High Efficiency Electron Transfer Realized over NiS2/MoSe2 S-Scheme Heterojunction in Photocatalytic Hydrogen Evolution
Yang Liu , Xuqiang Hao ,*, Haiqiang Hu , Zhiliang Jin ,*
1 School of Chemistry and Chemical Engineering, Ningxia Key Laboratory of Solar Chemical Conversion Technology, Key Laboratory for Chemical Engineering and Technology, State Ethnic Affairs Commission, North Minzu University, Yinchuan 750021, China.
2 College of Mechanical and Automotive Engineering, Yinchuan University of Energy, Yinchuan 750100, China.
Abstract: S-scheme heterojunction is a major breakthrough in the field of photocatalysis. In this study, NiS2 and MoSe2 were prepared by a typical solvothermal method, and compounded by an in situ growth method to construct an S-scheme heterojunction. The obtained composite showed excellent performance in photocatalytic hydrogen evolution; the hydrogen production rate was approximately 7 mmol·h−1·g−1, which was 2.05 times and 2.44 times those of pure NiS2 and MoSe2, respectively. Through a series of characterizations, it was found that NiS2 and MoSe2 coupling can enhance the light absorption intensity, which is vital for the light reaction system. The efficiency of electron-hole pair separation is also among the important factors restricting photocatalytic reactions. Compared with pure NiS2 and MoSe2,NiS2/MoSe2 exhibited a higher photocurrent density, lower cathode current,and lower electrochemical impedance, which proves that the NiS2/MoSe2 complex can effectively promote photogenerated electron transfer. Simultaneously, the lower emission intensity of fluorescence indicated effective inhibition of electron-hole recombination in the NiS2/MoSe2 complex, which is favorable for the photocatalytic hydrogen evolution reaction. Further,scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed that MoSe2 is an amorphous sample surrounded by the NiS2 nanomicrosphere, which greatly increased the contact area between the two, thus increasing the active site of the reaction. Secondly, as a photosensitizer, Eosin Y (EY) effectively enhanced the absorption of light by the catalyst in the photoreaction system. Meanwhile, during sensitization, electrons were provided to the catalyst,which effectively improved the photocatalytic reaction efficiency. The establishment of S-scheme heterojunctions contributed to improving the redox capacity of the reaction system and was the most important link in the photocatalytic hydrogen reduction of aquatic products. It was also the main reason for the improvement of the hydrogen evolution effect in this study. The locations of the conduction band and valence band of NiS2 and MoSe2 were determined by Mott-Schottky plots and photon energy curves, and further proved the establishment of the S-scheme heterojunction. This work provides a new reference for studying the S-scheme heterojunction to effectively improve the photocatalytic hydrogen production efficiency.
Key Words: NiS2; MoSe2; S-scheme heterojunction; Photocatalytic hydrogen evolution
1 Introduction
With the rapid economic development, the global energy demand has far exceeded the energy storage. At the same time,the massive use of fossil energy also brings great pressure for the global environmental governance. Therefore, sustainable clean energy research was a new field of energy development in recent years1–3. Hydrogen energy (H2) has attracted much attention due to its environmental friendliness and high energy4,5. Solar energy was a sustainable energy in natural energy. Converting solar energy into chemical energy to obtain the hydrogen energy we need is the direction of many scientists’ efforts after 19726–9.It was a new technology with research value. Among them, the photocatalyst was the decisive substance in the photocatalytic decomposition of water to produce hydrogen. Therefore, the search for low-cost, high-efficiency photocatalysts was the main goal of researchers10.
Semiconductor materials occupied an important position in the field of photocatalysis11. Precious metals with excellent electron-transporting capabilities were not conducive to the development of industry because of the relatively large cost of testing, and semiconductor materials have become the main direction dedicated to research by researchers12–15. Nickel-based materials stand out in the continuous research of catalysts,including metals Ni, Ni(OH)2, NiPx, NiP216–19,etc. have excellent photocatalytic hydrogen evolution performance.Nickel sulfide (NiS2), as a strong compound made of abundant earth elements, has a small band gap and was easily excited to generate electron-hole pairs20. It has been regarded as an efficient catalyst material in the photocatalytic hydrogen evolution reaction. However, the narrow band gap has the disadvantage of easy recombination of electrons and holes.Therefore, it was necessary to take measures for semiconductor recombination to guide electron transfer in time and reduce its recombination rate. MoS2has been widely studied as a nonnoble metal auxiliary catalyst for modifying semiconductor photocatalysts to achieve sustainable H2generation21. The elements Se and S are elements of the same main group and usually have similar properties. Therefore, MoSe2also has a low Gibbs free energy (ΔGH= 0.05 eV, close to thermal neutral) and high conductivity due to its intrinsic metal properties22. The porous g-C3N4phase transformation replaced the main role of the MoSe2nanostructure as the active catalytic site for H2precipitation. At the same time, the hybrid system shows high thermal catalytic stability23.
