Epithelial-mesenchymal transition-related circular RNAs in lung carcinoma
Meina Jiang, Shuai Fang, Xiaodong Zhao, Chengwei Zhou, Zhaohui Gong
1Department of Biochemistry and Molecular Biology, Ningbo University School of Medicine, Ningbo 315211, China; 2Zhejiang Province Key Laboratory of Pathophysiology, Ningbo University School of Medicine, Ningbo 315211, China; 3Department of Thoracic Surgery, The Affiliated Hospital of Ningbo University School of Medicine, Ningbo 315020, China
ABSTRACT The epithelial-mesenchymal transition (EMT) is a highly complex phenotypic conversion during embryogenesis, and is important for metastasis, which contributes to tumor deterioration and poor prognoses of cancer patients. Lung carcinoma has a high tendency to develop the EMT. Circular RNAs (circRNAs) are involved in EMT-related cell invasion and metastasis in various types of cancers.Moreover, circRNAs have been found to be a link to EMT-related transcription factors and EMT-associated signaling pathways. This review mainly focuses on the influence of EMT-related circRNAs on lung carcinomas. More specifically, the roles of EMT-inducingand EMT-suppressive circRNAs in lung carcinomas are discussed. With circRNAs potentially becoming promising biomarkers and therapeutic targets for cancer managements, they will hopefully stimulate the interest of medical workers in the early diagnosis,personalized treatment, and positive prognoses in the era of precision oncology.
KEYWORDS Epithelial-mesenchymal transition; circular RNA; transcription factor; metastasis; lung carcinoma
Introduction
The epithelial-mesenchymal transition (EMT) is a highly complicated phenotypic conversion during embryogenesis, and is a potential process in adults by which epithelial cells gradually downregulate the expressions of cytokeratins and E-cadherin,and upregulate the expressions of mesenchymal genes such as vimentin and fibronectin1. Generally, the EMT can be divided into three subtypes: type I EMT in embryonic development,type II EMT in fibrosis, and type III EMT in premalignant and malignant stroma2. It is worth noting that EMT is not a purely bipolar state with two well-defined cell populations of mesenchymal cells and epithelial cells. There is also an intermediate state, called partial, involving incomplete hybrid EMT states expressing various levels of epithelial and mesenchymal characteristics and preserving intermediate morphologies3-5.The EMT is involved in multiple tumor processes including tumor initiation, stemness, migration, cancerous progression,intravasation into the blood, malignant metastasis, and resistance to therapy6. However, EMT-inducing transcription factors (EMT-TFs), EMT-related signaling pathways, epigenetic controls, and post-transcriptional regulators have also been found to regulate the EMT7. Nonetheless, the roles of circular RNAs (circRNAs) as post-transcriptional regulators in modulating the EMT remain unclear.
With the rapid development of high-throughput RNA sequencing (RNA-seq), a variety of circRNAs have been characterized in humans. In the last few years, it has been reported that circRNAs possess EMT-associated functions and may have an effect on epithelial and mesenchymal cell characteristics including metastasis, migration, and invasion8. It is known that circRNAs are a type of covalent single chain closed-loop molecule lacking the 5′end cap and 3′end poly (A) tailsviaa form of alternative splicing, resulting in more stability than linear RNAs in the presence of RNase R9. The circRNAs can be divided into three classifications: exonic circRNAs (ecirc-RNAs), circular intronic RNAs (ciRNAs), and exon-intronic circRNAs (EIciRNAs). Based on their translational capabilities, circRNAs can be classified into noncoding circRNAs and protein-coding circRNAs10. In future studies, EMT-related circRNAs will therefore be extensively studied in the field of oncology.
With a 5-year survival rate of 16.6%, lung cancer is the most common cancer with two major categories of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), and is the leading cause of cancer-related deaths11,12. Despite great progress in experimental research and clinical treatments, lung carcinoma still has a poor diagnosis and prognosis. Thus, there is an urgent need to improve our understanding of the mechanisms of tumorigenesis in the lung13. Recently, studies have started to decipher the significance of EMT-related circ RNAs in various carcinomas. Lung carcinoma tends to develop as a result of the EMT and metastasis; however, the function of EMT-related circRNAs is still unclear. This review focuses on EMT-related circRNAs in lung carcinoma. It is hoped that it will stimulate interest in the early diagnosis, personalized treatment, and positive prognoses in the field of precision oncology.
