Trophic Niche Shifts in Mountain Feirana Frogs under Human-mediated Habitat Transformations
Shengnan YANG,Chunlan ZHANG,Wenbo LIAO,Na LI,4 and Junhua HU
1 Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
2 Guangdong Key Laboratory of Animal Conservation and Resource Utilization,Guangdong Public Laboratory of Wild Animal Conservation and Utilization,Institute of Zoology,Guangdong Academy of Sciences,Guangzhou 510260,Guangdong,China
3 Key Laboratory of Southwest China Wildlife Resources Conservation (Ministry of Education),China West Normal University,Nanchong 637000,Sichuan,China
4 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization &Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province,Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
Abstract Urbanization can induce environmental changes,disturbing habitat transformation process,and resulting in niche shift of species and local extinctions.Amphibians have experienced worldwide population declines,with habitat loss acting as one of the most disruptive causes.How amphibian species response to changing habitats could be reflected in their utilization and assimilation of resources.Using stable isotopes,we explored trophic niche variation between natural and transformed habitats for three closely related frog species in the genus Feirana (F.quadranus,F.taihangnica and F. kangxianensis).Our results indicated that the δ13C value was negatively correlated with body size (snout-vent length) and the δ15N value increased along with the ontogenetic process.The δ13C values were significantly different among habitat types,and the variation of δ15N values was relatively limited in different disturbed gradients.Urban groups displayed broader trophic niche width than both rural and natural groups.When species in sympatry,their resource utilization and trophic niche overlap probability were more similar in rural habitats than their natural counterparts.Our findings would be conducive to understand trophic niche and function variation in amphibians during the urbanization process,allowing for effective predictions of ecological consequences of habitat transformation.This study can also provide insight into conservation strategies for mountain amphibians in the Anthropocene.
Keywords amphibians,habitat transformation,habitat type,stable isotopes,trophic niche width,urbanization
1.Introduction
In light of present-day exceptionally high extinction rates of biodiversity,the pervasive population decline of wildlife is mainly caused by human activities,including anthropogenic climate change,environmental pollution,and habitat transformation (Pimmet al.
,2012;Battleset al.
,2018).Above all,the massive changes that the natural habitats are increasingly replaced with artificial structures have attracted wide attention,since habitat fragmentation and loss during global urbanization process acts as one of the main reasons for the decreasing biodiversity (Setoet al.
,2012;Lowryet al.
,2013).Notably,urbanization has altered and will continue to modify present environmental conditions via habitat transformation,which would pose an increasing threat to global existing biodiversity (Setoet al.
,2012).The transforming habitats bring novel challenges to most organisms,and result in local extinctions and range shifts in many species (Battleset al.
,2018).Nevertheless,numerous species can persist and even thrive in the changing environment,demonstrating a remarkable adaptation to urbanization (Bonieret al.
,2007;Lowryet al.
,2013).Empirical and experimental studies have documented ecological and evolutional adaptations of wildlife living in cities.Species persisting in urban conditions often exhibit special features (e.g.wider environmental tolerance and more diverse diets) to occupy broader niches than their natural counterparts,and to evolve into environmental generalists (Bonieret al.
,2007;Murrayet al.
,2015;Pagani-Núñezet al.
,2019;Lianget al.
,2020).Amphibians have experienced dramatic population declines all over the world during the past decades (Wake and Vredenburg,2008;Hofet al.
,2011).This is caused by multiple factors,in which habitat transformation is documented to be one of the most devastating reasons (Stuartet al.
,2004).The scaleless and highly permeable skin makes it difficult for amphibians to maintain a constant body temperature (Wells,2007).Their unshelled eggs and limited dispersal ability further make amphibians vulnerable and easily exposed to the changing environment (Stuartet al.
,2004;Wake and Vredenburg,2008).Being highly dependent on external habitats,amphibians may thus experience strong pressure from habitat transformations (Stuartet al.
,2004;Hofet al.
,2011).Using stable isotope analyses,previous studies have provided temporal integrated patterns of trophic niche variation under morphological constraint,seasonal change,intraspecific competition,and habitat partition for amphibians (Cloyed and Eason 2017;Huckembecket al.
,2018).However,compared with other vertebrates (e.g.Schmidtet al.
