Quinone oxidoreductase 1 is overexpressed in gastric cancer and associated with outcome of adjuvant chemotherapy and survival
Zhi-Nong Jiang, Syed Minhaj Uddin Ahmed, Qin-Chuan Wang, Hong-Fei Shi, Xiu-Wen Tang
Abstract
Key Words: Gastric cancer; Quinone oxidoreductase 1 ; Prognosis; Immunohistochemistry;Kaplan-Meier curves; Biomarker
INTRODUCTION
Gastric cancer (GC) is now the fourth most common cancer and the third most common cause of cancer-related deaths worldwide. It is estimated that 951600 new GC cases and 723100 deaths occurred in 2012 . East Asia, including China, is among the areas with the highest incidence of GC, accounting for > 40 % of all new cases[1 ,2 ].Presently, the prevalence rates of GC are declining in most parts of the world owing to the establishment of mass screening and advanced treatment techniques. Despite this decline, the clinical outcomes of GC patients remain disappointing. Moreover, the prognosis of GC strongly depends on the TNM stage, biological factors, and clinical pathological factors[3 ,4 ]. Therefore, finding a new biomarker for the diagnosis and treatment of GC is of great value.
Quinine oxidoreductase 1 (NQO1 ) is a cytoplasmic flavoenzyme located on chromosome 16 q22 . It is also known as DT-diaphorase, menadione reductase, or quinone reductase 1 . NQO1 directly reduces quinines to hyroquinones using reduced form of nicotinamide-adenine dinucleotide (NADH) or nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor[5 ,6 ]. Numerous functions of NQO1 have been reported, such as xenobiotic detoxification, superoxide scavenging,and maintenance of endogenous antioxidant vitamins[7 ]. It is likely that NQO1 plays an important role in protecting normal cells against oxidative damage and electrophilic attack. Paradoxically, despite the suggestion that it exerts a protective action and plays an antioxidant role, disruption of anNQO1genetic polymorphism increases the risk of xenobiotic-induced toxicity and cancer[8 ,9 ]. High levels of NQO1 expression have been found in many human tumors, including breast cancer, melanoma, lung cancer, cholangiocarcinoma, and pancreatic cancer[10 -13 ]. Garate et al[10 ] reported that NQO1 protein expression induces cell cycle progression and proliferation in melanoma cells. In a study by Linet al[14 ], NQO1 expression in GC patients, noncancer patients, and normal controls was quantified by immunohistochemistry (IHC).They reported that NQO1 played an important role in the progression of GC, and might be a potential prognostic biomarker and therapeutic target for GC. However,the role of NQO1 in cancer progression remains controversial, and data on its expression in GC are scarce. In this study, we analyzed the expression of NQO1 and its potential association with survival and pathological parameters in primary GC. We aimed to investigate the associations between NQO1 protein expression status and responses to 5 -fluorouracil (5 -FU)-based adjuvant chemotherapy in patients with GC and thereby determine the potential of NQO1 to serve as a prognostic biomarker and therapeutic target.
MATERIALS AND METHODS
Western blot analysis
Unless otherwise stated, all chemicals were procured from Sigma-Aldrich Co.(Shanghai, China). Antibodies against rabbit NQO1 were used as described previously[15 ,16 ]. The human gastric cancer cell line AGS was obtained from the American Type Culture Collection (Manassas, VA, United States). Cells were maintained in RPMI-1640 growth medium (Shenggong, Shanghai, China) supplemented with 10 % fetal bovine serum and antibiotics in a humidified atmosphere of 5 % CO2 and 95 % air at 37 °C[17 ].Cell extracts were prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and NQO1 immunoblotting was performed using a standard protocol with anti-NQO1 antibodies (dilution at 1 :2000 )[17 ,18 ].
Patients
A total of 175 samples of GC were used in this study. All patients were enrolled and treated between 1995 and 2011 at the Department of Surgical Oncology, Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China.All patients received surgical treatment and were periodically followed for the determination of their survival status.
Immunohistochemistry
Tumor samples were assembled as multiple tissue arrays, as described in our previous study[19 ]. IHC staining for NQO1 in paraffin-embedded tumor sections was performed using standard procedures. In brief, to eliminate endogenous peroxidase activity, the slides were deparaffinized in xylene, rehydrated through a graded series of ethanol solutions, and incubated with 3 % H2 O2 in methanol for 15 min at room temperature. Antigen retrieval was performed by placing the slides in 0 .01 M sodium citrate buffer (pH 6 .0 ) at 121 °C for 15 min. Next, the slides were incubated with the NQO1 antibodies (1 :5000 ) overnight at 4 °C. The immunoreactions were visualized with an ImmPRESS detection kit (Vector Laboratories), which uses a second antibody conjugated with horseradish peroxidase and a diaminobenzidine-based stain. Finally,the sections were counterstained with Mayer's hematoxylin, dehydrated, and mounted.