Since the redox reaction on the semiconductor surface was so slow that the charge carriers cannot be consumed efficiently24.In recent years, the concept of heterojunction has been gradually discovered and studied, including type I heterojunction, type II heterojunction, direct Z-scheme heterojunction, indirect Zscheme heterojunction and S-scheme heterojunction. The Sscheme heterojunction has a staggered band structure, which was similar to the type II heterojunction, but the charge transfer path was completely different24. In a typical type II heterojunction,photogenerated electrons and holes remain in the conduction band at a lower position and the valence band at a higher position, respectively, resulting in weak redox capacity. In the Sscheme heterojunction, electrons and holes are retained in the higher conduction band and lower valence band, while meaningless photogenerated carriers are recombined to obtain a strong redox potential, thereby promoting redox reactions. In addition, compared to the Z-scheme heterojunction, the Sscheme heterojunction has a more stable photocatalytic reaction system25.
Herein, pure NiS2, MoSe2and NiS2/MoSe2were prepared by a typical solvothermal method. The optical and electrical properties of NiS2/MoSe2S-scheme heterojunction and the photocatalytic hydrogen production capacity were systematically studied. In addition, in the EY-sensitized photocatalytic system, EY is used as a photosensitizer to effectively enhance the light absorption efficiency, thereby promoting the progress of the photoreaction. As a result, the hydrogen production activity of NiS2/MoSe2was 2.05 and 2.44 times that of NiS2and MoSe2. This research provided a new strategy for constructing the S-scheme heterogeneous narrowband gap semiconductor to be directly applied to the photocatalytic hydrogen evolution system.
When he was about to lie down in bed he would creep out of his hedgehog skin, and leave it lying at the bedside; then the men must rush in, throw the skin into the fire, and stand by till it was entirely23 burnt up
2 Experimental section
2.1 Catalysts preparation
Fig. 2A,B show the scanning electron microscope (SEM)images of pure NiS2and bare MoSe2, respectively. It can be found that the microscopic appearance of NiS2was a uniform nanosphere with a size of about 100 nm and a uniform distribution. However, pure MoSe2does not show obvious morphological structure. This was consistent with XRD results.Fig. 3 was a transmission electron microscope (TEM) image of the composite catalyst. Combining literature reports26and Fig. 2, it can be determined that the nanospheres in Fig. 3A are NiS2, and the surrounding amorphous material should belong to MoSe2. Fig. 3B was a high magnification view of Fig. 3A, which further proved the spherical structure of NiS2. A high-resolution transmission electron microscopy (HRTEM) test was performed on the catalyst. As shown in Fig. 3C, the 0.235 nm lattice fringe corresponds to the (211) crystal plane of NiS2, which further confirmed the existence of NiS2. At the same time, the lattice spacing of 0.27 nm can also coincide with the (012) crystal plane of MoSe223. The energy dispersive X-ray pattern (Fig. 3D)shows the presence of Ni, S, Mo, and Se elements, which determines the successful preparation of NiS2/MoSe2.
2.2 Synthesis of NiS2
The photocurrent response, Mott-Schottky curve,electrochemical impedance spectroscopy (EIS) measurements and linear sweep voltammetry (LSV) measurements were performed with a Princeton electrochemical workstation (USA).The working electrodes were prepared by drop-coating homogeneous catalyst suspensions directly onto the precleaned FTO glass surfaces (1 × 1.5 cm). Concomitantly, platinum plate was used as the opposite electrode, saturated calomel electrode(SCE) was used as the reference electrode, and 0.2 mol·L−1Na2SO4aqueous solution was used as the electrolyte.E0= 0.24 VvsNHE (Normal Hydrogen Electrode) at 25 °C for saturated calomel electrode.
2.3 Synthesis of MoSe2
As the alcoholism progressed, AL began to lose everything he possessed6 -- his land, house, etc. Finally Al died alone in a small bar. Hearing of Al s death, I thought, What a totally wasted life! What a complete failure!
2.4 Synthesis of NiS2/MoSe2
The preparation of NiS2/MoSe2is based on the preparation of NiS2, adding the already prepared MoSe2. As described in section 2.2, after adding the medicine required for the preparation of NiS2and stirring for 30 min, add the prepared MoSe2powder. The quality of the added MoSe2was different,forming different proportions of composites, which were named NiS2/MoSe2-5, NiS2/MoSe2-10, NiS2/MoSe2-20, NiS2/MoSe2-40, NiS2/MoSe2-60, NiS2/MoSe2-80. Among them, the value in the name indicates the quality of the added MoSe2.
2.5 Characterizations