,2011;Murrayet al.
,2015;Pagani-Núñezet al.
,2019),there are still considerable gaps in understanding how amphibians respond to ongoing habitat transformations in the Anthropocene.Feirana
frogs (family Dicroglossidae;Dubois,1992) include three living species:F.quadranus
(swelled-vented frog),F.taihangnica
(Taihangshan swelled-vented frog) andF.
kangxianensis
(Kangxian swelled-vented frog) (Feiet al.
,2009;Yanget al.
,2011).These frogs are distributed across the Qinling-Daba Mountains (QDM),with a substantially elongated contact zone between species in the Qinling Mountains (Feiet al.
,2009;Yanget al.
,2011;Hu and Jiang,2018;Huanget al.
,2020).Feirana
frogs are recorded and described as forest species,since they often inhabit streams and nearby ponds at elevations ranging from 330 to 1960 m in mountains (Feiet al.
,2009;Yanget al.
,2011).However,they are suffering from population declines due to illegal capture and habitat degradation (Feiet al.
,2009;Yanget al.
,2011).Human activities such as commercial logging,infrastructure and hydropower development have expanded rapidly,which hastened the process of habitat loss and fragmentation in the QDM.This can foster severe threats forFeirana
frogs from habitat transformation (Louckset al.
,2003;Zhanget al.
,2017).To understand the impacts of habitat transformation on amphibians,we here focus on trophic ecology ofFeirana
frogs across different degrees of human-disturbed environment in the Qinling Mountains.We aim to address three questions:(i) How does the ontogenetic trophic ecology shift in these frogs? (ii) What are the relationships between trophic niche characteristics and habitat transformation? (iii) Can habitat transformation process forecast trophic niche overlap between species? Our efforts are of significance for grasping trophic niche and function variation of amphibians during the urbanization process in the Anthropocene.2.Materials and Methods
2.1.Study area
The study area was located in the Qinling Mountains,which acts as a natural geographic and climate boundary between north and south China (Zhanget al.
,2017).The north slope of the Qingling Mountains has a warm temperate and semi-humid climate because of the cooling dry air from the northwest,while the south slope has a subtropical and humid climate under the effects of the humid air from the southeast (Zhang,2000).There are mainly three vegetation types along the elevation (from low to high):subtropical evergreen and deciduous broadleaf forests,temperate deciduous broadleaf and subalpine needle leaf forests,and subalpine scrub meadow (Louckset al.
,2003).Various climate and vegetation conditions generate substantial species richness and typical communities in this region,making it one of the most important treasured biodiversity hotspots in China (Myerset al.
,2000;Zhanget al.
,2017).2.2.Sample collection
We conducted field expeditions from July to August in 2018.Based on different urbanization gradients and known distributions ofFeirana
frogs (Feiet al.
,2009;Yanget al.
,2011),we selected six sampling sites in Shaanxi and Gansu provinces within the Qinling Mountains (Figure 1;Supplementary Material,Table S1).We divided these sampling sites into natural,rural and urban habitat types according to the landscape structure and the intensity of human activities (see also Marzluffet al.
,2001;Pagani-Núñezet al.
,2019;Lianget al.
,2020).Natural habitats were relatively undisturbed areas with homogeneous forests,rural habitats were combined with managed forest,farmland and scattered houses,and urban habitats were human-dominated areas inside cities and towns.We searched for frogs in and around streams with a flashlight half an hour after sunset.For each found individual,we measured snout-vent length to the nearest 0.02 mm with electronic digital calipers (YB5001B,Kraftwelle Company,Hangzhou,China) as body size.We collected muscle and bone collagen samples to compute trophic niche characteristics.These tissues can provide information on niche use at different temporal scales for each individual.Muscle tissue reflects resource utilization in a time window of weeks prior to sample collection,while bone collagen tissue reflects a broader time span across individuals’ development (Vanderet al.
,2015;Matsubayashiet al.
