山豆根多糖对猪圆环病毒Ⅱ型感染免疫细胞增殖活性及炎症相关因子的影响
曹迷霞 陈奇 杨剑 刘梦倩 贾妮娜 韦英益 胡庭俊
摘要:【目的】探究山豆根多糖(SSP)對猪圆环病毒Ⅱ型(PCV2)感染RAW264.7细胞增殖活性及炎症相关因子的影响,揭示SSP对PCV2感染免疫细胞炎症相关因子的调控作用。【方法】PCV2体外感染RAW264.7细胞建立炎症模型,以不同浓度(25、50、100、200、400、800和1600 μg/mL)SSP进行培养处理,然后采用CCK-8和ELISA分别测定SSP对PCV2体外感染RAW264.7细胞增殖活性及炎症相关因子(IL-1β、IL-8和MCP-1)分泌水平和胞内环氧合酶-1(COX-1)活性的影响。【结果】与细胞对照组相比,SSP浓度≤400 μg/mL对RAW264.7细胞增殖活性无显著影响(P>0.05),但SSP浓度达800和1600 μg/mL时RAW264.7细胞增殖活性极显著降低(P<0.01,下同),且随培养时间的延长,细胞增殖活性呈先降低后升高的变化趋势,于培养48 h时达最低值。PCV2感染RAW264.7细胞后其增殖活性极显著降低,炎症相关因子IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性极显著升高;100~400 μg/mL SSP能极显著提高PCV2感染RAW264.7细胞增殖活性,且能有效降低RAW264.7细胞的IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性。具体表现为:与PCV2模型组相比,100 ?g/mL SSP能显著降低PCV2感染RAW264.7细胞的IL-1β和MCP-1分泌水平(P<0.05,下同);200 ?g/mL SSP能极显著降低PCV2感染RAW264.7细胞的MCP-1分泌水平,同时显著降低细胞IL-1β和IL-8的分泌水平及胞内COX-1活性;400 ?g/mL SSP能极显著降低PCV2感染RAW264.7细胞的IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性。【结论】SSP对RAW264.7细胞增殖活性无显著影响,也未表现出细胞毒性作用,且100~400 μg/mL SSP能极显著提高PCV2感染RAW264.7细胞增殖活性,并通过调节PCV2感染免疫细胞的炎症相关因子水平而发挥抗炎作用。
关键词: 猪圆环病毒Ⅱ型;山豆根多糖;RAW264.7细胞;增殖活性;炎症相关因子
中图分类号: S853.74 文献标志码: A 文章编号:2095-1191(2021)02-0439-09
Abstract:【Objective】This study aimed to investigate the proliferation activity and inflammation-related factors of Sophora subprostrate polysaccharide(SSP) on porcine circovirus Ⅱ(PCV2) infected RAW264.7 cells, and to reveal its regulation on PCV2 infected immune cells inflammation-related factors. 【Method】 Established the inflammatory model of PCV2 infected RAW264.7 cells in vitro, and then cultured with different concentrations(25, 50, 100, 200, 400, 800 and 1600 μg/mL) of SSP. CCK-8 method was used to determine the proliferation activity of RAW264.7 cells and PCV2 infec-ted RAW264.7 cells with SSP at different concentrations. The effect of SSP at different concentrations on inflammation-related factors(IL-1β, IL-8, MCP-1 and COX-1) in PCV2 infected RAW264.7 cells were determined by enzyme linked immunosorbent assay(ELISA). 【Result】Compared with the cell control group, the results showed that the concentration of SSP≤400 μg/mL had no significant effect on RAW264.7 cell viability(P>0.05), but SSP concentrations at 800 μg/mL and 1600 μg/mL extremely significantly decreased the viability of RAW264.7 cells(P<0.01,the same below). After PCV2 infection, the proliferation activity of RAW264.7 cells was extremely decreased, the secretion levels of inflammatory cytokines IL-1β, IL-8 and MCP-1 and the activity of intracellular COX-1 were extremely increased. 100-400 μg/mL of SSP exttemely increased the viability of PCV2 infected RAW264.7 cells, and the secretion levels of IL-1β, IL-8, MCP-1 and the activity of COX-1 in RAW264.7 cells were decreased compared to PCV2 group. Compared with PCV2 model group, 100 μg/mL of SSP significantly decreased IL-1β and MCP-1 secretion levels in PCV2 infected RAW264.7 cells(P<0.05, the same below); 200 μg/mL of SSP extremely decreased the secretion level of MCP-1, significantly decreased the secretion levels of IL-1β and IL-8, and the activity of COX-1 in PCV2 infected RAW264.7 cells; SSP at 400 μg/mL of could extremely reduce the levels of IL-1β, IL-8 and MCP-1 and the activity of COX-1 in PCV2 infected RAW264.7 cells. 【Conclusion】 SSP has no significant effect on the proliferation activity of RAW264.7 cells, and shows no cytotoxicity. 100-400 μg/mL of SSP extremely increases the proliferation activity of PCV2 infected RAW264.7 cells, and play an anti-inflammatory effect via regulating the secretion levels of inflammation-related factors.
