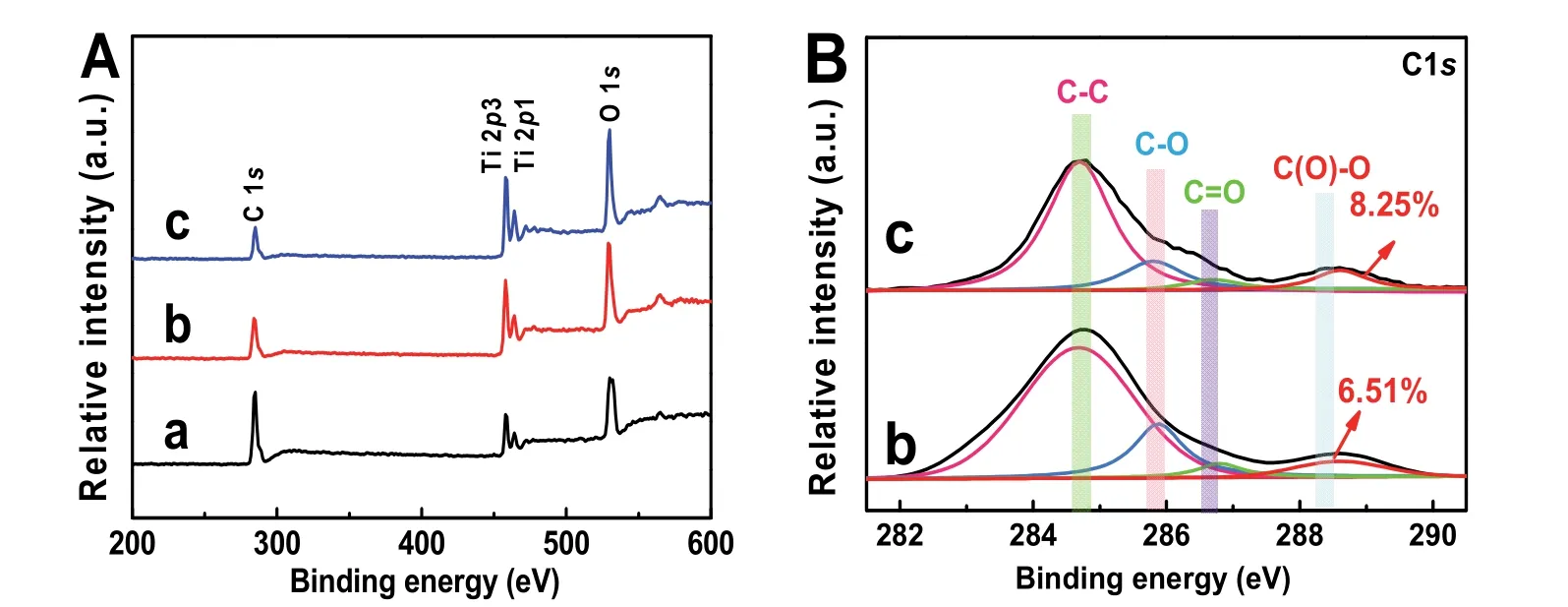
Fig. 5 (A) XPS survey spectra and the high-resolution XPS spectra of (B) C 1s for different samples: (a) TiO2, (b) TiO2/GO, and(c) TiO2/rGO-COOH(1%).
The chemical states and element components of TiO2/rGOCOOH samples can are suppliedviaXPS spectra (Fig. 5). Fig.5A displays the XPS survey spectra of TiO2, TiO2/GO and TiO2/rGO-COOH (1%) samples. It can be seen that all photocatalysts exhibit the characteristic peaks of Ti 2p, O 1sand C 1s(Fig. 5A). In the high-resolution XPS image of C element in the TiO2/GO (Fig. 5B-b) and TiO2/rGO-COOH (Fig. 5B-c)samples, carboxyl group (C(O)=O, ~288.5 eV), carbon-oxygen double bond (C=O, ~286.7 eV), carbon-oxygen bond (C―OH or C―O―C, ~285.8 eV), and carbon-carbon (C―C, ~284.7 eV) bond49are evidently found. Specially, the ratio of carboxyl peak area to the total area in the TiO2/rGO-COOH increases while the ratio of carbon-oxygen peak area reduces (Table 2),implying the successful carboxylated functionalization of graphene. In addition, the peak area of carboxyl groups markedly increases to 8.25% in the TiO2/rGO-COOH from 6.51% in the TiO2/rGO (Table 1). Therefore, the above XPS spectra obviously show that the successful covalently carboxylfunctionalized graphene has combined with TiO2.