,2017).After euthanasia,we clipped the fourth toe for collagen extraction,and the muscle tissue from right thighs of each post-metamorphic individuals was removed,washed with distilled water and stored in 2 mL tubes for subsequent stable isotope analysis (SIA).All samples were kept in a chilled cooler in the field and then stored in a -20 °C refrigerator in the laboratory.After sampling,all the voucher specimens were deposited in the Herpetological Museum of the Chengdu Institute of Biology (CIB),Chinese Academy of Sciences (CAS).All animal handling and processing were in accordance with the Law of the People’s Republic of China on the Protection of Wildlife and approved by the Animal Care Committee of CIB,CAS.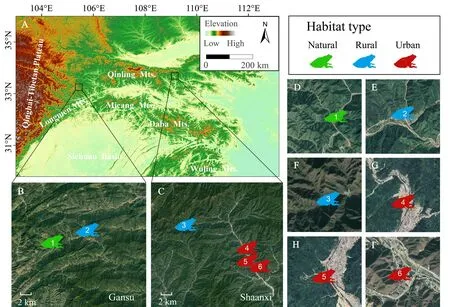
Figure 1 Geographical locations of the study area and the landscape structure of sampling sites.A) Study locations in the Qinling Mountains;B-C) Satellite images of the study area in Gansu and Shaanxi provinces;D-I) Close-ups of sampling sites.
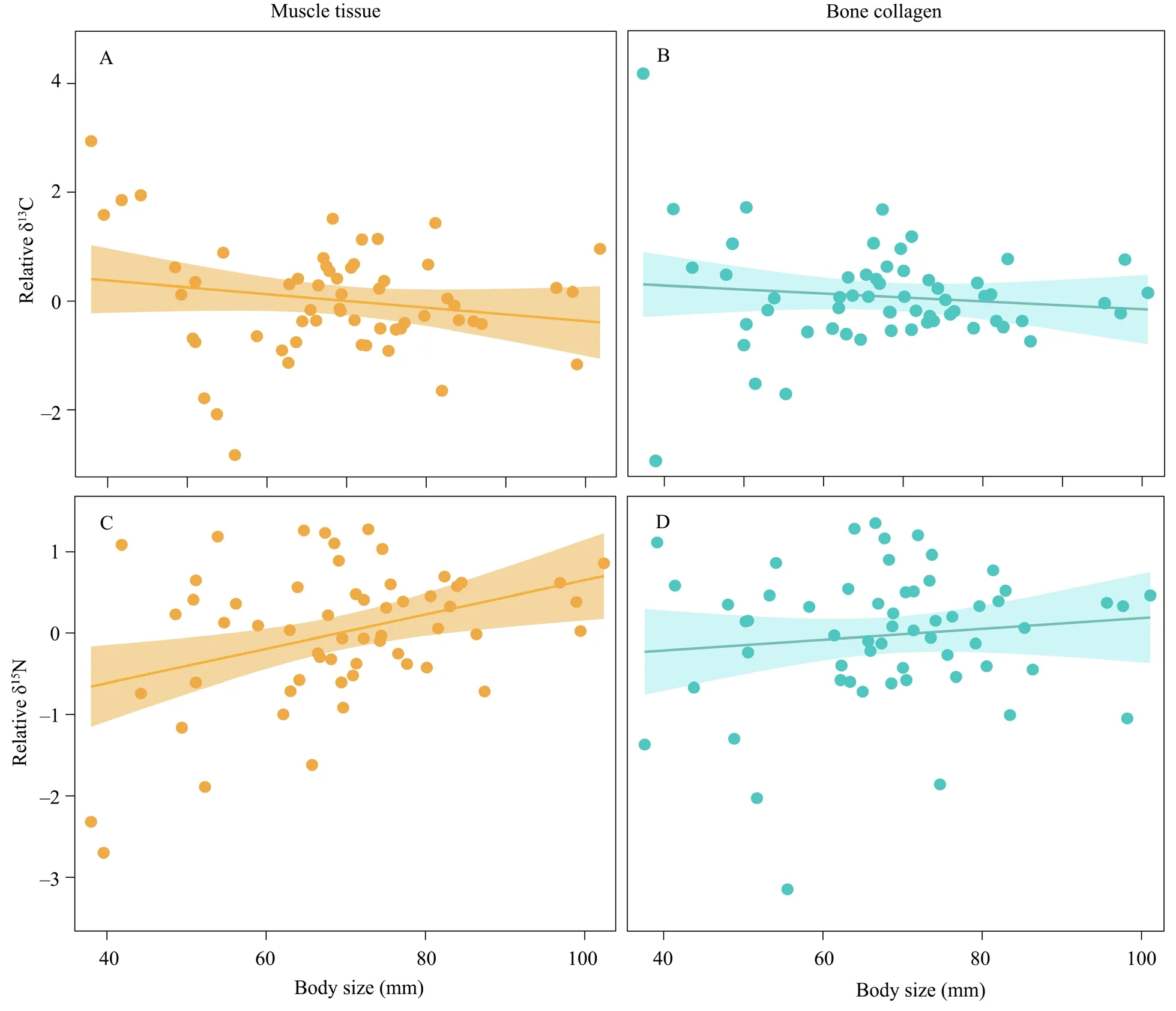
Figure 2 Variation trends of stable isotope values with body size in Feirana frogs.A and C) Relationships between isotope values and body size in muscle tissue,and B and D) in bone collagen.Lines represent the slope of the linear regression,and the shaded areas are the 95% confidence intervals.
