Trophic Ecology of Ansonia latidisca at Gunung Penrissen,Sarawak,North-Western Borneo
Jia Jet ONG and Indraneil DAS
Institute of Biodiversity and Environmental Conservation,Universiti Malaysia Sarawak,Kota Samarahan 94300,Sarawak,Malaysia
Abstract Dietary data on Ansonia latidisca,the little known Bornean Rainbow Toad,are presented,through an investigation of a population at Gunung Penrissen,Sarawak,Malaysia (north-western Borneo),at elevations between 1,100-1,229 m asl.Standard sampling techniques,including visual encounter surveys,were employed and individuals encountered stomach-flushed,marked,and released.The volume of food ingested by adults,apart from large females,did not vary monthly,and there was no significant difference between wet and dry periods,the dominance index between the two periods showing no significant difference,indicating that seasonal variation does not affect the dietary constitution of the species across months.The mean longest prey was recovered from a female in March,during the end of the wet season.Larger individuals did not consume larger prey in the species,although larger females did harvest the largest insects,and as may be expected,had fewer prey items in their stomachs at the time.Males fed on fewer prey items than females,the adult male diet predominantly comprised of members of the Hymenoptera,including formicids (ants),as indicated by percentage frequency of occurrence of 78.69,while for adult females,equivalent figure was 90.70.Coleopterans (beetles) were found secondary in importance (48.8% in females,5.85% in males),the rest categorized as tertiary.Plant items (including bryophytes) had a high frequency of occurrence (23.3% in females,4.64% in males).Females examined in the month of July had voluminous stomach contents,and may have eaten more to increase fat reserves for breeding in the upcoming wet season.Since the diet of all size classes consisted of hymenopterans (ants) and coleopterans,the study species is here considered to have a narrow food preference,and consequently,is a dietary specialist.
Keywords Ansonia latidisca,Borneo,diet,ecology,Malaysia,Sarawak
1.Introduction
Anuran amphibians are traditionally described as generalist predators,with opportunistic foraging behaviour (Santoset al.
,2004).A majority of the invertebrates reported in adult frog diets are arthropods,including arachnids,crustaceans,orthopterans,coleopterans,and especially,ants and mites (Cogălnicueanuet al.
,2000;Hirai and Matsui,2001;Kovacset al.
,2010;Maneyroet al.
,2004;Ogoanah and Uchedike,2010;Toft,1980a,1980b;Van Sluyset al.
,2006).Small vertebrates,such as fish and frogs,may be occasionally eaten,especially by largegrowing species (Santoset al.
,2004;Measeyet al.
,2015),and cannibalism has also been documented (Inger,1966a;Ogoanah and Uchedike,2010).Plant material and stones may sometimes occur in stomach samples in small amounts,and are probably taken incidental to food (Berry,1966),except in a couple of species (Das,1995;da Silvaet al.
,1989).Taking into account the number of prey items consumed and the contribution of each prey item to the overall diet,anuran amphibians can be organized in a feeding behaviour gradient,from generalist to specialist (da Rosaet al.
,2002).Many species show some degree of dietary specialization and recognized as specialist feeders that are often associated with high proportions of a particular food from the food spectrum available (Toft,1980a;Santoset al.
,2004).The two widely considered ends of the dietary continuum in tropical anurans include the “ant specialists”,which tend to be toxic to potential predators and active searchers of prey,taking many small prey that usually show a clumped distribution,such as ants and termites,and the “non-ant specialists or generalists”,that are cryptic,sit-and-wait foragers,which take relatively few,but larger prey types (Toft,1980a).Ants and other small insects make up the diet of probably allAnsonia
species (Ingeret al.
,2017),as in the bufonidae in general,as well as in members of the family Microhylidae (Ahmad Sahet al
.,2019).Amphibians as mesopredators of arthropods are gradually receiving their deserved attention (Hernández-Salinaset al.
,2018;Luria-Manzano and Ramírez-Bautista,2019;Ahmad Sahet al.
,2019),and apart for contribution to growth and reproduction,have been associated with predator defence (e.g.,Dalyet al.