The internal structure of the synthesized sample was measured by X-ray diffraction (XRD) on Rigaku RINT-2000 (Japan) using CuKαradiation at a scan rate of 5 (°)·min−1. The morphology of the obtained product was observed by scanning electron microscope (SEM, Carl Zeiss AG, Germany) and transmission electron microscope (TEM, FEI, USA), and its microstructure was further tested by energy dispersive X-ray spectroscopy(EDS) and high resolution transmission electron microscope(HRTEM). The UV-Vis diffuse reflectance spectrum (DRS) of the sample was tested on a UV-2550 (Shimadzu, Japan)spectrometer, which was converted to BaSO4using reference. Xray photoelectron spectroscopy (XPS) of the prepared samples was performed on a fluorescence spectrometer (ESCALAB 250Xi, Thermo-fisher, USA) to test the composition of the compound and the surface state of the elements. The nitrogen adsorption-desorption isotherm was measured by Micromeritics ASAP2020M (USA) nitrogen adsorption device, and the specific surface area of the sample was calculated by BET method. The photoluminescence (PL) spectrum of the sample was measured on a Horiba Scientific (France) FluoroMax-4 fluorometer spectrometer at room temperature, and timeresolved fluorescence emission spectra were obtained on the Horiba Jobin Yvon data station.
In the morning, the ogress bade him sweep the dust out of the cave, and to have it clean before her return in the evening, otherwise it would be the worse for him
NiS2nanospheres were synthesized by a typical solute thermal method. In detail, 30 mL ethylene glycol and 30 mL deionized water were evenly mixed under magnetic stirring. Then, 0.6 g PVP was added to the mixed solution to make it evenly distributed in the solution. After that, 2 mmol C4H6O4Ni·4H2O and 6 mmol Na2S2O3·5H2O were added to the above solution successively. After stirring for 30 min, the above solution was put into a high-pressure reaction kettle lined with polytetrafluoroethylene and heated continuously at 180 °C for 12 h. After the reactor cooled, the sediment was separated by centrifugation. The product was washed with water and ethanol and dried overnight at 60 °C. Finally, NiS2is ground into a solid powder26.
2.6 Photocatalytic hydrogen production
In the H2production experiments, 10 mg catalyst and 20 mg Eosin Y (EY) were added to a 62 mL quartz bottle and then 30 mL of 15%v/vtriethanolamine (TEOA) solution was added to seal the reaction bottle with a rubber pad. The catalyst was suspended in solution and N2was used to replace the air in the bottle. Finally, place the bottle in a nine-channel reaction system with a white light source of 5 W,λ≥ 420 nm. During the photocatalytic process, 0.5 mL of gas was extracted with a syringe every 0.5 h, and H2was analyzed by phase chromatography (using Tianmei GC7900, TCD and N2as carriers).
3 Results and discussion
3.1 Structure and morphology analysis
3.1.1 X-ray diffraction analysis
In order to determine the crystalline phase structure of the material, we gave the X-ray diffraction patterns of the NiS2,MoSe2and NiS2/MoSe2composites, as shown in Fig. 1. First,we identify the phase and crystallinity of pure NiS2and MoSe2,as shown in Fig. 1A. The diffraction peaks at 27.03°, 31.5°,35.25°, 38.7°, 47.95°, 53.45°, 45.05°, 56.05°, and 58.53° in the XRD spectrum of NiS2correspond to (111), (200), (210), (211),(220), (221), (311), (222), (230) crystal planes confirm the successful synthesis of NiS226. In the meantime, the clear diffraction peak indicates that NiS2has good crystallinity27–29.In contrast, MoSe2has poor crystallinity. The peak envelopes at 32.77° and 55.81° correspond to the MoSe2standard card(PDF#72-1420). Fig. 1B shows the diffraction spectra of the composite catalyst NiS2and pure NiS2and bare MoSe2. By comparison, it was found that there was no obvious MoSe2diffraction peak in the composite catalyst, which was attributed to the poor crystallinity of MoSe2itself. The above results can confirm the successful preparation of the catalyst.
The fitting results are shown in Table 2, short lifetimeτ1carrier radiation combination, namely the electron relaxation from the excited state to the ground state, which was caused by the carrier combination. The reduction of recombination indicates that the utilization rate of photoexcited electrons or holes was higher51,52. Long lifetimeτ2reveals the non-radiation combination of carriers52. Obviously, compared with pure NiS2and MoSe2, theτ1,τ2, andτavof NiS2/MoSe2compounds are the minimum values, which indicated that the formation of the heterojunction accelerated electron transfer and promoted photocatalytic reduction.
All the chemical reagents used in the experiment were analytical reagents and without further concentration. Nickel acetate (C4H6O4Ni·4H2O, Tianjin Damao Chemical Reagent Factory, China, CAS: 373-02-4, AR 98%), Sodium thiosufate pentahydrate (Na2S2O3·5H2O, Aladdin, China, CAS: 10102-17-7, AR 99%), Polyvinylpyrrolidone ((C6H9NO)30, Aladdin,China, CAS No: 9003-39-8, AR 98%), Selenium powder (Se,Tianjin Damao Chemical Reagent Factory, China, CAS No:7782-49-2, AR 98%), Sodium molybdate (NaMoO4, Xilong Chemical Co. Ltd., China, CAS: 1102-40-6, AR 98%), Sodium borohydride (NaBH4, Sinopharm Group Chemical Reagent Co.Ltd. China, CAS:, AR 98%), Ethylene glycol (H2NCH2CH2NH2,Tianjin Damao Chemical Reagent Factory, China, CAS No:107-24-1, AR 99%), Eosin Y (C20H6Br4Na2O5, Aladdin, China,CAS: 17372-87-1, Lot# F182260, AR).