2.3.Laboratory treatment
We removed the skin from toe clips manually and then separated ligaments and tendons from bone.Bone samples were then demineralized in 0.5 mol/L hydrochloric acid in a refrigerator for 24 h,after that they were dried in an oven for 48 h at 60 °C.We performed lipid extraction for bone collagen via three 24 h soaks in a 1:2 chloroform:methanol solution,then the bone collagen was thoroughly rinsed and heated in deionized water at 90 °C for about 12 h.All muscle and bone collagen samples were freezedried for 36 hours to constant weight and ground to a fine powder.2.4.Stable isotope dataset
Since stable isotopes of carbon and nitrogen can reflect resource utilization and trophic level of animals,they are widely used to quantify trophic niches at different scales,such as communities,species and individuals in the ecosystem (Newsomeet al.
,2007;Jacksonet al.
,2011;Dezeraldet al.
,2018).We measured the stable isotope values of each sampled individual collected from different habitat types (i.e.natural,rural and urban) to explore the trophic niche variation across urbanization gradients.We analyzed the δC and δN values for each sample at the Public Center of Experimental Technology in the CIB,CAS.The samples were combusted in a vario isotope cube elemental analyzer (vario ISOTOPE cube,Elementar,Germany) interfaced to an isotope ratio mass spectrometer (IsoPrime100,Isoprime,UK).Stable isotope ratios were expressed as standard delta notation (δ) in parts perthousand (‰),where δX=(R
/R
- 1) × 1000,in which δX
was δC or δN,R
andR
were the molar ratios ofC/C andN/N of the sample and the standard reference material,respectively.The reference material was Vienna-Pee Dee Belemnite for carbon and atmospheric Nfor nitrogen.2.5.Statistical analysis
As differences between sites can influence the characteristics of isotopic niches,and the two study areas in this study were far apart from each other,we transformed the raw stable isotope values of each individual according to the group centroid of each sampling site,and then these standardized stable isotope values were used for comparisons among habitat types (Schmidtet al.
,2011;Sagouiset al.
,2015).We used linear regression to test the variation of isotopic characteristics during the ontogenetic process.Body size was used as the independent variable and the relative δC or δN values were the dependent variables.The isotope values of muscle and bone collagen were respectively pooled across sites.After conducting Kolmogorov-Smirnov tests,Kruskal-Wallis tests were used to assess the difference of δC and δN among habitat types.Then we used the Bayesian geometric approach to quantify trophic niche of species at habitat level.We calculated the corrected standard ellipse area (SEAc) of frog groups in different habitat types using the SIBER package,and used SEAc to represent trophic niche width with 95% confidence intervals (Jacksonet al.