Key words: porcine circovirus Ⅱ; Sophora subprostrate polysaccharide; RAW264.7 cell; proliferation activity; inflammation-related factors
Foundation item: National Natural Science Foundation of China(31960715); Project of Guangxi Graduate Education Innovation(YCBZ2020004)
0 引言
【研究意义】猪圆环病毒(Porcine circovirus,PCV)是圆环病毒科(Circoviridae)圆环病毒属(Circovirus)的成员之一,无囊膜,含有共价闭合的环状单链DNA基因组,主要包括PCV I型(PCV1)、PCV II型(PCV2)和PCV III型(PCV3)等基因型(刘国阳等,2019;张柱青等,2019;苏芮等,2020)。其中,PCV1对猪为非致病性,而PCV2和PCV3均对猪表现出致病性(贺会利等,2017)。PCV2能引发一大类免疫抑制性的多系统传染病,主要侵害机体的免疫系统(邓文芳等,2020)。单核细胞和巨噬细胞是PCV2的靶细胞,PCV2感染可引起仔猪断奶后多系统衰竭综合症、皮炎和肾病综合征、增生性坏死性间质性肺炎和繁殖障碍等,是猪圆环病毒相关疾病(Porcine circovirus associated disease,PCVD)的主要病原(方博,2019;沈顺新和和玉丹,2020)。山豆根多糖(Sophora subprostrate polysaccharide,SSP)是一种从越南槐(Sophora tonkinensis Gapnep.)根和根茎中经干燥后提取获得的多糖,具有多种生物活性,如抗炎、抗氧化、抗病毒及抗肿瘤等(彭湘君等,2012)。山豆根多糖可通过调节一氧化氮(NO)分子水平而影响免疫细胞内的cAMP/cGMP和6-keto-PGF1信号体系,进而调节免疫功能(帅学宏等,2010)。已有研究证实,山豆多糖通过抑制PCV2的CAP基因复制而增强免疫功能,达到抗病毒效果(Sun et al.,2020)。因此,明确SSP对PCV2感染细胞增殖活性及其相关炎症因子分泌水平的调节作用,可为PCVD的综合防控提供新思路。【前人研究进展】炎症是机体对致炎因子产生的一种应答性反应,而炎症细胞因子是指参与炎症反应的各类细胞因子(Yao et al.,2016;Ho et al.,2020)。SSP可调节炎症细胞因子表达,缓解炎症症状。Su等(2013)研究表明,SSP可缓解PCV2诱导的RAW264.7细胞氧化应激,降低PCV2感染细胞中活性氧(ROS)和NO生成,抑制过氧化物酶(MPO)活性和诱导型一氧化氮合酶(iNOS)表达。路海滨等(2018)研究发现,以SSP处理Lewis肺癌小鼠后其血清肿瘤坏死因子α(TNF-α)和血管内皮生长因子(VEGF)水平均显著升高,即SSP可通过调节血清TNF-α和VEGF水平以缓解炎症反应,进而达到抗肿瘤效果。Yang等(2020)探究SSP对PCV2感染RAW264.7细胞炎症反应及组蛋白乙酰化修饰的影响,发现SSP通过增加组蛋白去乙酰化酶(HDAC)活性和HDAC基因表达,以降低H3和H4的乙酰化水平并激活NF-κB/MAPKs/c-Jun信号通路,即通过调节炎症细胞因子表达水平进一步抑制炎症反应。此外,陈云等(2014)研究表明,山豆根多糖硫酸酯(sBSRPS)可作为抗Ⅰ型鸭肝炎病毒(Duck hepatitis virus 1,DHV-1)药物的重要组分。Chen等(2014,2018)、Voronov等(2014)通过对比分析SSP和sBSRPS对DHV-1在鸭肝细胞上复制及释放的影响,发现SSP和sBSRPS均能较好地发挥体外抗DHV-1作用,且sBSRPS的抗病毒效果更明显,能有效抑制DHV-1的体外复制和释放。何淼等(2020)也研究证实,SSP和sBSRPS均具有明显的体外抗DHV-1活性,且sBSRPS的抗病毒效果优于SSP,可显著下调DHV-1的体外复制和释放,即sBSRPS在体外对DHV-1具有良好的拮抗作用。可见,sBSRPS具备良好的抗病毒活性,可作为主要成分用于研发抗病毒新型药物。【本研究切入点】PCV2作用于细胞或组织会促使炎症细胞因子上调表达,尤其是TNF-α、IL-1β、IL-6、IL-8和IL-10等细胞因子,但这些炎症细胞因子的分泌时间段及上调幅度各不相同,其变化可能是病毒通过刺激炎症反应,导致动物机体免疫系统发生紊乱,最终促进PCV2感染相关疾病的发生发展(李海花等,2016;石坤等,2016;崔贝贝,2017;谭红连等,2017;张蕾等,2018)。至今尚无研究报道SSP对PCV2感染免疫细胞增殖活性及炎症相关因子的调节作用。【拟解决的关键问题】通过建立PCV2体外感染RAW264.7细胞的炎症模型,以不同浓度SSP处理后采用CCK-8和ELISA分别测定SSP对PCV2体外感染RAW264.7细胞增殖活性及其炎症相关因子(IL-1β、IL-8和MCP-1)分泌水平和胞内环氧合酶-1(COX-1)活性的影响,旨在揭示SSP对PCV2感染免疫细胞炎症相关因子的调控作用。
1 材料与方法
1. 1 试验材料