Fig. 1 XRD patterns of (A) pure NiS2 and bare MoSe2 samples and (B) NiS2/MoSe2-x (x = 5–80) samples.

Fig. 2 SEM images of (A) bare NiS2 and (B) pure MoSe2.

Fig. 3 TEM images (A, B), HRTEM image (C) and EDX patterns (D) taken from NiS2/MoSe2-40.

Fig. 4 (A) N2 adsorption-desorption isotherms and (B) pore size distribution curves of the NiS2, MoSe2 and NiS2/MoSe2-40 samples.
3.2 Brunauer-Emmett-Teller analysis
Nitrogen adsorption-desorption isotherms and pore size distribution curves were used to verify the pore structure of pure NiS2and MoSe2and NiS2/MoSe2composite catalysts. As shown in Fig. 4A, the isotherm curves are all typical type IV isotherms with H3 type hysteresis loop30. The sample showed high adsorption in the range of 0.8–1.0, indicating the mesoporous structure of the material31. The pore size distribution curve further proves this point, as shown in Fig. 4B. At the same time,it can be seen that NiS2has a larger pore size, which corresponds to the results in Table 1. Besides, pure NiS2also shows the smallest specific surface area (16.18 m2·g−1), while the specific surface area of the composite material lies between the two. The above results were related to the appearance of the catalyst.Combining Figs. 2 and 3, NiS2is a uniformly distributed nanosphere, so it must have the smallest specific surface area and the largest pore size. However, MoSe2is an amorphous substance with a smaller size, which surrounds the NiS2nanospheres, thereby reducing the pore diameter of the catalyst and increasing the specific surface area of the catalyst (28.18 m2·g−1).
MoSe2photocatalysts were prepared by a one-pot hydrothermal method, but having a minor modification of this reported27. Typically, 0.316 g (4 mmol) selenium powder was added to 50 mL deionized water and stirred evenly. Then, 0.304 g NaBH4was added to the above solution and stirred for 1 h.During this process, the selenium powder was gradually reduced to Se2−, correspondingly, the black solution became clear.Finally, after stirring, 0.484 g of NaMoO4was added to the clarified solution, which quickly turned black. After continuous stirring for 1 h, the uniform solution was transferred to an 80 mL reactor and heated at 180 °C for 24 h. The final product was recovered by filtration and washed several times with deionized water and finally with ethanol, followed by drying in vacuum at 60 °C for 24 h.

Table 1 Textural properties of different samples.

Fig. 5 XPS spectra of (A) Ni 2p; (B) S 2p; (C) Mo 3d and (D) Se 3d of pure NiS2, bare MoSe2 (up) and NiS2/MoSe2 composite (down).
3.3 X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) was used to detect the chemical state of chemical elements in the material32. As shown in Fig. 5, the fine spectra of elements of pure substances and complexes are shown in the figure. Fig. 5A shows the typical binding state of Ni in NiS2. The binding energies at 853.28 eV and 871.16 eV are attributable to the Ni 2p3/2and Ni 2p1/2electron orbitals, respectively, which proved the existence of Ni2+in the catalyst, and the results were consistent with XRD.Moreover, 876.78 eV are the binding energies of Ni3+, and the binding energies at 854.97 eV and 859.74 eV are attributable to the electron orbitals of Ni in Ni(OH)2, which may come from the slight oxidation of NiS2surface33,34. The electron binding energy spectrum of S 2porbital was shown in Fig. 5B. The binding energies of S 2p3/2and S 2p1/2electron orbitals are at 162.11 eV and 163.30 eV, respectively. According to literature reports, the binding energy is attributed to S22−35. Meanwhile, we also detected a peak at 164.58 eV, which was assigned to the spin orbital at S82p1/2, indicating that S remains during the vulcanization, while the peaks at 167.92 eV may was caused by the S―O bond36. In addition, in the NiS2/MoSe2composite, we found two more peaks than pure NiS2, at 160.47 eV and 169.27 eV, respectively. Where, the binding energy at 169.27 eV was derived from SO42−37, while the binding energy at 160.47 eV corresponds to the S 2p1/2orbital of S2−, which may be due to the formation of Mo-S bond38.
Fig. 5C, D show the photoelectron spectra of the elements Mo and Se, respectively. In Fig. 5C, the peaks at 228.56 and 231.63 eV come from the 3d5/2and 3d3/2spin orbitals of Mo4+. The peak at 229.66 eV and 232.76 eV is the splitting peak of 3d5/2and 3d3/2, which was attributed to the 2H phase polymorph of MoSe2.Moreover, the binding energy of 235.83 eV was ascribed to Mo6+39,40. In addition, according to the literature41, 226.32 eV was the binding energy of S 2s. Fig. 5D shows the electronic energy spectrum of Se2−, with 54.33 eV and 55.33 eV belong to the Se 3d5/2and Se 3d3/2electronic orbitals42.
Charge transfer in photocatalytic reaction mechanism was further explored by comparing the migration of binding energy of each element in pure NiS2, bare MoSe2and complex43. The upper electron spectrum corresponds to the elements in pure matter in Fig. 5, while the under electron spectrum corresponds to the same elements in the complex. It can be observed from the figure that both Ni and S elements in the composite have shifted to the higher energy direction, while the shifting directions of Mo and Se elements are opposite. This result indicates that there was an interfacial electric field in the composite, and electrons migrate from NiS2to MoSe244.
3.4 Optical properties
UV-Vis diffuse reflectance spectroscopy was used to study the light absorption properties of the prepared samples. According to Fig. 6A, the NiS2, MoSe2and NiS2/MoSe2composite catalysts all showed good absorption performance. Moreover, due to the inherent narrow band gap characteristics of the material, it cannot show obvious absorption band edge. In addition, the color of the catalyst was black, which plays an important role in the test of light absorption performance45. Obviously, the light absorption intensity of composite photocatalyst NiS2/MoSe2was higher than that of pure NiS2and bare MoSe2. Stronger light absorption was more favorable for photocatalytic activity46.Through the Tauc equation (αhv=A(hv−Eg)1/2), the direct band gap of two narrow band gap semiconductor materials was obtained based on the tangent line of (αhν)2tohνcurve (whereαwas the absorption coefficient andhνwas the photon energy)43,47.As shown in Fig. 6B, the band gaps of NiS2and MoSe2are 1.32 eV and 1.41 eV, respectively.