,2011).To quantify the trophic niche overlap of sympatric species,we respectively calculated trophic niche characteristics of sympatric frogs from rural and natural habitats using the probabilistic method in the nicheRover package.All the analyses were performed in R v3.5.3 (R Core Team,2019).3.Results
The characteristics of δC and δN had contrasting variation patterns in the ontogenetic process (Figure 2).Along with the increase of body size,the relative δC of muscle tissue decreased (t
=-0.82,P
=0.42;Figure 2A),and the value in bone collagen showed a similar decreasing pattern (t
=-1.35,P
=0.18;Figure 2B).There was a significantly positive correlation between the relative δN value and body size in muscle tissue,and it also increased in bone collagen during the development process (muscle:t
=2.95,P
< 0.01;bone collagen:t
=0.85,P
=0.40;Figures 2C,D).The δC values of two sample tissues significantly differed among habitat types (muscle:H
=14.26,P
< 0.01;bone collagen:H
=11.49,P
< 0.01).Specifically,the muscle samples from urban habitats had significant higher value than their rural (Z
=-2.90,P
=0.01) and natural counterparts (Z
=-3.66,P
< 0.01) while the isotope characteristics were similar between rural and natural habitats (Z
=-0.94,P
=1.0).For bone collagen,even the isotope ratio increased from natural to transformed habitats,the remarkable difference of δC was only detected between urban and natural groups (Z
=-3.38,P
< 0.01).Rural samples showed no significant difference from natural (Z
=-1.86,P
=0.20) and urban ones (Z
=-1.79,P
=0.22).There was also no significant variation in the δN values neither in muscle tissue (H
=1.52,P
=0.47) nor in bone collagen (H
=0.23,P
=0.89) among habitat types.The trophic niche width (SEAc) varied across habitats types (Figure 3).The results of SIBER showed that frog groups from urban habitats had the broadest trophic niche (SEAc=6.30),followed by the natural (SEAc=1.58) and the rural groups (SEAc=0.94).In both urban and rural habitats,the isotope ratios of δC showed broader range than δN,while the variation of δN contributed more on trophic niche width in natural habitat.
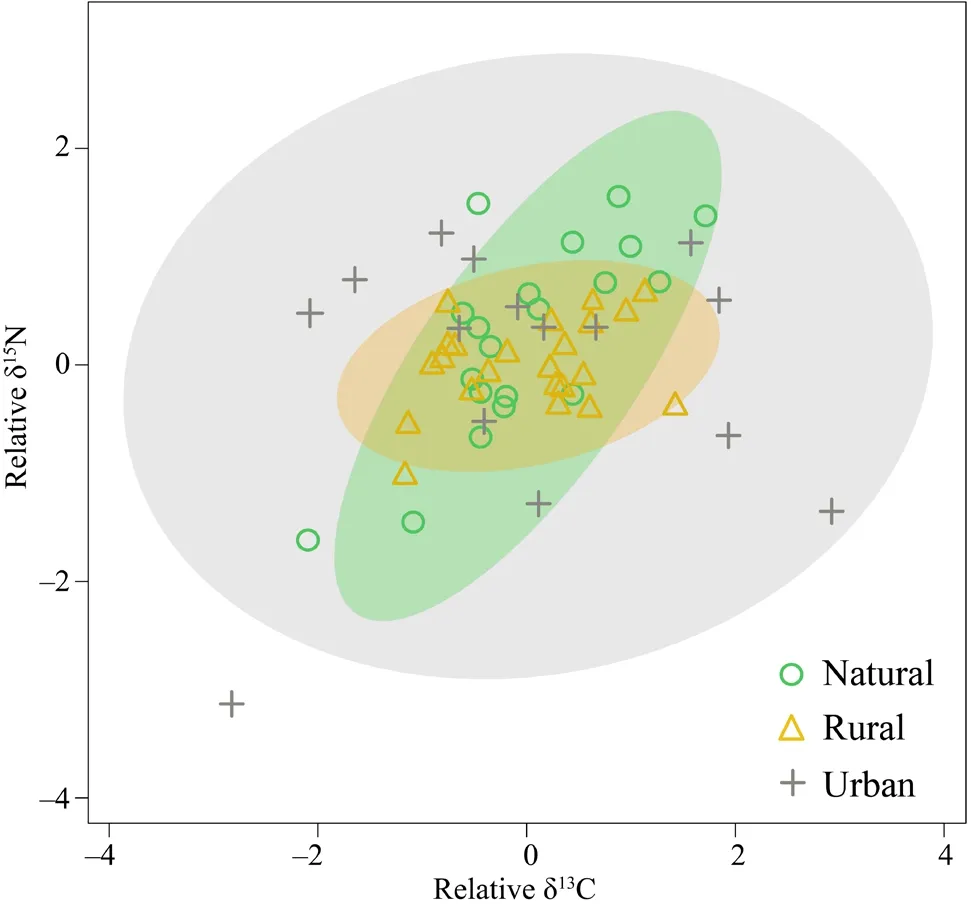
Figure 3 Characteristics of trophic niche in different habitat types based on isotopic biplot of relative δ13C and δ15N values.The isotopic variance is illustrated as a size of ellipses,and shaded areas are the 95% confidence intervals.