PCV2(SH株)为南京农业大学农业农村部动物疫病诊断与免疫重点开放实验室分离获得,后经本课题组采用猪肾细胞系(PK-15细胞)增殖保存;RAW264.7细胞购自武汉大学细胞库;SSP由广西大学动物科学技术学院兽医药理与毒理学实验室提取获得;脂多糖(LPS)购自上海索莱宝生物科技有限公司;细胞培养相关试剂包括DMEM高糖培养基(Gibco)、胎牛血清(Gibco)、青链霉素混合液(北京康为世纪生物科技有限公司)、蛋白酶K[生工生物工程(上海)股份有限公司]、饱和酚(上海索莱宝生物科技有限公司)、CCK-8试剂盒(上海碧云天生物技术有限公司);LA Taq DNA聚合酶购自TaKaRa公司;小鼠IL-1β(Lot.M191008-001a)、IL-8(Lot.M191008-104a)、MCP-1(Lot.M191008-113a)和COX-1(Lot.M191008-135a)等ELISA試剂盒购自深圳欣博盛生物科技有限公司。
1. 2 RAW264.7细胞复苏传代与培养
RAW264.7细胞复苏后用含10% FBS-DMEM完全培养液稀释并移至细胞培养瓶中,置于37 ℃、5% CO2培养箱中培养,待瓶底细胞贴壁融合至70%~80%后进行传代,连续稳定传代3次后即可用于后续试验。
1. 3 PCV2增殖及鉴定
将RAW264.7细胞稀释至5×104个/mL,按100 μL/孔添加至96孔细胞培养板中。将-80 ℃保存的PCV2接种至RAW264.7细胞,2 h后弃培养液,PBS洗涤3次后加入含5% FBS的DMEM培养液,置于37 ℃、5% CO2的培养箱中培养24 h,收集细胞悬液,反复冻融3次后5000 r/min离心5 min,提取DNA。采用PCR检测病毒核酸,根据GenBank已公布PCV2的ORF-2基因设计引物(F:5'-CACTTCTTTCGTTTTC AG-3'和R:5'-TTTATCACTTCGTAATGGT-3'),并委托深圳华大基因科技服务有限公司合成。PCR反应体系25.0 μL:Ex Taq DNA聚合酶12.5 μL,上、下游引物各1.0 μL,DNA模板2.0 μL,ddH2O 8.5 μL。扩增程序:94 ℃预变性3 min;94 ℃ 40 s,55.5 ℃ 40 s,72 ℃ 50 s,进行30个循环;72 ℃延伸7 min。PCR扩增产物以1.0%琼脂糖凝胶电泳进行检测。
1. 4 CCK-8测定SSP对RAW264.7细胞增殖活性的影响
将RAW264.7细胞稀释至5×104个/mL,按100 μL/孔添加至96孔细胞培养板中,置于37 ℃、5% CO2培养箱中培养,使其贴壁长成单层细胞融合至70%。如表1所示,分别设细胞对照组、LPS阳性对照组及不同浓度药物组,其中,细胞对照组只加入10% FBS-DMEM完全培养液,LPS阳性对照组加入终浓度为1 μg/mL的LPS(以10% FBS-DMEM完全培养液稀释),不同浓度药物组加入终浓度分别为25、50、100、200、400、800和1600 μg/mL的SSP(以10% FBS-DMEM完全培养液稀释),每组4个重复孔,进行3个平行试验。在37 ℃、5% CO2培养箱中培养12、24、48和72 h后,每孔分别加入10.0 μL CCK-8溶液继续孵育4 h,于450 nm处测定吸光值。
1. 5 CCK-8测定SSP对PCV2感染RAW264.7细胞增殖活性的影响
根据1.4的试验结果,筛选出SSP浓度25、50、100、200和400 μg/mL作为后续试验剂量。RAW264.7细胞传代培养后,调整其细胞浓度为5×104个/mL,按100 μL/孔添加至96孔细胞培养板中,置于37 ℃、5% CO2培养箱中培养过夜使其贴壁融合至70%。分别设细胞对照组、PCV2模型组及不同浓度药物组(表2),每组4个重复,进行3个平行试验。PCV2模型组和不同浓度药物组先用103 TCID50 PCV2悬液孵育感染2 h,弃病毒悬液后以PBS洗涤3次,再加入终浓度分别为25、50、100、200和400 μg/mL的SSP,细胞对照组和PCV2模型组加入10% FBS-DMEM完全培养液,置于37 ℃、5% CO2培养箱中培养,于培养12、24、48和72 h后,各处理组每孔分别加入10.0 μL CCK-8溶液继续孵育4 h,于450 nm处测定吸光值。
1. 6 SSP对PCV2诱导RAW264.7细胞炎症相关因子分泌水平的影响
取对数生长期的RAW264.7细胞,调整细胞浓度为1×105个/mL,添加至12孔细胞培养板中,1000 μL/孔,置于37 ℃、5% CO2培养箱中培养过夜,待其贴壁后進行试验处理,设细胞对照组、LPS阳性对照组、PCV2模型组及不同浓度(100、200和400 ?g/mL)药物组(表3),每组3个重复,进行3个平行试验。细胞对照组加入500 ?L DMEM培养液,LPS对照组加入500 ?L终浓度为1 ?g/mL的LPS, PCV2模型组和不同浓度药物组加入500 ?L的103 TCID50 PCV2悬液感染孵育2 h,弃病毒悬液,PBS洗3次;不同浓度药物组分别加入1000 μL含不同终浓度(100、200和400 ?g/mL)SSP的10% FBS-DMEM完全培养液,细胞对照组、LPS阳性对照组和PCV2模型组则加入1000 μL的10% FBS-DMEM完全培养液。置于37 ℃、5% CO2培养箱中继续培养24 h,收集细胞培养上清液置于1.5 mL灭菌EP管中,4 ℃下1500 r/min离心5 min,收集上清液,按ELISA试剂盒说明进行IL-1β、IL-8、MCP-1和COX-1测定。
2 结果与分析
2. 1 PCV2在RAW264.7细胞内的增殖鉴定结果