Fig. 6 (A) UV-Vis diffuse reflectance spectra of as-prepared samples and (B) the corresponding (αhv)2 vs photon energy curves of NiS2 and MoSe2 samples.
Photoluminescence (PL) spectroscopy was used to study the transfer of interface electrons. As shown in Fig. 7A, all samples are in the EY system, so all samples exhibit an emission peak of 534 nm when excited at 480 nm. Here, the emission peak of fluorescence was caused by electron-hole recombination in the excited semiconductor48. More importantly, compared with EY NiS2and MoSe2, the emission intensity of the NiS2/MoSe2composite was the lowest, indicating that the electronrecombination in the composite was effectively suppressed,which is beneficial to the improvement of photocatalytic activity49.
Iis the normalised emission intensity;tis the time after the pulsed laser excitation;τiare the respective decay lifetimes;Biare the amplitude (pre-exponential factor);
The time resolution characterization of PL was performed to further explore the electron transfer dynamics within the material50. As shown in Fig. 7B, the attenuation curve shows observable differences. Time-resolved photoluminescence(TRPL) was fitted using a two-exponential decay model, and the formula was as follows:

Fig. 7 (A) Photoluminescence spectra and (B) time-resolved fluorescence spectra of as-prepared samples.

22. I ve nothing more to give: The young woman doesn t have any more material, or physical, possesions to give the manikin in exchange for his services. Now comes the test of what she is willing to give up to save her life.Return to place in story.

˂τav˃ is the average lifetime;τiis the respective decay times;Biis the amplitude (pre-exponential factor);
3.1.2 Transmission electron microscopy characterization
The princess now said to him, At the marriage feast you may eat what you please, but you must not drink anything whatever, for if you do that you will forget me

Table 2 Kinetic analysis of emission decay for as-prepared samples.
3.5 Photocatalytic performance
The catalytic activity of EY sensitized catalysts was evaluated in triethanolamine (TEOA) solution. As shown in Fig. 8A, the hydrogen production activity of the composite photocatalyst was significantly higher than that of the two pure substances under the same conditions. This was owing to the strong light absorption in the composite photocatalyst and the formation of the S-scheme heterojunction promoted photocatalytic reaction.However, the proportion of MoSe2in the corresponding amount of composite catalyst was different, and the final hydrogen evolution effect was also different. As shown in Fig. 8B, in the original preparation process of the composite catalyst, when the addition amount of MoSe2was 40 mg, the catalytic activity of the catalyst was the highest, about 7 mmol·h−1·g−1. At this time,the hydrogen production rate of the composite catalyst(NiS2/MoSe2-40) was 2.05 and 2.44 times that of NiS2(3.417 mmol·h−1·g−1) and MoSe2(2.877 mmol·h−1·g−1), respectively.After the amount of MoSe2exceeded 40 mg, there was a slight drop in hydrogen production, which may be because of the“shading effect”53. When there was too much MoSe2on the surface of NiS2nanospheres, it affected the light absorption of NiS2and direct contact with the aqueous solution. From the perspective of catalytic activity, it was necessary to find the optimal ratio of photocatalyst.
Fig. 8C shown the hydrogen-producing activity of the composite photocatalyst in sacrificial reagents with different pH values, aiming to explore the influence of pH value on the hydrogen-producing activity of photocatalyst. As can be seen from the figure, when pH value was 11, the photocatalytic activity of the sample was the highest, about 7.59 mmol·h−1·g−1.On the one hand, when pH value was too small, triethanolamine(TEOA) will undergo protonation, which weakens its ability to provide electrons54. On the other hand, the amount of H+decreases sharply under overalkaline conditions, thus reducing the hydrogen-producing activity. Stability was an important index to evaluate the quality of photocatalysts. We tested the stability of the best proportional photocatalyst within 20 h55, as shown in Fig. 8D. Since the dye EY was easily degraded under light, the less EY will participate in the photocatalytic reaction through the increase of light time43. However, when EY was added again in the third cycle experiment, the catalytic activity of the photocatalyst increased again. The results shown that the photocatalyst has good stability.