For the sympatric species,F.quadranus
had a higher proportion of being in the trophic niche ofF.kangxianensis
andF.taihangnica
(Figure 4).The overlap probability thatF.quadranus
was found in the trophic niche occupied byF.kangxianensis
was 74.1% in natural habitat,while the ratio was 40.9% in reverse (Figures 4A,B).For rural habitat,the proportion ofF.
quadranus
occupying the niche ofF.taihangnica
was 62.7%,and the probability was 52.8% on the contrary (Figures 4C,D).
Figure 4 Overlap probability in trophic niche of Feirana species in different degrees of human-mediated disturbances.Solid lines represent mean overlap estimates and dashed lines represent 95% confidence intervals.A) In natural habitat,the probability that the niche of F.quadranus is found in the niche occupied by F.kangxianensis;B) the counter situation in natural habitat.C) In rural habitat,the probability that the niche of F.quadranus is found in the niche of F.taihangnica;and D) the counter situation in rural habitat.
4.Discussion
Trophic niche variation can represent species’ response strategy to changing habitats,demonstrating animals’ utilization and assimilation of environmental resources (Murrayet al.
,2015;Dezeraldet al.
,2018).Our results demonstrated how trophic niche characteristics changed between urban and less transformed habitats.Urban groups displayed broader trophic niche than both rural and natural groups,and the δC value showed significant differences among habitats types.Trophic niche overlap was found in sympatric species,and frogs in rural habitat had more similar resource utilization than the natural ones.Body size is a common restriction on amphibians’ diets as they grow (Wells,2007).In this study,both muscle and bone collagen tissues showed decreasing trends of δC when body size increasing.As theC/C fractionation is more conspicuous with rapid flow,the δC value is usually lower in lotic ecosystems than in lentic (Ishikawaet al.
,2013).Accordingly,our results indicated thatFeirana
frogs may prey more frequently at riffle during their development process.In general,larger individuals had enriched δN values,indicating that they occupy higher trophic positions (Cloyed and Eason,2017).There were significant differences of δC values among habitats types in both muscle and bone collagen.Given the variation of δC values depends on the consumption of resources from aquatic versus terrestrial habitats,higher δC value in urban habitat may be interpreted by more allochthonous resource input from natural to human-disturbed environment (Ishikawaet al.
,2013).Semiaquatic organisms like many frog species rely on the features of both water and land resources.In urban habitats,artificial structures shrink the buffer wetland between upland and water bodies,promoting the resource transport processes between aquatic and terrestrial ecosystems,and increasing the possibility of amphibians foraging on terrestrial habitats (Ficetolaet al.
,2011;Ishikawaet al.
,2013).Thus,the interplay between terrestrial and aquatic systems might influence their foraging strategies and resource assimilation process,giving rise to the discrete trophic niche characteristics among different habitats (Ficetolaet al.
,2011;Cloyed and Eason,2017).As the nitrogen isotope usually accounts for what has been assimilated by individuals over a period of growth,and shows greater enrichment from resource to consumer with each trophic level,the δN values are widely used to indicate species’ trophic position within a food web (Newsomeet al.
,2007;Jacksonet al.
,2011;Dezeraldet al.
,2018).In this study,we found no significant variation of δN values in neither muscle nor bone collagen samples.This may indicate that these frog groups occupy similar trophic level in the Qinling Mountains,and maintain basically stable positions in the food web,which make them a reliable medium of energy flow and material transfer process within the ecosystem (Matsubayashiet al.
,2017;Dezeraldet al.
,2018).Urban habitats provide challenging survival conditions for most wildlife since they represent a recent and dramatic shift from historical habitats where species have evolved in (Martin and Bonier,2018).Our results showed that frogs living in the urban conditions had broader niche width than the rural and natural groups.This is consistent with previous studies which analyzed niche variation patterns influenced by habitat transformation in different taxa (Murrayet al.