Biosynthesis, characteristics, and functions of circRNAs
In the past, numerous circRNAs have been found at lower levels of expression by using outdated detection technologies.As a result, they have been recognized as nonspecific byproducts14. However, it is now known that circRNAs play more critical roles with their unique biosynthesis and characteristics, when compared to cognate linear RNAs. Most circRNAs are created by back-splicing in pre-mRNA, of which 3′ splice donors covalently link to 5′ splice acceptors in the opposite direction (Figure 1A). The back-splicing includes four paradigms such as exon-skipping, lariat-driven, intron- pairingdriven circularization, and RNA binding protein-driven circulation15. Furthermore, circRNAs have four distinct peculiarities. First, they are conservative despite the length of their evolution16. Second, they are specifically expressed spatiotemporally in altered cell types and tissues17. Third,they are abundant in all species containing plants, archaea,mice, and humans9,18-20. Finally, they have a high stability because of their closed-loop construction and short free terminals. Although circRNAs are stable, the understanding of circRNA degeneration is still unclear. Some hypotheses suggest that endonucleases or N6-methyladenosine (m6A) may initiate degradation of circRNAs. Alternatively, circRNAs may be degraded when combined with miRNAs during Ago2-facilitated cleavage21,22.
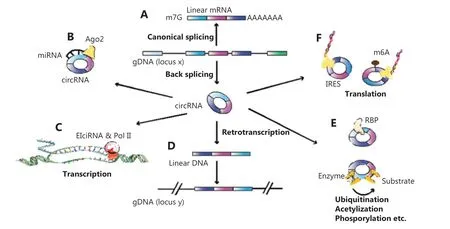
Figure 1 Biosynthesis and functions of circular RNAs (circRNAs). (A) Pre-mRNAs generate mRNAs through canonical splicing while circRNAs are generated through back-splicing. (B) CircRNAs bind to miRNAs. (C) Exon-intronic circRNAs bind to pol II to regulate transcription.(D) The circRNA-derived pseudogenes insert into the genome and switch the genomic DNA configuration through circRNA-retrotranspostion.(E) CircRNAs control protein-protein interactions in a similar manner to reservoirs or scaffoldings. (F) CircRNAs initiate translation through internal ribosome entry sites or via N6-methyladenosine.
Regarding biological functions, circRNAs play different roles in different subcellular locations. In the cytoplasm,circRNA segregates miRNA as a “sponge” to modulate the activity of miRNA and the miRNA-targeted gene (Figure 1B). For example, Zhang et al.23reported that circFOXO3(hsa_circ_0006404) promoted the expression of nuclear factor of activated T cells 5 (NFAT5) as a sponge of both miR-138-5p and miR-432-5p in glioblastoma tissues.In the nucleus, circRNA regulates the splicing process or gene transcription (Figure 1C). Huang et al.24showed that circERBB2, a circRNA generated from theERBB2cognate gene, accumulated in the nucleus to bind with PA2G4, a nucleolus-associated protein, to regulate ribosomal DNA transcription and cell proliferationviathe circERBB2-PA2G4-TIFIA axis in gallbladder cancer. Like linear mRNAs, circRNAs can also generate pseudogenes.The circRNA- derived pseudogenes are capable of inserting into the genome and switching the genomic DNA configuration during circRNA retrotransposition25(Figure 1D).Moreover, circRNAs, in a similar manner to reservoirs or scaffoldings, can bind proteins in exact subcellular positions to control protein-protein interactions26(Figure 1E).Presently, it is well-known that circRNAs involve one of the spliced production coding peptides. For example, Ye et al.27showed that circFBXW7 (hsa_circ_0001451) encoded a 185 amino acid peptide (FBXW7-185aa) inhibiting migration and proliferation in triple-negative breast cancer cells.Yet unlike canonic cap-dependent translations, circRNAs commence translationviathe internal ribosome entry site(IRES) or through m6A27,28(Figure 1F).