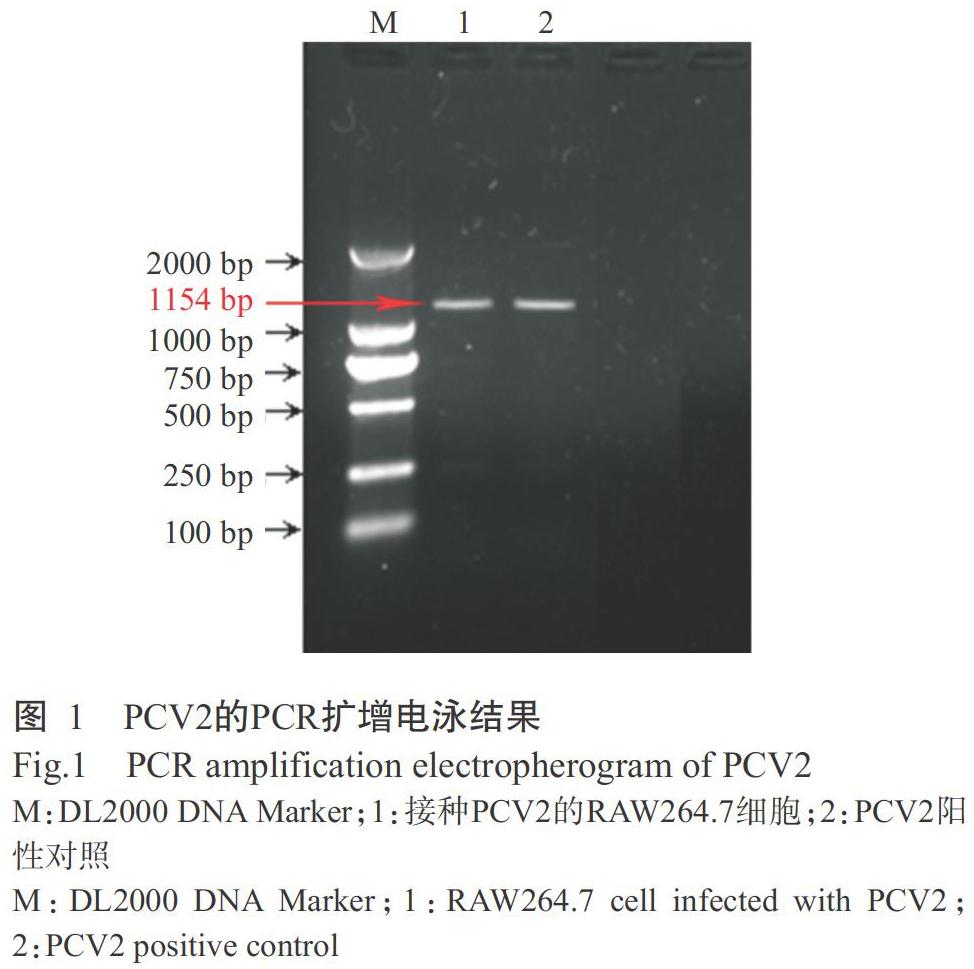
RAW264.7细胞接种PCV2后,反复冻融裂解RAW264.7细胞,释放病毒,提取病毒DNA,检测所提取DNA在260 nm和280 nm处的吸光值,得知A260/A280比值为1.81,表明提取获得的病毒DNA不存在蛋白质污染,纯度较高。以病毒DNA为模板进行PCR扩增,扩增产物经1.0%琼脂糖凝胶电泳检测得到单一明亮的目的条带(图1),扩增片段大小(1154 bp)与预期结果相符,即RAW264.7细胞成功感染PCV2。
2. 2 SSP对RAW264.7细胞增殖活性的影响
采用CCK-8检测SSP对RAW264.7细胞增殖活性的影响,结果(表4)显示,LPS处理RAW264.7细胞在各时间点的增殖活性均高于细胞对照组,且随着培养时间的延长,细胞增殖活性呈先升高后降低的变化趋势,以培养24 h时的细胞增殖活性最高,与细胞对照组间存在极显著差异(P<0.01,下同)。SSP浓度≤400 μg/mL时,在各时间点的RAW264.7细胞增殖活性均无显著差异(P>0.05,下同);但SSP浓度达800和1600 μg/mL时,各时间点的RAW264.7细胞增殖活性极显著低于细胞对照组,且随着培养时间的延长,细胞增殖活性呈先降低后升高的变化趋势,于培养48 h时达最低值。因此,在后续研究中SSP使用浓度不宜超过400 μg/mL。
2. 3 SSP对PCV2感染RAW264.7细胞增殖活性的影响
由表5可知,PCV2感染RAW264.7细胞后,其增殖活性极显著降低,且随着培养时间的延长,细胞增殖活性整体上呈逐渐降低趋势,于培养72 h时降至最低值。以25~50 μg/mL SSP培养PRV2感染RAW264.7细胞能有效提高细胞增殖活性,且培养72 h的细胞增殖活性极显著高于PCV2模型组;以100~400 μg/mL SSP培养PRV2感染RAW264.7细胞能极显著提高细胞增殖活性,且随着培养时间的延长,其细胞增殖活性越高,故选择100~400 ?g/mL SSP进行后续研究。
2. 4 SSP对PCV2感染RAW264.7细胞炎症相关因子分泌水平的影响
由表6可知,PCV2感染RAW264.7细胞后,其细胞IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性均极显著升高。与PCV2模型组相比,100 ?g/mL SSP能显著降低PCV2感染RAW264.7细胞的IL-1β和MCP-1分泌水平(P<0.05,下同);200 ?g/mL SSP能极显著降低PCV2感染RAW264.7细胞的MCP-1分泌水平,同时显著降低细胞IL-1β和IL-8的分泌水平及胞内COX-1活性;400 ?g/mL SSP能极显著降低PCV2感染RAW264.7细胞的IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性。表明100~400 ?g/mL的SSP能通过降低PCV2感染RAW264.7细胞的IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性,以缓解病毒引起的炎症反应。
3 讨论
山豆根化学成分含量丰富,药用价值高。SSP是山豆根的主要活性成分之一,具有调节免疫、抗炎、抗癌、抗病毒及抗氧化等多种生物活性(Cheng et al.,2013;Ji et al.,2014)。本研究通过测定SSP对RAW264.7细胞增殖活性的影响,以明确SSP对RAW264.7细胞增殖活性及细胞毒性影响的浓度范围,从而筛选出SSP对PCV2感染RAW264.7细胞增殖活性影响的最适宜浓度,进一步揭示SSP的抗炎作用,结果显示,在25~400 ?g/mL浓度范围内SSP对RAW264.7细胞增殖活性无显著影响,也未表现出细胞毒性作用,但SSP浓度为800~1600 ?g/mL时极显著抑制RAW264.7细胞增殖活性,与Gan等(2018)的研究结果相似,即1.78~35.60 μmol/L SSP对鸡胚肝细胞无细胞毒性,且对黄曲霉毒素诱导的鸡胚肝细胞损伤起保护作用。
炎症是动物机体对各种致炎因素引起损伤产生的防御性反应,而炎症相关因子在炎症过程中发挥关键作用,如IL-1β、IL-6、IL-8、COX-1和MCP-1等可促进炎症细胞聚集、活化及炎症介质释放(Kandalam and Clark,2010;Yin et al.,2018;Xiao et al.,2020)。IL-1β是一种重要的促炎因子,在细胞免疫中扮演重要角色,能与TNF-α产生相互协同作用,通过激活靶细胞内NF-κB信号通路而参与炎症反应,在机体免疫调节过程中发挥重要作用(Wulster-Radcliffe et al.,2004;Li et al.,2020);MCP-1是炎症反应的促发剂,可促进炎症细胞聚集,且具有多种生物活性,包括促进肿瘤细胞生长、刺激免疫应答及参与炎症反应等(Ou et al.,2020;Weber et al.,2020);IL-8在免疫应答的全过程中发挥重要作用,能吸引炎症细胞进入组织部位,激活巨噬细胞及增强其杀伤活性(Mohamed et al.,2020);COX-1與炎症发生密切相关,受致炎因素刺激后,可分泌前列腺素(PGE2),活化小神经胶质细胞,进而释放促炎介质IL-1、IL-6、TNF6、NO及PG等参与炎症反应(Liedtke et al.,2012)。已有研究表明,PCV2感染免疫细胞可导致炎症相关因子的表达发生改变,尤其是IL-6、IL-8、IL-10、TNF-α和免疫调节因子IFN-γ(Borghetti et al.,2013)。汪伟等(2016)对PCV2体外感染3D4/21细胞的研究发现,PCV2感染对炎症相关因子IL-1β和IL-8的表达起促进作用。在本研究中,采用103 TCID50的PCV2感染RAW264.7细胞后,其细胞IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性均极显著升高,说明PCV2能刺激RAW264.7细胞分泌炎症相关因子,从而促进炎症反应的发生,即PCV2感染诱导RAW264.7细胞炎症模型构建成功。