,1994).Ansonia latidisca
,the Bornean Rainbow Toad (Figure 1),is known to be restricted to two locations in north-western Borneo-Gunung Damus in western Kalimantan and Gunung Penrissen in western Sarawak (Inger,1966;Puiet al
.,2011).The species is currently listed as Endangered in the IUCN Red List of Threatened Species (Ingeret al.
,2018).Little is known of the life history of the species,although like its congeners,it is expected to show riparian-adapted tadpoles,with modified mouthparts and other torrenticole features (see Inger,1985,1992;Inger and Dring,1988).Its advertisement call has been recently described (Ong and Das,2019),although further details of its breeding biology remain unstudied.This communication presents information on the trophic ecology ofAnsonia latidisca
,as a contribution to its conservation and management.Questions asked include:What are the primary components of the diet of the species? Does food volume or type vary seasonally? Are there sexual or ontogenetic differences in diet,in terms of prey type and quantity? And finally,is the species a dietary generalist or specialist?
2.Materials and Methods
2.1.Study Area
Gunung Penrissen is located in western Sarawak,between latitudes 01.12 °N to 01.14 °N and longitudes 110.21°E to 110.23°E,and the range forms the natural boundary between Malaysia’s Sarawak State and Indonesia’s Kalimantan Barat Province (Figure 2).It is drained mainly by Sungei Semadang and forms the headwaters of Batang Kayan.The massif is comprised of a matrix of sandstone and karst features,rising to the rugged ridges of the Penrissen Range (Wilford and Kho,1965).
Figure 1 Ansonia latidisca,from Gunung Penrissen,Sarawak,northwestern Borneo.

Figure 2 Digital elevation model of Gunung Penrissen and adjacent areas of western Sarawak,on western Borneo.Triangle refers to location of Bungo Dam.Image generated by Hans Hazebroek.
Western Sarawak experiences two predominant monsoon periods,i.e.,the Northeast Monsoon which prevails from November to March and the Southwest Monsoon,from June to September (Malaysia Meteorological Department,2016).Inland areas of Sarawak,such as the Penrissen Range,generally experience evenly distributed annual rainfall.Slightly less rainfall is received during the period June to August,which corresponds to the prevailing south-westerly winds.April,May,October are considered non-monsoonal periods.However,these are generalities,and heavy rainstorms can be experienced during the Southwest Monsoon.Daily temperature fluctuates from 17.5-25.5°C in the study site.
The forest type at the higher elevation of Gunung Penrissen (800-1,300 m asl) is predominantly primary highland mixed dipterocarp forest,with trails that permit access to the summit of Penrissen.Old growth trees are sparsely distributed along the trails,but understory plants are abundant,ranging from seedlings of trees,wild gingers,aroid plants,rattans,shrubs,climbers,ferns and mosses.Due to high humidity levels,tree trunks are festooned with mosses and lichens.
Surveys were conducted along forest transects.Four transect lines (P,Q,R and S) were established,each of 500 m length,except transect line ‘Q’,which was 250 m,on account of a landslide in late February 2012 that impeded the extension of the transect.
Transect P
(999-1,141 m asl;Highland mixed dipterocarp forest).Relatively flat terrain,with steep slopes after the first 200 m.A few large dipterocarp trees with large crowns,with diameter at breast height (DBH) range 90-120 cm and height range 20-40 m observed.The forest canopy cover is ca.60%-70% and there are two permanent streams within the transect.Low leaf litter coverage,of average depth ca.2.5 cm.(900-1,000 m asl;HMDF).
Relatively flat area within first 200 m of transect line,with steep slopes till the end.Canopy cover ca.70%-85% with few large trees along trail,mostly comprisingShorea
sp.(Dipterocarpaceae).Leaf litter heavy,average litter depth 6 cm.Low number of understory plants along trail due to steep slopes.(1,146-1,229 m asl).
Forest type gradually changes to intermediate mossy forest at an altitude of 1,100 m asl.Canopy cover ca.60%-85%,with dense leaf litter,average litter depth 6.5 cm.Abundance of understory plants,ranging from seedling of trees,shrubs,grasses,ferns and fern allies,Pandanus
species,palms,wild gingers,mosses,lichens and pitcher plants.At highest elevation within transect line,trees prone to lightning strikes.(1,146-1,204 m asl).