The circRNA link to the EMT in multiple cancers
The EMT comprises fine-tuned phenotypic shifts, in which cells imperceptibly decline expressing E-cadherin and lose cellular adhesion and apical-basal polarity, while showing increased expressions of N-cadherin, extracellular matrix degradation, and cytoskeletal reorganization. Such shifts are initiated by EMT-TFs, which are regulated by EMT-related signaling pathways. EMT-inducing signs are spatiotemporally specific when interacting with numerous regulators and signaling pathways. Although the contributions of the EMT to drug resistance and chemotherapy tolerance have been well-studied, the EMT promotion of tumor metastasis is still controversial29. However, increasing evidence has suggested that circRNA-mediated EMT is an essential part of tumor occurrence and development (Table 1).
The circRNA link to EMT-TFs
Vimentin is a type of EMT protein marker, which is present in mesenchymal cells and is involved in cancer metastasis and poor prognoses. Cullin2 (Cul2), as a tumor suppressor protein, is a principal part of the multiple cullin-RING-based ECS (Elongin B/C-Cul2/5-SOCS-box protein) E3 ubiquitin-protein ligase complexes engaging in cell cycle control and vasculogenesis30.Twist, a vital EMT-TF, combines with the promoter of Cul2 to increase the expression of circCul2 (hsa_circ_10720), which binds to a set of miRNAs to increase vimentin expression in hepatocellular carcinoma (HCC)31(Figure 2A). In addition to Twist, the EMT is also initiated by Snail, which is a conservative protein of the zinc finger transcription factor family, containing zinc finger domains to bind DNA at the C-terminus, and containing the SNAG domain to interact with epigenetic remodeling complexes at the N-terminus32,33. In urothelial carcinoma of the bladder (UCB), circPRMT5 (has_circRNA_101320)generated fromPRMT5on chromosome 14q11.2, promotes the EMT and aggressiveness through the circPRMT5/miR-30c/SNAIL1/E-cadherin pathway34(Figure 2B). In melanomas, circRNA_0084043 contributes to cell metastasis and growthviathe miR-153-3p/snail axis35(Figure 2C). In cervical cancer, circRNA_000284 induces cell invasion and proliferationviathe circRNA-000284/miR-506/Snail-2 axis36. Apart from Twist and Snail, ZEB2 has been associated with epithelial polarities and diverse malignancies. Li et al.37found that circNUP214 (hsa_circ_0089153), as an oncogenic molecule, sponged miR-145 to upregulate the expression of ZEB2 and thereby promote cell proliferation, migration, and invasion in papillary thyroid cancer (PTC). Taken together, the results have shown that circRNAs can bind the classic EMT-TFs to modulate the EMT process and cancer development.
The circRNA link to EMT-related signaling pathways