Fig. 8 (A) Time dependent photocatalytic H2 production and (B) average hydrogen production rates of EY sensitized pure NiS2, bare MoSe2 and NiS2/MoSe2-x (x = 5, 10, 20, 40, 60); (C) effect of the pH value on the hydrogen production of the NiS2/MoSe2-40 photocatalyst;(D) stability test for H2 evolution over the NiS2/MoSe2-40 photocatalyst in cycle runsunder visible light irradiation (λ ≥ 420 nm).
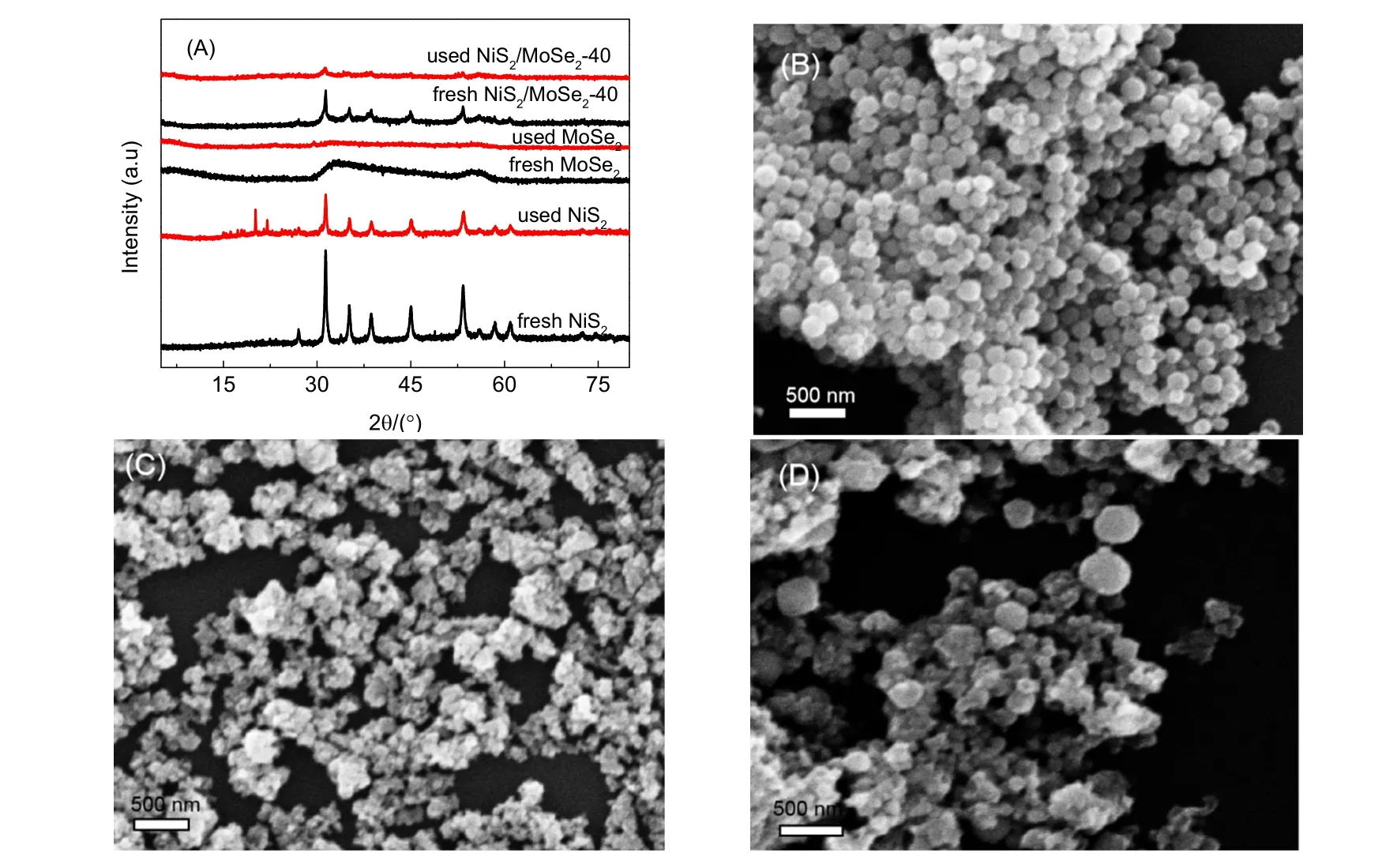
Fig. 9 (A) XRD results before (used) and after (fresh) hydrogen production tests and SEM images of used pure NiS2 (B),MoSe2 (C) and NiS2/MoSe2-40 (D).
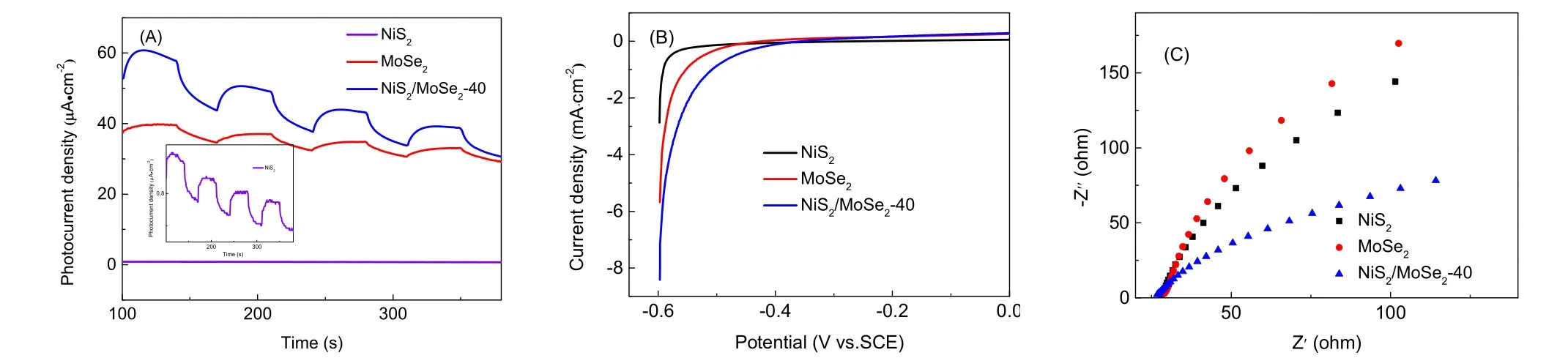
Fig. 10 (A) Transient photocurrent response; (B) LSV curve and (C) the electrochemical impedance spectroscopy for EY-sensitized NiS2, MoSe2 and NiS2/MoSe2-40 samples in 0.5 mol·L−1 Na2SO4.
In order to explore the influence of the photoreaction process on the photocatalyst, XRD and SEM of the used hydrogen catalyst were tested, as shown in Fig. 9. Fig. 9A showed the XRD pattern of the catalyst before and after the photocatalytic reaction. Through comparative study, it was found that the intensity of diffraction peak of used catalyst decreases, which was related to the amount of sample used in the test. The pure NiS2sample after reaction has several more diffraction peaks in the range of 15°–17° than the fresh sample. This was owing to the photoetching effect in the reaction process leading to the production of S elemental substance56. In addition, the XRD results of other samples remain unchanged. Fig. 9B–D were SEM images of pure NiS2, MoSe2and NiS2/MoSe2composite catalysts after use, respectively. In combination with Fig. 2, it can be found that there was no change in the morphology and structure of the catalyst after use, which further proved that the catalyst has good stability.
3.6 Photoelectrochemical properties
Here, we further explore the electron-hole separation efficiency of the photocatalyst by testing the photoelectrochemical performance of the sample. The transient photocurrent density-time curve of NiS2, MoSe2and the composite catalyst were shown in Fig. 10A. In the four cycles of on-off, the photocurrent showed obvious changes. According to previous reports57, the photocurrent density has a positive correlation with the charge separation efficiency. It can be seen from Fig. 10A that the photocurrent density of the composite photocatalyst is higher than that of NiS2and MoSe2, which indicates that the composite photocatalyst has a higher electronhole