,2015;Battleset al.
,2018;Pagani-Núñezet al.
,2019).The high structural heterogeneity of human-disturbed habitats can promote species’ utilization of empty niche space (Martin and Bonier,2018).The variations of habitat structure in cities alter the availability of resources and nutrient input in the ecosystem,and occupying the empty niche can reduce the risk from intense competition between colonial species and other species in new environment conditions (Cloyed and Eason,2017;Battleset al.
,2018).Trophic niche overlap was found in sympatric species under different degrees of human-mediated disturbances.Feirana
frogs are closely related with similar features in morphology and microhabitat utilization (Hu and Jiang,2018;Yanget al.
,2019;Huanget al.
,2020).The niche filtering theory indicates that,in a given environment,the profitable traits are selected by local conditions,which lead to a coexistence of species with more homogeneous ecological needs and characteristics (Hu and Jiang,2018;Yanget al.
,2019).The focal species in rural environment had more similar resource utilization and trophic niche overlap probability than their natural counterparts,which might be explained by reduction of available resources and intensification of interspecific competition (Battleset al.
,2018;Huanget al.
,2020).It is noteworthy thatF.quadranus
occupied broader trophic niche than other two species in both natural and rural habitats,and it was also the onlyFeirana
species found in the urban environment.As dominant urban species may competitively displace taxa that occupy similar niches,specialist species with narrow niches would be lost and these generalist species with broad niches will persist when habitats are modified (Martin and Bonier,2018;Pagani-Núñezet al.
,2019).As semiaquatic species,amphibians are important consumers in both aquatic and terrestrial habitats,and can play a key role in linking energy flow between the two systems (Ficetolaet al.
,2011;Cloyed and Eason,2017).Habitat loss and anthropogenic factors are highly responsible for global reduction in amphibians (Wake and Vredenburg,2008;Hofet al.
,2011).In this study,we used stable isotopes to provide quantitative estimates of trophic ecology for three closely related frog species,demonstrated the trophic niche variation in different habitat types,and illustrated a trophic niche expansion trend in urban groups.Understanding the varied trophic characteristics in different degrees of human-mediated disturbance will allow for predicting the ecological adaption strategies of amphibians,and making scientific management and conservation strategies for vulnerable ectotherms in the Anthropocene.Remarkably,even though the urban groups showed a significant higher ratio in carbon isotope and expressed an expansion trend than the rural and natural counterparts,the relatively limited sample sizes may suggest further investigations involving more populations and additional comparisons that help to speculate a general ecological variation pattern of wildlife in urbanization process.Acknowledgements
This study was supported by the National Natural Science Foundation of China (32071544,31770568 and 31572290),the ‘Light of West China’ Program of the Chinese Academy of Sciences (2019XBZG_XBQNZG_A_003),the Engineering Technical Center of Wildlife Survey,Monitor and Ecological Restoration,Guangdong,China,and GDAS Special Project of Science and Technology Development (2018GDASCX-0107).We are grateful to Chunpeng GUO and Xue GOU for their help in the field surveys,and the two reviewers,Xiaoyi WANG,Maojun ZHONG and Ka Wah LEUNG for their helpful comments and suggestions on the manuscript. Asian Herpetological Research2021年2期
Asian Herpetological Research2021年2期
- Asian Herpetological Research的其它文章
- Trophic Ecology of Ansonia latidisca at Gunung Penrissen,Sarawak,North-Western Borneo
- Group Living on Trees Does Not Elevate Inbreeding Risk in Shedao Pit Vipers Gloydius shedaoensis
- Higher Body Temperatures and Earlier Parturition in Response to Hypoxia Experienced by Pregnant Lizards
- Male Advertisement Call of the Endangered Leptobrachella tengchongensis (Anura:Megophryidae) from Mount Gaoligongshan,Yunnan Province,China
- Evolution of Phenotype and Mitochondrial Genome Reveals Limbless and Body-elongated Squamates may Change Their Energy Basis for Locomotion
- Phylogeny and Biogeography of Common Toad (Bufo bufo) in Xinjiang,China