Table 2 XPS elemental analyses of the samples.

Table 3 The H2-production performance and quantum efficiency of different samples.
The light absorption of covalently carboxyl-functionalized GO-modified TiO2can be analyzedviaUV-Vis characterization(Fig. 6). In comparison with blank TiO2(Fig. 6a), the TiO2/GO(Fig. 6b) manifests definitely enhanced light absorption with light yellow color (inset in Fig. 6b), indicating the modification of GO on the surface TiO2. When the TiO2is modified with the rGO-COOH, the further enhanced absorption intensity is observed in TiO2/rGO-COOH samples (Fig. 6c–e). With increasing amount of rGO-COOH, they possess a constantly improved visible-light absorption with the color change from light grey to deep grey (the inset of Fig. 6c–e) in the range of 400–800 nm, which results from the modification of carboxylfunctionalized graphene. On the basis of the analysis of UV-Vis spectra, it is also revealed that the carboxyl-functionalized graphene has been successfully modified onto titanium dioxide.
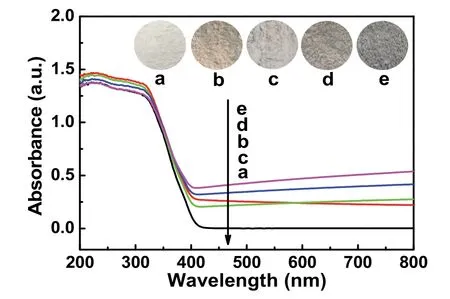
Fig. 6 UV-Vis absorption spectra of different samples: (a) TiO2,(b) TiO2/GO, (c) TiO2/rGO-COOH (0.5%), (d) TiO2/rGO-COOH (1%),and (e) TiO2/rGO-COOH (2%).

Fig. 7 The photocatalytic H2-evolution activity of different samples:(a) TiO2, (b) TiO2/GO, (c) TiO2/rGO-COOH (0.5%),(d) TiO2/rGO-COOH (1%), and (e) TiO2/rGO-COOH (2%).
3.3 Photocatalytic hydrogen-production activity and mechanism of TiO2/rGO-COOH
The hydrogen-evolution performances of TiO2/rGO-COOH samples are evaluated under the irradiation of ultraviolet light,as shown in Fig. 7 and Table 3. The TiO2(Fig. 7a) displays a finite hydrogen-evolution activity (56.7 μmol·h−1·g−1, AQE =0.55%). After further modification by GO, the TiO2/GO (Fig.7b) manifests an apparently boosted efficiency (123.6 μmol·h−1·g−1) superior to the TiO2. When further edgecarboxylated GO, TiO2/rGO-COOH photocatalysts manifest boosted performance. Especially, the TiO2/rGO-COOH (1%)(Fig. 7d) attains the maximum hydrogen-production rate (254.2 μmol·h−1·g−1, AQE = 2.47%), which is 4.48 and 2.06 fold superior to TiO2and TiO2/GO, respectively. Although the TiO2/rGO-COOH (2%) (Fig. 7e) displays a minorly decreased hydrogen-evolution activity, it still maintains an excellent efficiency than that of TiO2. Hence, the above results definitely indicate that the modification of carboxyl-functionalized graphene onto TiO2can greatly boost the hydrogen-generation rate of TiO2. Photocatalytic results distinctly indicate the hydrogen-production activities of TiO2are greatly boosted through the covalently carboxyl-functionalized GO. Therefore,it is highly desired for exploring the promotingmechanism of the TiO2/rGO-COOH photocatalyst. The carboxyl groups of carboxyl-functionalized graphene as catalytic sites on the basis of GO as an effective hydrogen-production center is illustrated for the greatly boosted performance of the TiO2/rGO-COOH photocatalyst, as shown in Fig. 8. In this case, the photogenerated electrons can be transferred from the CB(conduction band) of TiO2to the electron-transfer mediator GO nanosheets, and then steadily transport to the carboxyl groups in the rGO-COOH42. Meanwhile, the unsaturated oxygen atoms in carboxyl groups of carboxyl-functionalized graphene as an interfacial catalytic center can effectually enrich H+owing to its excellent nucleophilicity and boost the following hydrogenproduction process on the interface55. As a result, the high photocatalytic performance of TiO2/rGO-COOH is attributed to carboxyl groups of carboxyl-functionalized graphene.