近年来,有关中药活性多糖对免疫细胞炎症相关因子分泌的调节作用研究逐渐增多。李胜亮等(2006)研究发现,经10 mg/L LPS刺激后肺血管内巨噬细胞释放TNF-α、IL-6和IL-8增多;仲芳等(2009)研究表明,姜黄素可下调LPS诱导人类肾小管近端上皮细胞MCP-1和IL-8的分泌;陈潇等(2012)在研究灵芝多糖对动脉粥样硬化预防与治疗作用机理时发现,其高剂量组的血清TNF-α分泌水平较模型组显著降低,表明灵芝多糖可有效抑制炎症相关因子的分泌;王松等(2012)研究显示,复方甘草酸苷可明显降低LPS诱发RAW264.7细胞生成促炎因子NO、TNF-α、IL-1β和IL-6,并促进抗炎因子IL-10表达;Liu等(2015)研究发现,酸枣多糖对四氯化碳(CCl4)诱导的小鼠肝毒性损伤有显著治疗作用;Xue等(2015)研究表明,黄芪多糖可通过抑制氧化应激和阻断NF-κB途径来抑制PCV2复制。本研究结果显示,PCV2感染RAW264.7细胞后极显著提高其IL-1β、IL-8和MCP-1分泌水平及胞内COX-1活性,而100~400 ?g/mL SSP能显著或极显著降低PCV2感染RAW264.7细胞IL-1β、IL-8、MCP-1分泌水平及胞内COX-1活性,与Sun等(2020)的研究结果相似。可见,SSP是通过调节PCV2感染免疫细胞的炎症相关因子水平而发挥抗炎作用。
4 结论
SSP对RAW264.7细胞增殖活性无显著影响,也未表现出细胞毒性作用,且100~400 μg/mL SSP能显著提高PCV2感染RAW264.7细胞增殖活性,并通过调节PCV2感染免疫细胞的炎症相关因子水平而发挥抗炎作用。
参考文献:
陈潇,王爽,孟国梁,常珊珊,陈惠,郑惠华,徐济良. 2012. 灵芝多糖对动脉粥样硬化大鼠炎症因子表达的影响[J]. 中药新药与临床药理,23(3):251-254. doi:10.3969/j.issn.1003-9783.2012.03.004. [Chen X,Wang S,Meng G L,Chang S S,Chen H,Zheng H H,Xu J L. 2012. Effects of Ganoderma lucidum polysaccharids on expression of inflammatory factors in atherosclerosis rats[J]. Traditional Chinese Drug Research and Clinical Pharmacology,23(3):251-254.]
陈云,曾玲,熊文,张玲,张清,王德云,刘家国. 2014. 山豆根多糖及其硫酸酯体外抗DHV-1感染细胞作用的比较[J]. 南京农业大学学报,37(4):117-122. doi:10.7685/j.issn.1000-2030.2014.04.017. [Chen Y,Zeng L,Xiong W,Zhang L,Zhang Q,Wang D Y,Liu J G. 2014. Comparison of bush sophora root polysaccharide and its sulfate against DHV-1 infecting cell in vitro[J]. Journal of Nanjing Agricultural University,37(4):117-122.]
崔貝贝. 2017. IL-10在PCV2感染与复制中的调控作用[D]. 杨凌:西北农林科技大学. [Cui B B. 2017. The regulation of IL-10 in the PCV2 infection and replication[D]. Yangling:Northwest A & F University.]
邓文芳,宋文博,胡星星,贾双,陈翔鸿,喻红艳,汤细彪. 2020. 2017~2018年华中地区猪圆环病毒2型分子流行病学分析[J]. 中国动物传染病学报,28(2):32-38. [Deng W F,Song W B,Hu X X,Jia S,Chen X H,Yu H Y,Tang X B. 2020. Molecular epidemiological analysis of porcine circovirus virus type 2 in central China during 2017-2018[J]. Chinese Journal of Animal Infectious Di-seases,28(2):32-38.]
方博. 2019. 猪圆环病毒2型及其综合防控[J]. 广东饲料,28(7):50-51. [Fang B. 2019. Prevention and control of porcine circovirus type 2[J]. Guangdong Feed,28(7):50-51.]