Small trees (≤ 5 cm in DBH) recorded along transect line.Few mid-sized trees (30-45 cm and 15-20 m in height) present.Canopy cover is ca.65%-80%.Relatively high leaf litter layer,ca.6.5 cm,and high understory plant density,with seedlings of trees,wild gingers,mosses and lichens,climbers,creepers,aroid plants and rattans in abundance.2.2.General Field Methods
Field work was conducted from January to December 2012,with a total of 42 nights spent in visual encounter surveys (Heyeret al.
,1994),involving 2-3 field investigators,walking along the transect between 1800-2400 h,when the species was active (exposed and sometimes calling).Additional surveys involving two field personnel over a period of 20 field days were carried out at other potential habitats,between August and September 2010,and covered wider range of habitats at different altitudes (particularly 800-1,000 m),the then known elevational range for the species.These were unproductive in terms of encounters with the target species.Individuals encountered were marked with narrow strips of colour-coded balloon rubber and released at the point of capture.The following data were taken for every individual encountered:measurements (including snout-vent length (SVL) and mouth width (HW),using a Mitutoyovernier caliper);sex and stage (male,female or juvenile);and dietary contents by the stomach flushing method.Each individual was weighed to the nearest 0.1 gm,using a 10 gm Pesolaspring balance.Sexes of adult toads were determined by the presence of single median external vocal sacs,restricted to adult males that were smaller than adult females.Individuals of indeterminable sex (typically,the smallest size-classes) were categorized as juveniles.
2.3.Dietary Study Techniques
Stomach flushing method was modified from the protocol of Mahan and Johnson (2007) and Solé and Rödder (2010).A 10 ml syringe was loaded with water and injected into the toad’s stomach via a piece of plastic tubing.Stomach content was flushed onto a glass petri dish.The stomach contents were stored in plastic numbered vials and preserved in 70% ethanol (Limaet al.
,2010).In the laboratory,samples were spread onto a petri dish and examined under low magnification,using an OlympusSZX9 dissecting microscope,to the ordinal level of classification.Parts of unidentified insect are categorized as ‘unknown arthropods’ and organic material refers to plants that were presumably consumed incidentally.The size of fragmented specimens was estimated based on other specimens with intact bodies.Total volume of food ingested by each individual was estimated by the displacement of water in a graduated cylinder (Das,1995).Analysis of data pertaining to contents of stomach samples included:
Percent frequency of occurrence (%F
)=Number of targeted individual in which a given food item was found / Number of individuals that provided dietary samples × 100Thereafter,food groups were classified as primary when registered in > 50% of stomachs,secondary when between 25%-50%,and tertiary when < 25% of stomach samples,following Santoset al.
(2004).Non-Metric Dimensional Scale (NMDS) analyses was used to visualise the findings,using IBM SPSSStatistics 20.Individual frog prey dimensions were averaged to calculate mean prey length (APL) and total stomach content volume (Woodheadet al.
,2007).Correlation and linear regressions were performed after log transformation of data.The Berger-Parker diversity index values was used to compare for differences in the degree of dominance of food types in adult stomach samples throughout the sampling period:
d=N
/N
where,N
is the total number of individuals andN
,the number of individuals in the most abundant resource type.The reciprocal form of the measure was used,whereby the index increases with increasing prey diversity and a decrease in dominance (Magurran,2004).For seasonal comparisons,data were grouped in two periods:wet (January-March and November-December) and dry (April-October),although some precipitation was encountered year round during this study.