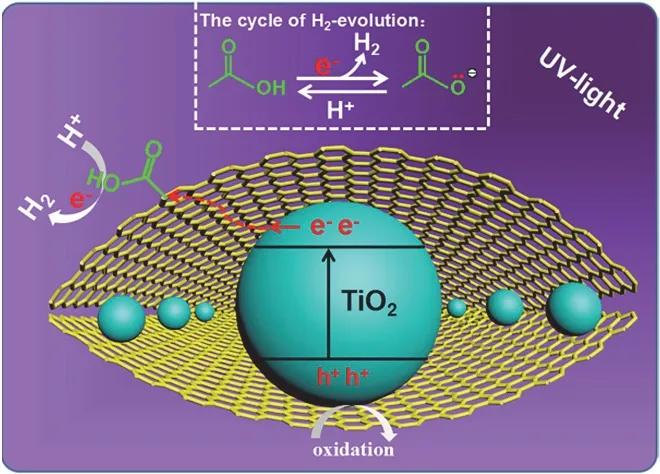
Fig. 8 Photocatalytic H2-evolution mechanism of TiO2/rGO-COOH.

Fig. 9 (A and B) Linear sweep voltammetry (LSV) curves, (C) transient photocurrent responses and (D) electrochemical impedance spectra for different samples: (a) GO, (b) rGO-COOH, (c) TiO2, (d) TiO2/GO, and (e) TiO2/rGO-COOH(1%).
To confirm that the carboxyl groups can efficiently enhance the hydrogen-generation reaction, the photoelectrochemical capabilities of the various photocatalysts are evaluated (Fig. 9).Fig. 9A displays the typical linear sweep voltammograms of GO and GO-COOH samples. Compared with the GO, the rGOCOOH exhibits the more positive potential, suggesting that its carboxyl work as a hydrogen-generation catalytic center to boost photocatalytic hydrogen-generation reaction56. To deeply compare the impact of rGO-COOH on the hydrogen-production reaction, the LSV tests of TiO2, TiO2/GO and TiO2/rGOCOOH(1%) samples are performed, as shown in Fig. 9B. After further carboxyl-functionalized, the TiO2/rGO-COOH(1%)exhibits a highest current intensity, which is ascribed to the carboxyl-functionalized graphene can effectively promote H2-evolution reaction. To deeply evaluate the capture, separation and transfer rate of photoexcited carriers in TiO2/rGOCOOH(1%), thei–tand EIS curves of different photocatalyst manifest in Fig. 9C and D, respectively. The TiO2/rGO-COOH(1%) sample displays highest photocurrent intensity and smallest arc radius, indicating a fast charge transfer between graphene and TiO2and following rapid interfacial catalytic H2-evolution reaction57. Hence, the photoelectrocatalytic results in the TiO2/rGO-COOH fully demonstrate the covalently carboxylfunctionalized GO dramatically promote the separation and transport performance of photogenerated carriers of pristine TiO2.
4 Conclusions
In conclusion, the covalently carboxyl-functionalized graphene (rGO-COOH) by ring-opening and esterification reactions has been successfully grafted on the TiO2surface to acquire high-efficiency TiO2/rGO-COOH. The experimental results show that TiO2/rGO-COOH samples exhibit an obviously promoted hydrogen-evolution activity. Its highest H2-evolution rate is 254.2 μmol·h−1·g−1with an AQE of 2.47%, which corresponds to 4.48 and 2.06 fold superior to the TiO2and TiO2/GO, respectively. On the basis of the abovementioned results, a proposed mechanism can illustrate the enhancement in hydrogen-generation performance of TiO2/rGO-COOH, namely,the carboxyl groups as effective H2-evolution active sites can adsorb hydrogen ions to facilitate the hydrogen-generation performance of TiO2. The paper provides new insights on the covalent functionalization of grapheneviafacile organic reactions to prepare effectual graphene-supported semiconductor photocatalytic material in the hydrogengeneration field.
- 物理化学学报的其它文章
- Fluorinated TiO2 Hollow Photocatalysts for Photocatalytic Applications
- Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution
- 微波辅助快速制备2D/1D ZnIn2S4/TiO2 S 型异质结及其光催化制氢性能
- TiO2-Supported Single-Atom Catalysts for Photocatalytic Reactions
- S-Scheme Heterojunction Photocatalyst for CO2 Photoreduction
- Review of Z-Scheme Heterojunctions for Photocatalytic Energy Conversion