何淼,张宝康,粟灵琳,杜红旭,明珂,白景英,刘家国,王德云,武毅. 2020. 磷酸化修饰对山豆根多糖抗Ⅰ型鸭肝炎病毒效果的影响[J/OL]. 南京农业大学学报. doi:10. 7685/jnau.202003030. [He M,Zhang B K,Su L L,Du H X,Ming K,Bai J Y,Liu J G,Wang D Y,Wu Y. 2020. Effect of phosphorylation on resisting duck hepatitis virus I effect of Bush Sophora Root polysaccharide[J/OL]. Journal of Nanjing Agricultural University. doi:10.7685/jnau. 202003030.]
贺会利,李军,潘艳,胡帅,冯世文,彭昊,李常挺,陈泽祥,杨威. 2017. 广西首例猪圆环病毒3型的发现及其衣壳蛋白序列分析[J]. 南方农业学报,48(8):1499-1503. doi:10.3969/j.issn.2095-1191.2017.08.27. [He H L,Li J,Pan Y,Hu S,Feng S W,Peng H,Li C T,Chen Z X,Yang W. 2017. The first report of porcine circovirus type 3 infection in Guangxi and sequence analysis of its capsid protein[J]. Journal of Southern Agriculture,48(8):1499-1503.]
李海花,张蕾,杨春蕾,赵向华,乔家运,王文杰. 2016. PCV2通过NF-κB/NLRP3信号通路调控体外培养PAMs分泌IL-1β[J]. 中国畜牧兽医,43(9):2366-2372. doi:10.16431/j.cnki.1671-7236.2016.09.022. [Li H H,Zhang L,Yang C L,Zhao X H,Qiao J Y,Wang W J. 2016. PCV2 regulates PAMs secreting IL-1β through the NF-κB/NLRP3 signalling pathway in vitro[J]. China Animal Husbandry & Veterinary Medicine,43(9):2366-2372.]
李胜亮,张淑琴,秦翠平,陈彬,陈正堂,金敬顺,伍伟玲. 2006. 脂多糖致肺血管内巨噬细胞释放炎症因子变化的研究[J]. 中国危重病急救医学,18(3):136-138. doi:10.3760/j.issn:1003-0603.2006.03.003. [Li S L,Zhang S Q,Qin C P,Chen B,Chen Z T,Jin J S,Wu W L. 2006. Changes in inflammatory cytokines released by pulmonary intravascular macrophages after stimulation with lipopolysaccharide[J]. Chinese Critical Care Medicine,18(3):136-138.]
刘国阳,唐波,常晨,华涛,侯继波,张道华. 2019. 猪圆环病毒2型与猪细小病毒混合感染对仔猪致病性的研究[J]. 江西农业学报,31(9):79-85. doi:10.19386/j.cnki.jxnyxb. 2019.09.14. [Liu G Y,Tang B,Chang C,Hua T,Hou J B,Zhang D H. 2019. Athogenicity of porcine circovirus type 2 and porcine parvovirus co-infection in piglets[J]. Acta Agriculturae Jiangxi,31(9):79-85.]
路海濱,高洋,禹珊珊,赵倩倩,宋玉明. 2018. 山豆根多糖对Lewis肺癌小鼠抑瘤作用及免疫功能影响的实验研究[J]. 中药材,41(6):1459-1462. doi:10.13863/j.issn.1001-4454.2018.06.043. [Lu H B,Gao Y,Yu S S,Zhao Q Q,Song Y M. 2018. Experimental study of the effect of the Sophorae tonkinensis polysaccharide on the tumor inhibition and immune function in lewis lung cancer bearing mice[J]. Journal of Chinese Medicinal Materials,41(6):1459-1462.]
彭湘君,李银保,李青松. 2012. 山豆根多糖的研究进展[J]. 湖北农业科学,51(24):5559-5561. [Peng X J,Li Y B,Li Q S. 2012. The pesearch progress of polysaccharide from radix Sophorae tonkinensis[J]. Hubei Agricultural Sciences,51(24):5559-5561.]
沈顺新,和玉丹. 2020. 猪圆环病毒病的危害及其防控[J]. 今日畜牧兽医,36(4):27-28. [Shen S X,He Y D. 2020. The harm of porcine circovirus disease and its prevention and control[J]. Today Animal Husbandry and Veterinary Medicine,36(4):27-28.]
石坤,魏建超,邱亚峰,张克龙,李宗瑞,刘茜倩,李蓓蓓,刘珂,邵东华,陈立志,马志永. 2016. PCV2与PRRSV体外共感染对肺泡巨噬细胞系炎症反应的影响[J]. 中国动物传染病学报,24(3):16-20. [Shi K,Wei J C,Qiu Y F,Zhang K L,Li Z R,Liu X Q,Li B B,Liu K,Shao D H,Chen L Z,Ma Z Y. 2016. Inflammatory response of a pulmonary alveolar macrophage in vitro response to porcine circovirus tyre 2 and porcine reproductive and respiratory syndrome virus co-infection[J]. Chinese Journal of Animal Infectious Diseases,24(3):16-20.]
帅学宏,苏子杰,胡庭俊,曾芸,韦英益,李跃华. 2010. 山豆根多糖对鸡脾脏淋巴细胞信号转导相关分子水平的影响[J]. 动物医学进展,31(1):36-41. doi:10.16437/j.cnki. 1007-5038.2010.01.009. [Shuai X H,Su Z J,Hu T J,Zeng Y,Wei Y Y,Li Y H. 2010. Effects of Sophora subprostrate polysaccharide on signal transduction related molecules in chicken splenic lymphocytes[J]. Progress in Veterinary Medicine,31(1):36-41.]