In addition to the EMT-TFs, EMT-related signaling pathways had been reported to be linked to cancer-associated circ RNAs.In triple-negative breast cancer (TNBC), circANKS1B (hsa_circ_0007294), derived from exons 5-8 of theANKS1Bgene,is an independent risk factor of the overall survival of patients with TNBC, because of the promotion of tumor metastasis and invasion. Mechanistically, circANKS1B segregates miR-152-3p and miR-148a-3p, and upregulates the expression of transcription factor, USF1, which increases the expression
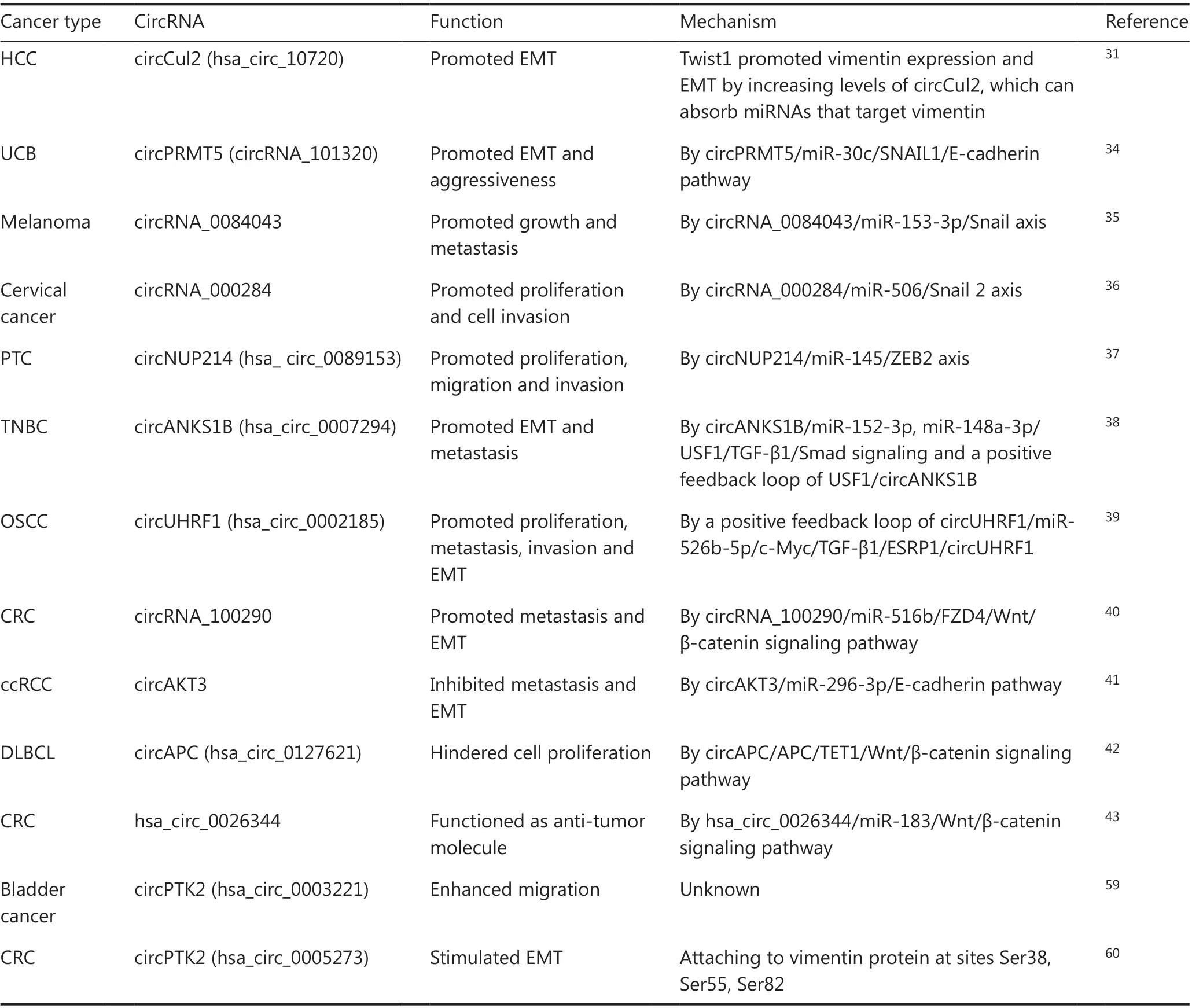
Table 1 CircRNAs modulating the epithelial-mesenchymal transition (EMT) in various carcinomas
HCC, hepatocellular carcinoma; UCB, urothelial carcinoma of the bladder; PTC, papillary thyroid cancer; TNBC, triple-negative breast cancer;
OSCC, oral squamous cell carcinoma; CRC, colorectal cancer; ccRCC, clear cell renal cell carcinoma; DLBCL, diffuse large B-cell lymphoma.of TGF-β1 by binding with the TGF-β1 promoter, and then initiating TGF-β1/Smad signaling to stimulate the EMT. In addition, there is a positive feedback loop where USF1 upregulates circANKS1B expression by regulating the splicing factor ESRP138(Figure 2D). In the oral squamous cell carcinoma(OSCC), circUHRF1 (hsa_circ_0002185) is in excess and promotes migration, invasion, proliferation, and the EMTin vitroandin vivoviaa positive feedback loop pathway of circUHRF1/miR-526b-5p/c-Myc/TGF-β1/ESRP1/circUHRF139(Figure 2E). In colorectal cancer (CRC), circRNA_100290 acts as an oncogene to increase metastasis and the EMT by sponging miR-516b, upregulating FZD4 expression, and then initiating the FZD4-induced Wnt/β-catenin signaling pathway40(Figure 2F). Other than pro-oncogenic circRNAs,investigators have also discovered some EMT suppressive circ-RNAs. For example, in clear cell renal cell carcinoma (ccRCC),overexpression of circAKT3 decreases the EMT and metastasisviathe circAKT3/miR-296-3p/E-cadherin pathway41(Figure 2G). In diffuse large B-cell lymphoma (DLBCL),circAPC (hsa_circ_0127621), derived from theAPCexon 7 to exon 14, decreases cell proliferation. CircAPC binds the DNA demethylase, TET1, and binds to the APC promoter to enhance the expression of APC, which decreases the Wnt/βcatenin signaling pathway in the nucleus. In addition, circAPC acts as a sponge of miR-888 to upregulate APC expression in the cytoplasm42(Figure 2H). Notably, the EMT and metastasis induced by CCL20 and CXCL8 treatment are decreased by overexpression of anti-tumor hsa_circ_0026344, which binds to miR-183 and inhibits the Wnt/-catenin pathway43. In general, circRNAs modulate EMT and migration mainly through two signaling pathways: the TGF-β1 signaling and Wnt/β-catenin signaling pathways.