3.Results
Individuals ofAnsonia latidisca
were found every month during the study year (2012),at transects on elevations between 1,100-1,229 m asl.However,no female was found after September 2012 along designated transects,coinciding with the rainy season.3.1.Dietary Description
A total of 46 adults (30 males and 16 females) were examined for their stomach contents (including six recaptured individuals).Of these,93.48% (43 individuals;28 males and 15 females) had food items in their stomachs.Three individuals with empty stomachs are not included in the dietary analyses.The number of prey items (t
-test,df
=10,t
=0.793,p
> 0.05,) and average stomach content volume (t
-test,t
=0,p
> 0.05) also showed no significant difference in terms of number of prey items or in stomach content volume of recaptured toads of both sexes.Consequently,all further calculations included first-time and recaptured toads,as no marked difference was indicated in their dietary pattern between captures.Diet was identified to ordinal level of classification and a total of 582 food items were recovered,including representatives from the class Arachnida (Araneae and Acarina),Insecta (Blattodea,Coleoptera,Hymenoptera,Isoptera and Thysanura),in addition to Diplopoda.The remaining categories were plant remnants (assumed to be incidentally ingested) and indeterminate insects and their larvae (Figures 3-4).
Table 1 shows that formicids (Insecta:Hymenoptera),specifically,ants were the most commonly found stomach item,comprising 78.69% by percentage of abundance of the total diet and showing a percent frequency of occurrence of 90.70%.Coleopterans (beetles) were found to be the secondary (F
= 48.84%),and plants were considered as incidental ingestion (F
= 23.26%),presumably taken inadvertently,as reported for many other anuran amphibians (Berry,1966).There is a significant difference in prey number between the sexes (Table 2;Mann-WhitneyU
test,p
< 0.05).Stomachs of the larger-growing females contained more food (n
=418);volume food range 0.05-1.5 mL;mean prey item per stomach 22.80±S.E.8.87;n
=15 than did stomachs of males (n
=164) range 0.05-0.3 mL;mean prey item per stomach 5.86±S.E.0.75;n
=28.Two dimensions were derived to discriminate the relationship of different food categories using nearest scaling with low stresses (Stress-1=0.021,Stress-2=0.025,S-stress=0.0003) and high coefficient (Tucker’s coefficient of congruence=0.999).Positive loading (Table 3;Figure 5) of dimension 1 indicates that one variable (Hymenoptera) is discriminated as most important in the diet.Dimension 2,on the other hand indicated two positive loadings,of hymenoptera and plant,as shown in Table 2.As dimension 1 and 2 show high positive value (2.214 and 0.336,respectively),only one of 13 food categories was found meaningful.These results suggest that hymenopterans constitute the primary diet of the species.