苏芮,王东亮,陈指龙,范秀军,邓治邦,杨青. 2020. PCV2 ORF1~ORF4基因所编码蛋白功能的研究进展[J]. 经济动物学报,24(1):46-51. doi:10.13326/j.jea.2018.1357. [Su R,Wang D L,Chen Z L,Fan X J,Deng Z B,Yang Q. 2020. Research progress on major function proteins encoded by PCV2 ORF1-ORF4 gene[J]. Journal of Economic Animal,24(1):46-51.]
谭红连,杨剑,尹丹,郝祝兵,韦英益,胡庭俊. 2017. 马尾藻多糖对PCV-2体外感染3D4/2细胞活性及炎症相关因子的影响[J]. 江苏农业科学,45(23):166-168. doi:10.15889/j.issn.1002-1302.2017.23.046. [Tan H L,Yang J,Yin D,Hao Z B,Wei Y Y,Hu T J. 2017. Effects of Sargassum sargassum polysaccharides on cell activity and inflammatory related factors of 3D4/2 cells infected with PCV-2 in vitro[J]. Jiangsu Agricultural Science,45(23):166-168.]
汪伟,王小敏,何孔旺,温立斌,倪艳秀. 2016. 猪圆环病毒2型体外刺激3D4/21猪肺泡巨噬细胞的炎症相关细胞因子mRNA转录分析[J]. 西南农业学报,29(5):1225-1228. doi:10.16213/j.cnki.scjas.2016.05.042. [Wang W,Wang X M,He K W,Wen L B,Ni Y X. 2016. Inflammation-associated cytokines mRNA transcriptional profiles of porcine alveolar macrophages cell lines 3D4/21 stimulated by porcine circovirus type 2[J]. Southwest China Journal of Agricultural Sciences,29(5):1225-1228.]
王松,王微,罗猛,赵修华,祖元刚,赵艳丽. 2012. 复方甘草酸苷制剂对脂多糖诱导小鼠RAW264.7细胞分泌炎症因子的调节作用[J]. 药学进展,36(10):465-470. doi:10.3969/ j.issn.1001-5094.2012.10.005. [Wang S,Wang W,Luo M,Zhao X H,Zu Y G,Zhao Y L. 2012. Regulating effect of gompound glycyrrhizin on LPS-induced inflammatory factor release from murine RAW264.7 cells[J]. Progress in Pharmaceutical Sciences,36(10):465-470.]
張蕾,代松宝,张丽琳,时培殿,王家顺,任婕,黄金海. 2018. PCV2逃逸宿主天然免疫的分子机制研究[J]. 华北农学报,33(2):149-156. doi:10.7668/hbnxb.2018.02.021. [Zhang L,Dai S B,Zhang L L,Shi P D,Wang J S,Ren J,Huang J H. 2018. Molecular mechanisms of PCV2 escape from innate immunity[J]. Acta Agriculturae Boreali-Sinica,33(2):149-156.]
张柱青,吕其壮,卓严玲,邓启霞. 2019. 猪圆环病毒2型Cap蛋白体外表达技术研究进展[J]. 河南农业学报,48(9):1-6. doi:10.15933/j.cnki.1004-3268.2019.09.001. [Zhang Z Q,Lü Q Z,Zhuo Y L,Deng Q X. 2019. Research pro-gress on the expression technology of the Cap protein of porcine circovirus type 2 in vitro[J]. Journal of Henan Agricultural Sciences,48(9):1-6.]
仲芳,陈慧,韩琳,靳远萌,王伟铭,陈楠. 2009. 姜黄素对脂多糖刺激的肾小管近端上皮细胞分泌的相关炎症因子的影响[J]. 肾脏病与透析肾移植杂志,18(3):236-241. doi:10.3969/j.issn.1006-298X.2009.03.007. [Zhong F,Chen H,Han L,Jin Y M,Wang W M,Chen N. 2009. Curcumin inhibits expression of pro-inflammation factors in renal tubular epithelial cells induced by lipopolysaccharide[J]. Chinese Journal of Nephrology,Dialysis & Transplantation,18(3):236-241.]
Borghetti P,Morganti M,Saleri R,Ferrari L,de Angelis E,Cavalli V,Cacchioli A,Corradi A,Martelli P. 2013. Innate pro-inflammatory and adaptive immune cytokines in PBMC of vaccinated and unvaccinated pigs naturally exposed to porcine circovirus type 2(pcv2) infection vary with the occurrence of the disease and the viral burden[J]. Veterinary Microbiology,163(1-2):42-53. doi:10. 1016/j.vetmic.2012.12.007.
Chen Y,Xiong W,Zeng L,Wang D Y,Liu J G,Wu Y,Hu Y L. 2014. Comparison of Bush Sophora Root polysaccharide and its sulfate?s anti-duck hepatitis A virus activity and mechanism[J]. Carbohydrate Polymers,102:333-340. doi:10.1016/j.carbpol.2013.11.065.
Chen Y,Yang Y H,Yuan W J,Wang Z H,Ming K,Zeng L,Liu J G. 2018. Effects of Bush Sophora Root polysaccharide and its sulfate on DHAV-1 replication[J]. Carbohydrate Polymers,19:508-514. doi:10.1016/j.carbpol.2018. 06.039.
Cheng H R,Feng S L,Shen S A,Zhang L,Yang R W,Zhou Y H,Ding C B. 2013. Extraction,antioxidant and antimicrobial activities of Epimedium acuminatum Franch. polysaccharide[J]. Carbohydrate Polymers,96(1):101-108. doi:10.1016/j.carbpol.2013.03.072.
Gan F,Yang Y L,Chen Y,Che C P,Pan C L,Huang K H. 2018. Bush Sophora Root polysaccharide could help prevent aflatoxin B1-induced hepatotoxicity in the primary chicken hepatocytes[J]. Toxicon,150:180-187. doi:10. 1016/j.toxicon.2018.05.019.
Ho D R,Chang P J,Lin W Y,Huang Y C,Chen C S,Lin J H,Huang K T,Chan W N,Chen C S. 2020. Beneficial effects of inflammatory cytokine-targeting aptamers in an animal model of chronic prostatitis[J]. International Journal of Molecular Sciences,21(11):3953. doi:10.3390/ijms21113953.