Cytoplasmic NQO1 staining was considered as positive for NQO1 expression.NQO1 expression was semiquantitatively assessed by two experienced pathologists who were blinded to the clinical parameters. Analyses of NQO1 expression were based on both the staining intensity and proportion of positive cells. The staining intensity was scored from 0 to 3 + as follows: 0 for no staining, 1 + for weak staining, 2 +for moderate staining, and 3 + for strong staining[20 ]. The proportion of NQO1 -positive cells was expressed as a percentage and divided into four grades: grade 0 , 0 %positive; grade 1 , 1 %-30 % positive; grade 2 , 31 %-60 % positive; and grade 3 , > 60 %positive cells. The total score was obtained by multiplying these two parameters(range 0 -9 ); for statistical purposes, the samples were divided into two groups: Low expression level (0 -4 ) and high expression level or overexpression (> 4 ) groups. As the median score of NQO1 expression in the GC samples was 4 .5 , a cut-off value of 4 was selected[20 ].
Data analysis
The correlation between the expression of NQO1 and clinical and pathological parameters was determined by statistical analysis using SSPS 16 .0 for Windows (SPSS,incorporated, Chicago, IL, United States). Theχ2or Fisher’s exact test, Kaplan-Meier survival analysis, and Cox regression analysis were used as appropriate.Pvalues <0 .05 were considered significant.
RESULTS
NQO1 antibody is specific and sensitive for IHC staining
Protein extracts from AGS human GC cells were separated by SDS-PAGE, and the proteins were detected by Western blot analysis. The results showed that NQO1 can be readily detected, and no other protein cross-reacted with the NQO1 antibodies(Figure 1 ). Therefore, these antibodies are sensitive and specific for IHC staining to detect NQO1 expression in paraffin-embedded tumor sections.
Patient characteristics
A total of 175 patients were enrolled in this study. The baseline characteristics are described in detail and summarized in Table 1 . The patients ranged from 26 to 84 years of age. The study population consisted of 124 men and 51 women. The clinical and pathological parameters consisted of age, gender, tumor size, tumor location, tumor stage, tumor depth, lymph node metastasis, histological differentiation, and survival data. The number of patients with a tumor size < 5 cm and ≥ 5 cm was 63 and 99 ,respectively. The stage of GC was determined at the time of analysis, according to the American Joint Commission on Cancer system AJCC TNM 8 th edition. Of the 175 samples, 69 cases were stage I-II and 102 were stage III-IV GC. Among all patients, 67 underwent total gastrectomy, 83 received distal subtotal gastrectomy, 1 received partial resection, and 14 received proximal subtotal gastrectomy. The information of the remaining 10 patients was missing. None of the patients received chemotherapy prior to surgery. A total of 84 patients were treated with 5 -FU + platinum-based adjuvant chemotherapy. The patients were followed until the last follow-up date or death. Among the 175 patients, there were 75 death events and 65 recurrence events in total. The follow-up period ranged from 0 .5 -182 mo, and the median follow-up time was 22 .4 mo.
Overexpression of NQO1 protein is associated with GC prognosis
IHC staining showed that the NQO1 protein was mainly localized in the cytoplasm of GC cells (Figure 2 ). The level of NQO1 protein expression was high in 49 .43 % of the samples, with an overall score of > 4 , while 71 (40 .57 %) samples showed low NQO1 expression levels, with an overall score of ≤ 4 . To evaluate the role of the NQO1 protein in GC progression, we analyzed the correlation between its expression and the clinical and pathological parameters (Table 2 ). The number of patients with high levels of NQO1 expression and lymph node metastasis was higher than that of patients with low NQO1 expression, but this difference was not significant (P = 0 .06 ; Table 2 ). In addition, there were no significant correlations between NQO1 and other clinical parameters (Table 2 ). However, the follow-up results showed that the mean survival time for patients with low levels of NQO1 expression was 34 mo, while for those with NQO1 overexpression, it was 22 mo. Kaplan-Meier survival analysis revealed that patients with NQO1 overexpression had significantly poorer survival rates than those with low levels of NQO1 expression (P = 0 .02 ; Figure 3 ).