Table 1 Percentage of abundance (A),percentage of frequency of occurrence (F) and number of prey individuals per taxon (N) in stomach samples of Ansonia latidisca.
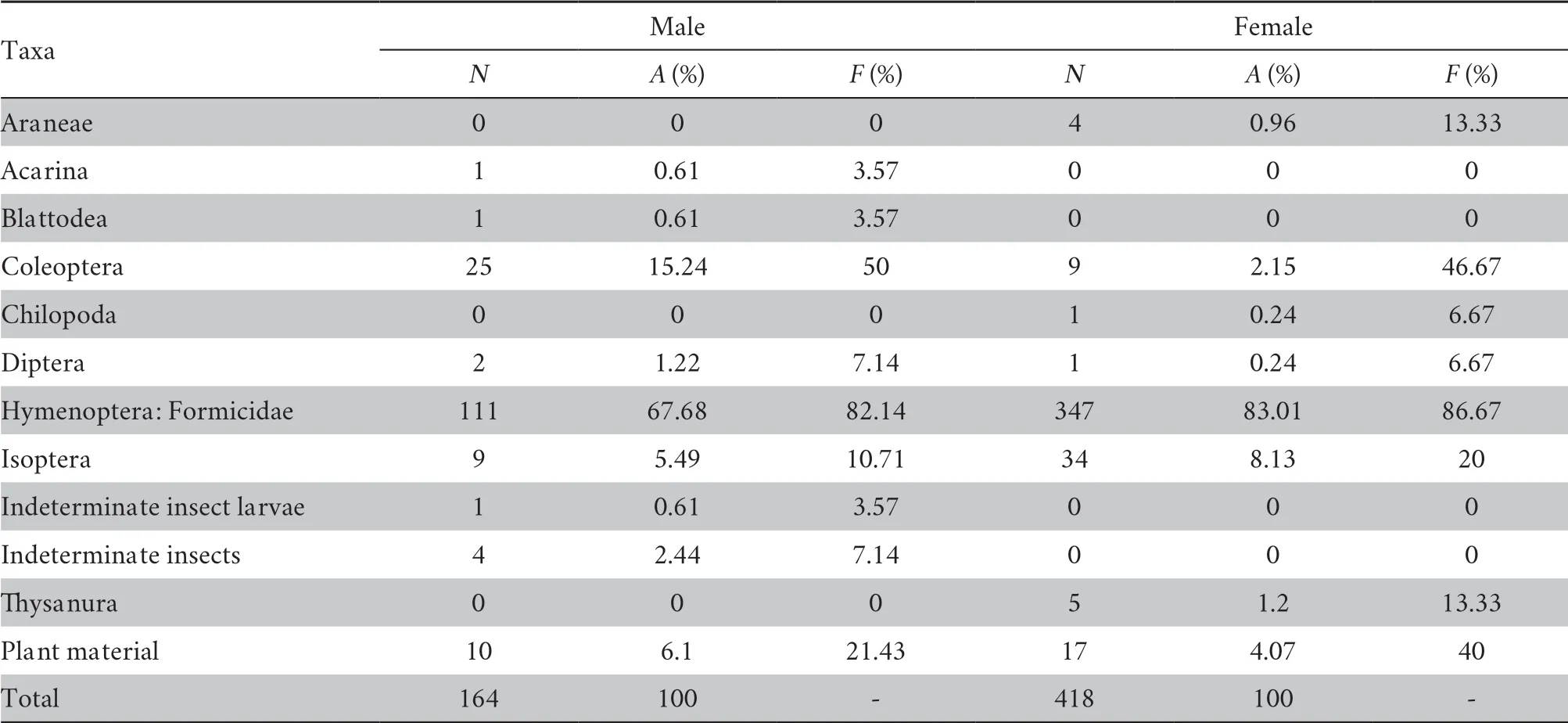
Table 2 Percentage of abundance (A),percentage of frequency of occurrence (F) and the number of prey individuals per taxon (N) in stomach samples of males and females of Ansonia latidisca.

Table 3 Final coordinates of two dimensions for food categories in stomach samples of Ansonia latidisca sampled.FCD1 (final coordinate dimension 1);FCD2 (final coordinate dimension 2).

Figure 3 Final coordinate dimension 1 of food categories found in the stomach of Ansonia latidisca,January to December 2012.

Figure 4 Final coordinate dimension 2 of food categories found in the stomach of Ansonia latidisca,January to December 2012.
3.2.Temporal Variation
The volume of food ingested by adults did not vary monthly (one-way ANOVA,F
=1.053,p
> 0.05),and there was no significant difference between wet and dry periods (χ=0.659,df
= 1,p
> 0.05;Figure 6).The mean longest prey was recovered from a female in March,during the wet season.Monthly Berger-Parker Index indices of adults show the greatest dominance values (0.53) during February,that may relate to the fact that the highest number of individuals (n
= 15) was found during this period.There was a strong positive correlation between dominance index and number of individuals found in a particular month (Pearsonr
=0.750;p
< 0.01),indicating the increasing stomach prey diversity with larger samples.The dominance index between dry and wet periods (t
-test,df
=10,t
=0.122,p
> 0.05),on the other hand,shows no significant difference,indicating absence of seasonal variation in diet.3.3.Prey Use by Sex and Size
Relationships between average prey length (APL),number of prey items (NPI) and snout-vent length (SVL) in the sexes were tested after log transformation (Figure 7).The data show no obvious trend for larger individuals consuming larger prey.Only females show an increase in lnNPI consumed with a corresponding increasein body size (here,lnSVL),indicating that their larger body size allow them to ingest more prey items than males.lnAPL and lnNPI were negatively correlated (Pearsonr
=-0.318),both when considering males and females together (p
< 0.05),and for the female data alone (p
< 0.05),indicating that individuals which consumed larger prey had on average consumed fewer prey items.The largest prey item (prey length 19.43 mm) recovered was the only centipede (class Chilopoda),found in a stomach of a female (Field ID:BH 14;SVL 42.8 mm).