Ji P,Wei Y M,Xue W X,Hua Y L,Zhang M,Sun H G,Song Z X,Zhang L,Li J X,Zhang W Q. 2014. Characterization and antioxidative activities of polysaccharide in Chinese angelica and its processed products[J]. International Journal of Biological Macromolecules,67:195-200. doi:10.1016/j.ijbiomac.2014.03.025.
Kandalam U,Clark M A. 2010. Angiotensin II activates JAK2/STAT3 pathway and induces interleukin-6 production in cultured rat brainstem astrocytes[J]. Regulatory Peptides,159(1-3):110-116. doi:10.1016/j.regpep.2009. 09.001.
Li Y F,Huang B,Ye T T,Wang Y,Xia D J,Qian J. 2020. Physiological concentrations of bilirubin control infla-mmatory response by inhibiting NF-κB and inflammasome activation[J]. International Immunopharmacology,84: 106520. doi:10.1016/j.intimp.2020.106520.
Liedtke A J,Crews B C,Daniel C M,Blobaum A L,Kingsley P J,Ghebreselasie K,Marnett L J. 2012. Cyclooxygenase-1-selective inhibitors based on the (E)-2'-des-methyl-sulindac sulfide scaffold[J]. Journal of Medicinal Chemistry,55(5):2287-2300. doi:10.1021/jm201528b.
Liu G P,Liu X Q,Zhang Y C,Zhang F,Wei T,Yang M,Wang K M,Wang Y J,Liu N,Zhao Z X. 2015. Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao[J]. International Journal of Biological Macromolecules,76:169-175. doi:10.1016/j.ijbiomac.2015.01.061.
Mohamed H T,El-Ghonaimy E A,El-Shinawi M,Hosney M,G?tte M,Woodward W A,El-Mamlouk T,Mohamed M M. 2020. IL-8 and MCP-1/CCL2 regulate proteolytic activity in triple negative inflammatory breast cancer a mechanism that might be modulated by Src and Erk1/2[J]. Toxicology and Applied Pharmacology,401:115092. doi:10.1016/j.taap.2020.115092.
Ou X C,Ying J W,Bai X D,Ruan D K. 2020. Activation of SIRT1 promotes cartilage differentiation and reduces apoptosis of nucleus pulposus mesenchymal stem cells via the MCP1/CCR2 axis in subjects with intervertebral disc degeneration[J] International Journal of Molecular Medicine,46(3):1074-1084. doi:10.3892/ijmm.2020.4668.
Su Z J,Wei Y Y,Yin D,Shuai X H,Zeng Y,Hu T J. 2013. Effect of Sophora subprosrate polysaccharide on oxidative stress induced by PCV2 infection in RAW264.7 cells[J]. International Journal of Biological Macromolecules,62:457-464. doi:10.1016/j.ijbiomac.2013.09.026.
Sun N,Zhang H,Sun P P,Khan A,Guo J H,Zheng X Z,Sun Y G,Fan K H,Yin W,Li H Q. 2020. Matrine exhibits antiviral activity in a PRRSV/PCV2 co-infected mouse model[J]. Phytomedicine,77:153289. doi:10.1016/j.phymed.2020.153289.
Voronov E,Carmi Y,Apte R N. 2014. The role IL-1 in tumor-mediated angiogenesis[J]. Frontiers in Physiology,5:114. doi:10.3389/fphys.2014.00114.
Weber R,Riester Z,Hüser L,Sticht C,Siebenmorgen A,Groth C,Hu X Y,Altevogt P,Utikal J S,Umansky V. 2020. IL-6 regulates CCR5 expression and immunosuppressive capacity of MDSC in murine melanoma[J]. Journal for Immunotherapy of Cancer,8(2):e000949. doi:10. 1136/jitc-2020-000949.
Wulster-Radcliffe M C,Ajuwon K M,Wang J Z,Christian J A,Spurlock M E. 2004. Adiponectin differentially regulates cytokines in porcine macrophages[J]. Biochemical and Biophysical Research Communications,316(3):924-929. doi:10.1016/j.bbrc.2004.02.130.
Xiao W D,Fu L,Gu C W,Wang R,Zhao M D,Wang J,Jia X B,Chen S Y,Lai S J. 2020. β-Glucan augments IL-1β production by activating the JAK2/STAT3 pathway in cultured rabbit keratinocytes[J]. Microbial Pathogenesis,144:104175. doi:10.1016/j.micpath.2020.104175.
Xue H X,Gan F,Zhang Z Q,Hu J F,Chen X X,Huang K H. 2015. Astragalus polysaccharides inhibits pcv2 replication by inhibiting oxidative stress and blocking NF-κB pathway[J]. International Journal of Biological Macromolecules,81:22-30. doi:10.1016/j.ijbiomac.2015.07.050.
Yang J,Cao M X,Hu W Y,Wei Y Y,Hu T J. 2020. Sophora subprosrate polysaccharide suppress the inflammatory reaction of RAW264.7 cells infected with PCV2 via regulation NF-κB/MAPKs/c-Jun signal pathway and histone acetylation modification[J]. International Journal of Biological Macromolecules,159:957-965. doi:10.1016/j.ijbiomac.2020.05.128.
Yao Y,Zhu Y Y,Ren G X. 2016. Antioxidant and immunoregulatory activity of alkali-extractable polysaccharides from mung bean[J]. International Journal of Biological Macromolecules,84:289-294. doi:10.1016/j.ijbiomac. 2015.12.045.
Yin L F,Dai Q J,Jiang P P,Zhu L,Dai H F,Yao Z G,Liu H,Ma X P,Qu L X,Jiang J K. 2018. Manganese exposure facilitates microglial JAK2-STAT3 signaling and consequent secretion of TNF-a and IL-1β to promote neuronal death[J]. Neurotoxicology,64:195-203. doi:10.1016/j.neuro.2017.04.001.
(責任编辑 兰宗宝)