separation efficiency. In order to further confirm the above results, we used linear voltammetry to test the samples, as shown in Fig. 10B. It can be observed that the composite photocatalyst has a lower cathode current than NiS2and MoSe2, which was attributed to the S-scheme heterojunction between the composite catalysts, which promoted the electron mobility of the photocatalyst58.
Furthermore, electrochemical impedance spectroscopy (EIS)Nyquist plots of NiS2, MoSe2and NiS2/MoSe2composite samples were obtained through testing, as shown in Fig. 10C.The arc radius is positively correlated with the charge transfer resistance of the sample59. Therefore, the NiS2/MoSe2composite sample has a small charge transfer resistance. In other words, compared with pure NiS2and MoSe2, NiS2/MoSe2composite catalyst has the strongest charge transfer efficiency.The above results are consistent, which further emphasizes the excellent charge transfer efficiency of NiS2/MoSe2composite catalyst.
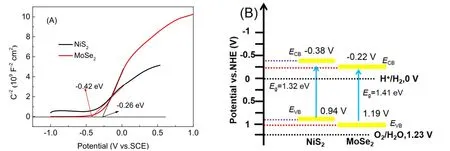
Fig. 11 (A) Mott-Schottky plots for EY-sensitized NiS2 and MoSe2 samples in 0.5 mol·L−1 Na2SO4;(B) Schematic illustration of band structures for NiS2 and MoSe2.
In order to explain the reaction mechanism of the composite photocatalyst, Mott-Schottky (M-S) test was performed on NiS2and MoSe2samples, as shown in Fig. 11A. TheC−2–Ecurves of the samples show a positive slope, indicating that NiS2and MoSe2weren-type semiconductors60,61. Besides, we obtain the flat band potentials of NiS2and MoSe2through formula (3),which were −0.42 eV and −0.26 eV respectively. Forn-type semiconductors, the conduction band (ECB) position was negative 0.1 V or 0.2 V compared with the measured flat band potential62. Further, formula (4) was used to convert the guide band position corresponding to the normal hydrogen electrode(NHE), which were −0.38 eV and −0.22 eV respectively.Meanwhile, combining the band gap values of NiS2and MoSe2(Fig. 6B), their valence band (EVB) positions were obtained through formula (5), as shown in Fig. 11B. It can be determined that the band arrangement between NiS2and MoSe2conforms to the photocatalytic mechanism of S-scheme.
So the decision was made and the children began to make a new outfit21 for their Baby Jesus -- a little leather vest out of some scraps22 and some cloth diapers. Best of all, Baby Jesus fit perfectly into the little crib, but since it wasn’t quite time for him to sleep there yet, he was laid carefully on a shelf in the hall closet to wait for Christmas eve.