Figure 5 Monthly variation in mean stomach volume of prey in stomachs of adult Ansonia latidisca with standard deviation,January to December 2012.

Figure 6 Monthly variation in the composition of diet of adult Ansonia latidisca,as determined by the inverse of the Berger-Parker index of dominance,January to December 2012.

Figure 7 Relationship between mean prey length (APL),number of prey items (NPI) and snout-vent length in Ansonia latidisca (log transformed data).Square symbols and dashed lines are females;round symbols and continuous lines are males.(A) Relationship between lnAPL and lnSVL.Slopes of regression lines:y=-2.3559x + 11.0912,p=0.149,for females;y=-1.7604x + 8.2326,p=0.368,for males.Relationship not significant if data are pooled (p=0.886).(B) Relationship of lnNPI and lnSVL.Slopes of regression lines:y=1.8380x -5.6027,p=0.726,for females;y=-0.3485x + 2.8244,p=0.969.Relationship insignificant if data are pooled (p=0.066).(C) Relationship of lnAPL and lnNPI.Slopes of regression lines:y=-1.9217x + 5.6576,p < 0.05,for females;y=-0.4279x + 2.2359,p=0.172,for males.Relationship significant if data are pooled (p < 0.05).
Males had fewer prey than females in stomach samples (Table 2),and the adult diet predominantly comprised formicids (ants),as indicated by percentage frequency of occurrence of 90.7%.Coleopterans (beetles) were found secondary in importance (48.8%).Plant items (including bryophytes) showed a high frequency of occurrence (23.3%).These studies reveal that formicids (specifically,ants) formed a major part of the diet (indicated by high percentage — 90.7% in frequency of occurrence) and is the primary food of the target species.
4.Discussion
Data obtained from stomach-flushing of 46 adults (30 males and 16 females) revealed 12 food categories.Formicids formed the bulk of the diet,with coleopterans being secondary and other arthropod groups being tertiary.
Based on the dietary composition,Ansonia latidisca
is assumed to be an ant specialist,with females ingesting more prey than males.There was no relationship between predator length (here,mouth width) and prey length as all size classes consumed hymenopterans and coleopterans.Dietary diversity,as recovered from the diversity index,appeared unaffected across months.The spike in feeding during the month of July and the disappearance of females after August is suspected to be associated with the breeding season over the months from August to December,when they presumably use streamside habitats.Knowledge of dietary requirements is fundamental for understanding amphibian life history,population fluctuations over time and effects of habitat modification (Andersonet al.
,1999).Dalyet al.
(1994;2007) suggested that toxic frogs obtain toxic substances through exogenous sources by sequestering alkaloids from arthropod prey,with mites and ants contributing most of the alkaloids.Toft (1980a) classified these organisms into two guilds:ant specialist and non-ant specialist,based on their dietary pattern.It is thus of theoretical interest to ascertain the dietary composition,foraging and feeding habits.It is hyphothesized thatAnsonia latidisca
belongs to the ant specialist ecological guild amongst the local amphibian ecological assemblage.Comparable research conducted in the New World (including Panama,Costa Rica and Peru) has shown that Neotropical bufonids tend to be ant specialists,eating relatively few types of prey other than ants (although some eat small mites as well;Toft,1980a;1980b).On the contrary,non-ant specialists tend to eat a wide variety of prey taxa (Toft,1980a;Lieberman,1986;Parmelee,1999).Result from the study corresponds to the aforementioned ones from the Neotropics,proportions of hymenopterans ingested generally within the range for dendrobatids,as well as Asian bufonids,which have been described as ant specialists (frequency of occurrence,52%-99%;Toft,1980a;Direpet al.
,2009;Ahmad Sahet al.