We further used the Cox proportional hazard regression method to examine the correlations between various clinical indexes and survival (Table 3 ). Univariate analysis revealed significant associations of tumor size (P< 0 .001 ), tumor stage (P<0 .001 ), and NQO1 expression (P = 0 .01 ) with overall survival, whereas the other factors showed no significant association. However, multivariate analysis of these factors,including NQO1 expression, indicated that age, tumor stage, and NQO1 were independent prognostic factors for the overall survival of GC patients [hazard ratio(HR) = 1 .90 , P = 0 .01 ; HR = 2 .64 , P < 0 .001 ; and HR = 1 .56 , P = 0 .05 , respectively].
NQO1 overexpression predicts a favorable response to chemotherapy in GC patients
We also performed the survival analysis of patients from the various treatment subgroups based on the NQO1 expression. Among 84 patients treated with 5 -FUbased chemotherapy, 52 (61 .09 %) had high levels of NQO1 expression and 32 (38 .10 %)had low levels of NQO1 expression. Similarly, high and low levels of NQO1 expression were found in 60 % and 40 % of patients who did not receive treatment,respectively. Patients with low levels of NQO1 expression showed poor response to adjuvant chemotherapy and similar survival, compared to those that underwent surgery alone (log rankP= 0 .82 ) (Figure 4 A). However, high levels of NQO1 expression indicated good response to adjuvant chemotherapy and longer overall survival compared with those in patients that underwent surgery alone (log rankP=0 .03 ) (Figure 4 B). In addition, in the Kaplan-Meier analysis, we stratified NQO1expression based on chemotherapy. Interestingly, the results showed that in patients who underwent surgery