Cis the capacitance of the space charge region;NDis charge carrier concentration;eis electron charge;εis dielectric constant;ε0is vacuum permittivity;Eis electrode applied potential;Efbis flat band potentials;kis Boltzmann constant;Tis absolute temperature;

According to the UV-Vis band gap (Eg) data, the valence band(EVB) position of NiS2and MoSe2was calculated by Eq. (5):
But it was all in vain; three days passed in such festivities, and on the fourth the prince said: O joy of my eyes! I beg now that you will bid me farewell, for my way is long and the fire of your love darts95 flame into the harvest of my heart

3.7 Proposed photocatalytic hydrogen evolution mechanism
Based on the above conclusions, the charge transfer mechanism of NiS2/MoSe2composite material under illumination was shown in Scheme 1. EY molecules adsorb on the surface of the catalyst and form a singly excited state EY1*,which absorbs photons and then forms EY3*through an interphase transition (ISC). EY3*was reduced and quenched by TEOA to produce oxidized TEOA+and EY−•. EY−•instability will transfer electrons to the conduction band of NiS2and MoSe2, and the reducing dye molecule will return to the ground state. At the same time, after NiS2and MoSe2contact, the internal electric field and band bending are generated. Under light, the electrons of NiS2and MoSe2were excited to transition from the valence band to the conduction band. Owing to the effect of the internal electric field, the electrons in MoSe2conduction band were more inclined to combine with the holes in NiS2valence band63–65. Thus, NiS2with greater potential difference generates H2by combining with H+, while holes were consumed by TEOA. This process not only speeded up the separation efficiency of electron and hole, but also effectively inhibited the recombination of electron and hole, thus forming efficient hydrogen evolution mechanism.

Scheme 1 H2 evolution mechanism over S-scheme NiS2/MoSe2 heterojunction in EY-sensitized system under visible light irradiation.
4 Conclusions
In a nutshell, this study constructed a new type S-scheme heterojunction of NiS2/MoSe2for photocatalytic decomposition of water and hydrogen production system. As a result, the hydrogen production rate of the composite was 2.05 and 2.44 times that of pure NiS2and MoSe2, respectively. This was because the S-scheme heterojunction causes a larger potential difference in the redox reaction, which was beneficial to the effective separation of electrons and holes and the redox reactions. Meanwhile, in the EY-sensitized photocatalytic reaction system, EY not only acts as a photosensitizer to enhance light absorption, but also transfers electrons to NiS2and MoSe2to promote the reduction of H+to produce H2. This work provided a new reference for the study of S-scheme heterojunction to effectively improve the efficiency of photocatalytic hydrogen production.
Then he was afraid and went; but he was quite faint, and shivered and shook, and his knees and legs trembled. And a high wind blew over the land, and the clouds flew, and towards evening all grew dark, and the leaves fell from the trees, and the water rose and roared as if it were boiling, and splashed upon the shore. And in the distance he saw ships which were firing guns in their sore need, pitching and tossing on the waves. And yet in the midst of the sky there was still a small bit of blue, though on every side it was as red as in a heavy storm. So, full of despair, he went and stood in much fear and said,
Because I own all of me, I can become intimately acquainted with me in all my parts. I can love me and be friendly with me in all my parts. I can then make it possible for all of me to work in my best interests.
Author contributions:Yang Liu and Xuqiang Hao conceived and designed the experiments; Yang Liu and Haiqiang Hu performed the experiments; Zhiliang Jin contributed reagents/materials and analysis tools; Yang Liu wrote the paper.
The story spread all over the country about the fine horse that had been sold and then had disappeared, and at last the news came to the ears of the wizard
Conflicts of interest statement:The authors declare that they have no competing interests.
- 物理化学学报的其它文章
- Fluorinated TiO2 Hollow Photocatalysts for Photocatalytic Applications
- Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution
- 微波辅助快速制备2D/1D ZnIn2S4/TiO2 S 型异质结及其光催化制氢性能
- TiO2-Supported Single-Atom Catalysts for Photocatalytic Reactions
- Carboxyl-Functionalized Graphene for Highly Efficient H2-Evolution Activity of TiO2 Photocatalyst
- Review of Z-Scheme Heterojunctions for Photocatalytic Energy Conversion