,2019).Results from stomach flushing of adultA.latidisca
reveal 12 categories of food,dominated by ants.The dietary profile fits the description of an ant specialist,and the species thus,appear to demonstrate an active preference for feeding on small prey showing clumped distribution.Optimal foraging theory suggests that larger animals should select large prey and overlook small prey items,which tend to have low nutritional values,to warrant expenditure of energy in their pursuit and consumption (Schoener,1979).However the major limitation is that within-species,evidence is hard obtain,and may not be applicable to all species.Prey size did not appear to be a function of predator body size in this study,and larger toads (especially females) tend to eat more prey.The results of this study conforms to that of Woodheadet al.
(2007),who studied the Malagasy poison frog,Mantella aurantiaca
,but contrasts with data from other studies which found frogs of greater snout-vent length consuming fewer but larger prey (e.g.,Berry,1966;Das,1995;Hirai,2002).In fact,a large portion in the diet of large and small individuals ofA.latidisca
is made up of formicids and coleopterans,prey types showing clumped distribution.According to prey item attributes,an active capture strategy is proposed forAnsonia latidisca
.It is also possible that high proportion of ants found in stomach samples simply reflects a high abundance of ants in their environment.The spike in stomach content volume during July was on the account of inclusion of three females with high food volume.The females might have eaten more to increase their fat reserves for breeding later in the wet season.The disappearance of females over the months of August to December is suspected to be associated with the breeding season,as they presumably use streamside habitats although no female was spotted along the stream during that time.The climate at the higher elevation of Gunung Penrissen is humid,with little yearly fluctuation and the area is considerably wet throughout the year due to frequent mist.Since formicids (ants) are the primary food source that are available year round,lack of seasonal variation in diet is understandable.
In summary,the studies reported here add to the body of knowledge on the ecology of a relatively poorly known Bornean endemic amphibian species,providing new information on its dietary ecology.
This montane species inhabits mossy forests at elevations between 1,100-1,229 m asl.Stomach-flushed individuals reveal that apart from large females,that are presumably about to breed,food ingestions did not vary monthly,with no significant difference between wet and dry periods.Males fed on fewer prey items than females,the diet of both dominated by insect groups belonging to the Hymenoptera and Coleopterans.Plants (including bryophytes) in dietary samples are assumed to be incidentally ingested,although deliberate consumption of plants may also be possible.As the diet of all post-metamorphic stages consisted of ants and beetles,Ansonia latidisca
shows a narrow food preference,and is a dietary specialist.Acknowledgements
Field work was supported by a Fundamental Research Grant,FRGS/07(04)787/2010(68) from the Ministry of Higher Education,Government of Malaysia,and The Rufford Small Grants Foundation.We thank Y.M.Pui,A.A.Tuen,S.T.Pang,A.Sapis,A.M.Sapis,E.Juing,E.Deka,I.Wadell,W.C.Lou,S.Shonleben,A.Z.Poh,M.Jenang,C.Peter,C.Emang,E.Odan,J.Ngeian,M.Buloh,L.Jack and T.H.Tan.The Borneo Highlands Golf and Country Resort provided logistic support.The first author thanks the Ministry of Higher Education for granting him a MyBrain15 scholarship.We thank H.P.Hazebroek for generating the digital elevation model (Figure 2) of our study area,and Y.M.Pui for help with the preparation of text figures.Finally,we are grateful to the Sarawak Forest Department for a research permit (NCCD.907.4.4(Jld.VI)-106) to conduct these studies,and three anonymous reviewers for their thoughtful comments. Asian Herpetological Research2021年2期
Asian Herpetological Research2021年2期
- Asian Herpetological Research的其它文章
- Group Living on Trees Does Not Elevate Inbreeding Risk in Shedao Pit Vipers Gloydius shedaoensis
- Trophic Niche Shifts in Mountain Feirana Frogs under Human-mediated Habitat Transformations
- Higher Body Temperatures and Earlier Parturition in Response to Hypoxia Experienced by Pregnant Lizards
- Male Advertisement Call of the Endangered Leptobrachella tengchongensis (Anura:Megophryidae) from Mount Gaoligongshan,Yunnan Province,China
- Evolution of Phenotype and Mitochondrial Genome Reveals Limbless and Body-elongated Squamates may Change Their Energy Basis for Locomotion
- Phylogeny and Biogeography of Common Toad (Bufo bufo) in Xinjiang,China