only, significant difference in overall survival was observed(log rankP= 0 .003 ), whereas in patients who underwent surgery plus chemotherapy,no difference was found (log rankP= 0 .61 ) (Figure 4 C and D). These findings indicated that GC patients with NQO1 overexpression may be suitable for adjuvant chemotherapy.

Table 1 Clinical characteristic of the gastric cancer patients

Chemotherapy Adjuvant chemotherapy 8448 .00 Without chemotherapy 8045 .71 Unknown 116 .29
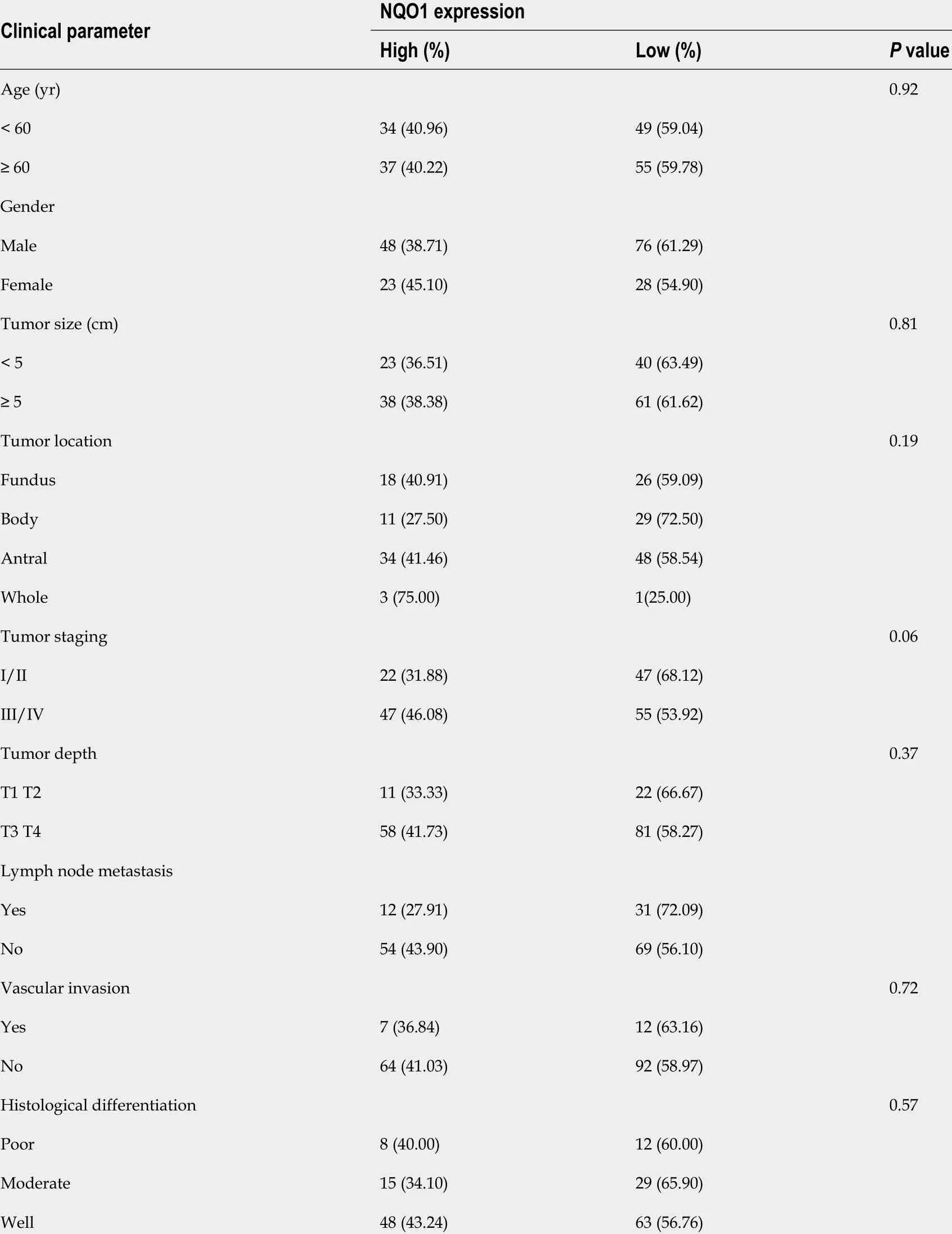
Table 2 Association of quinine oxidoreductase 1 expression with clinical and pathological features of gastric patients

Table 3 Univariate and multivariate analyses of prognostic factors in gastric cancer patient
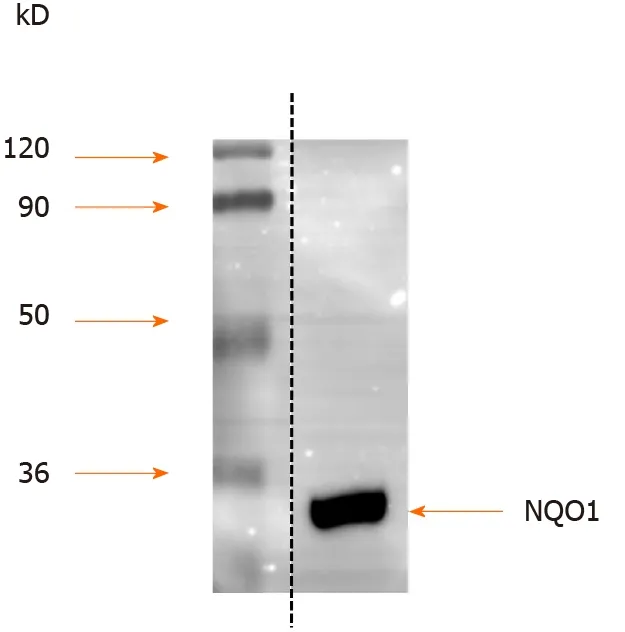
Figure 1 Western blot analysis of quinine oxidoreductase 1 expression in gastric carcinoma cell line AGS. Protein extracts from AGS human gastric cancer cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins were detected by Western blot using anti-quinine oxidoreductase 1 antibodies. Molecular masses in kDa are indicated to the left. NQO1 : Quinine oxidoreductase 1 .

Figure 2 Representative images of immunohistochemical staining for quinine oxidoreductase 1 in gastric cancer samples. A: Strong (+++);B: Moderate (++); C: Weak (+); D: Negative staining (original magnification × 400 ).

Figure 3 Kaplan-Meier survival curves of postoperative outcomes of gastric cancer patients according to quinine oxidoreductase 1 expression status. The correlation of high and low quinine oxidoreductase 1 expression with the overall survival rate was statistically significant (P = 0 .02 ). NQO1 :Quinine oxidoreductase 1 ; OS: Overall survival; CI: Confidence interval.
DISCUSSION
The catalytic properties of NQO1 were first reported by Ernster and Navazio[21 ] in 1958 . NQO1 is a homodimeric flavoprotein that reduces quinines to hydroquinones in an obligatory two-electron reduction step[6 ]. After years of research, cumulative data have shown that NQO1 protects cells from exogenous substances, oxidants, and ultraviolet and ionizing radiation by inducing a series of protective reactions[10 ].Consistent with this finding, recent research by Nagataet al[22 ] and Malik et al[23 ]showed that the C609 T polymorphism in the NQO1 gene might be linked to the risk of cancer-associated death. In addition, anNQO1polymorphism that causes inactivation of the enzyme predicts a poor outcome in breast cancer[24 ]. Moreover, NQO1 plays a prominent role in regulating the stability of p53 by inhibiting its degradation and that of other labile proteins, such as ornithine decarboxylase[25 ,26 ]. Based on this evidence,NQO1 plays an important role in cancer progression.
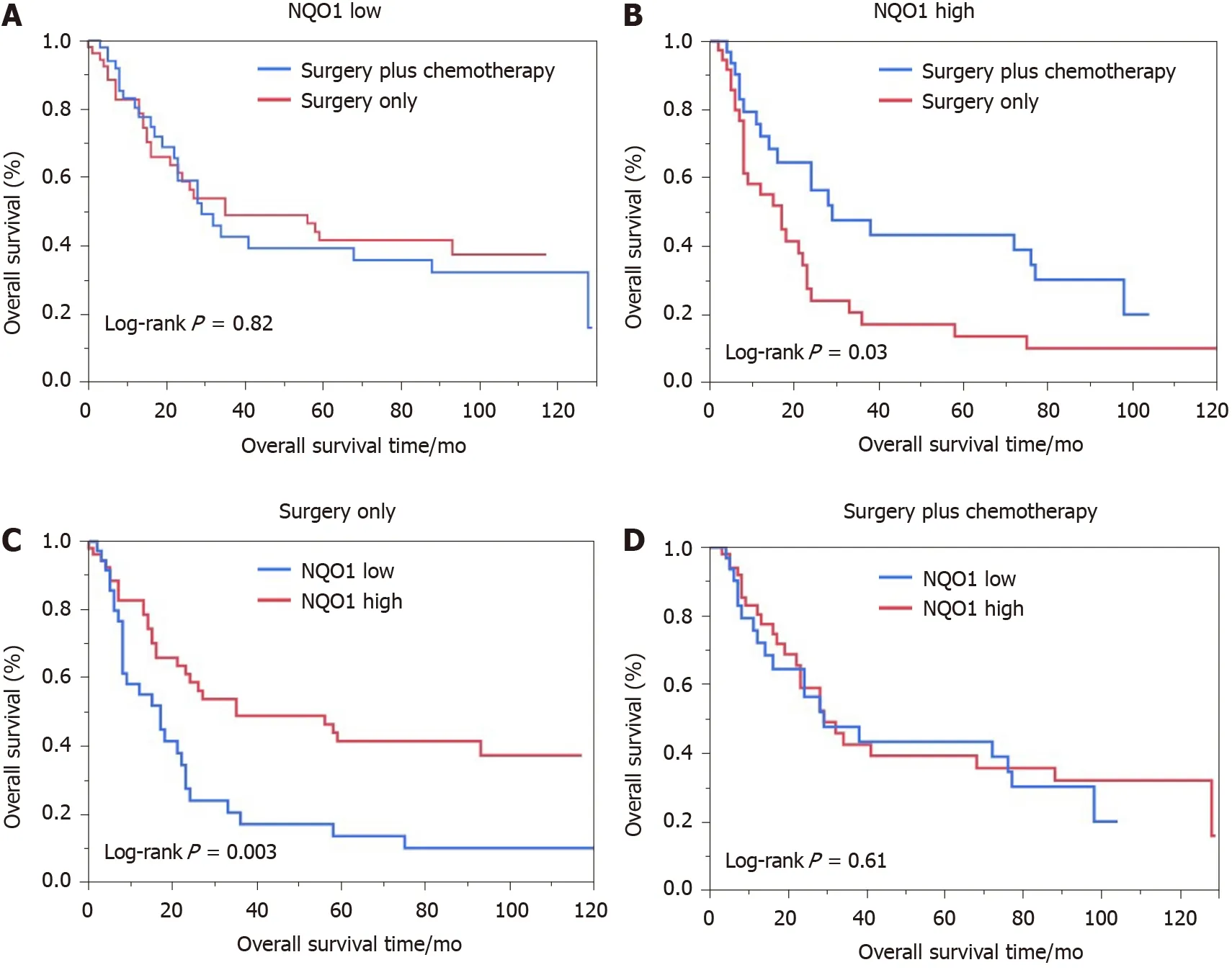
Figure 4 Kaplan-Meier analysis of overall survival rates in patients with gastric cancer. A: Low oxidoreductase 1 expression; B: High quinine oxidoreductase 1 expression; C: Surgery only; D: With adjuvant chemotherapy. NQO1 : Quinine oxidoreductase 1 .
Recent studies have reported that NQO1 protein expression is significantly elevated in the cytoplasm and nucleus in many human solid tumors[27 ]. For instance, Yanget al[28 ] showed that NQO1 is highly expressed in breast cancer tissues and may be associated with breast cancer progression. Chenget al[29 ] demonstrated strong NQO1 expression in primary melanomas compared with that in dysplastic nevi, and that NQO1 may be involved in the initiation of melanoma development. Similarly, the expression of NQO1 in the cervical epithelium indicated that it might be responsible for cervical carcinogenesis[30 ]. Malkinson et al[31 ] reported elevated NQO1 expression in human lung cancer tissues. Liet al[32 ] showed that NQO1 is upregulated in nonsmall cell lung cancer and identified it as a biomarker of poor prognosis. In addition,the gene encoding NQO1 is thought to participate in the pathogenesis of lung cancer[33 ,34 ]. Consistently, Ji et al[35 ], Awadallah et al[13 ], and Lyn-Cook et al[36 ]demonstrated that elevated NQO1 expression is associated with pancreatic ductal adenocarcinoma and may be a potential prognostic biomarker for pancreatic cancer.
In the present study, elevated NQO1 expression was observed in cancer tissues. In addition, a higher rate of NQO1 protein overexpression in patients with lymph node metastasis than in those without metastasis was observed. This is partly consistent with the findings of Linet al[14 ], which suggested that NQO1 upregulation contributes to tumorigenesis and progression of GC.
With regard to survival, we found that GC patients with overexpression of NQO1 protein had a significantly lower overall survival than those with low NQO1 expression. Buranratet al[12 ] showed a significant correlation between overexpression of NQO1 protein and short overall survival in cholangiocarcinoma patients, enhancing the possibility of using NQO1 as a tumor biomarker. Univariate survival analysis revealed that tumor size, tumor location, tumor stage, histological differentiation, and NQO1 expression status were significantly associated with the overall survival of GC patients. Likewise, multivariate survival analysis revealed that NQO1 was an independent prognostic biomarker, along with the tumor stage and patient age.Interestingly, it was also notable that patients with elevated NQO1 expression may be more sensitive to 5 -FU-based chemotherapy than those with low NQO1 expression.Although the mechanism underlying this phenomenon remains unclear, the results indicated that NQO1 may serve as a biomarker for the early diagnosis and prognosis of GC, and as a potential molecular target for GC therapy. However, the role of NQO1 in GC has not been fully elucidated. Further manipulation ofNQO1gene expression in different gastric cancer cell lines and investigation of the characteristics and mechanism of its effects on gastric cancer are needed in additional studies.
CONCLUSION
NQO1 is involved in the initiation and progression of GC. High NQO1 expression levels indicates a worse overall survival and better response to 5 -FU therapy. NQO1 could be a useful prognostic biomarker and therapeutic target for the appropriate management of patients with GC.
ARTICLE HIGHLIGHTS
Research conclusions
This study found that high expression of NQO1 is closely related to a poor prognosis and better response to 5 -fluorouracil in patients with gastric cancer.
Research perspectives
Our study found prognostic value of NQO1 protein expression in patients with gastric cancer. However, our results need to be validated by independent groups or cohorts in a prospective study.
ACKNOWLEDGEMENTS
We thank Ms Shiping Yan and Mr. Ahmed Hammad for their assistance with the preparation of the manuscript.
 World Journal of Gastroenterology2021年22期
World Journal of Gastroenterology2021年22期
- World Journal of Gastroenterology的其它文章
- Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma
- High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: A proof-of-concept study
- Liver injury in COVID-19 : Detection, pathogenesis, and treatment
- Enhancer of zeste homolog 2 contributes to apoptosis by inactivating janus kinase 2 / signal transducer and activator of transcription signaling in inflammatory bowel disease
- Interplay between nuclear factor erythroid 2 -related factor 2 and inflammatory mediators in COVID-19 -related liver injury
- Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression