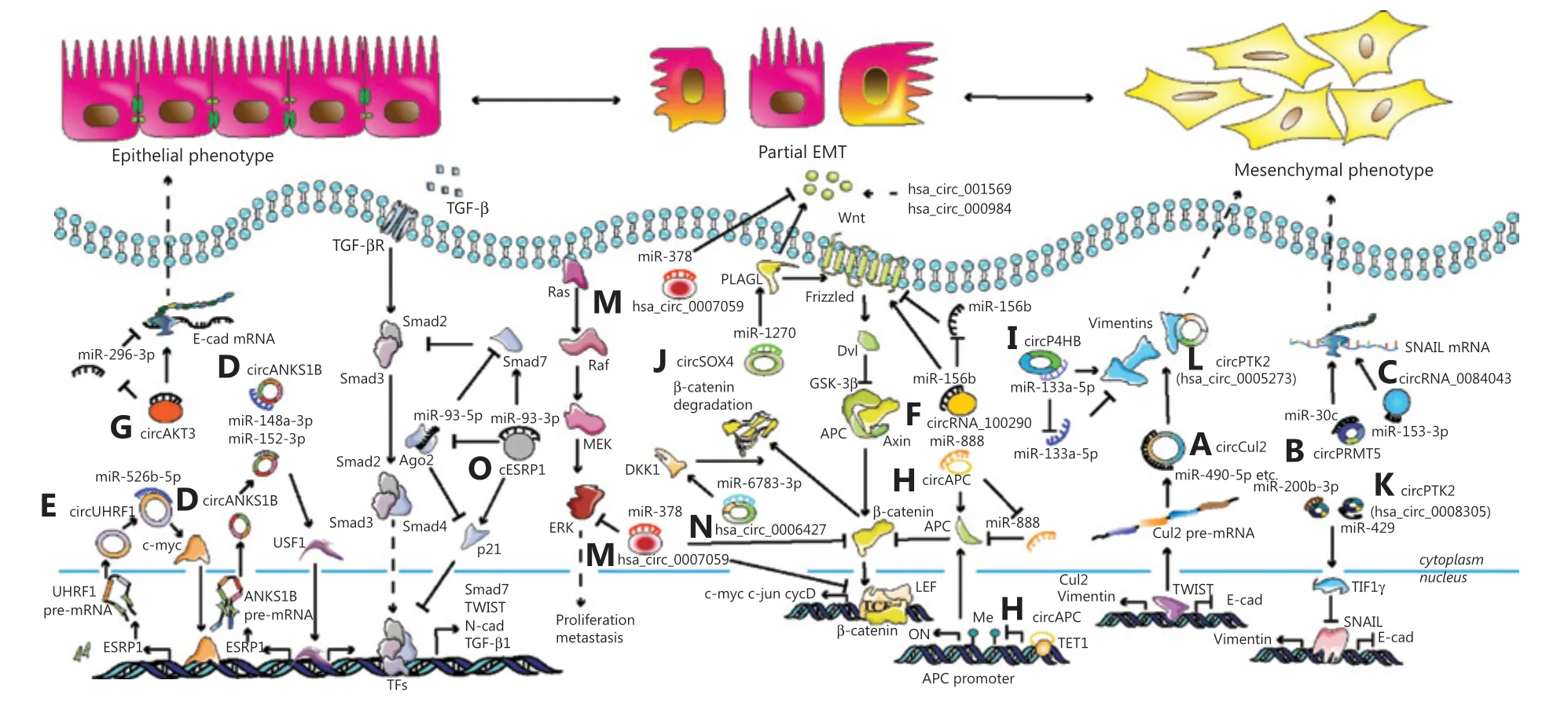
Figure 2 Mechanisms of the circRNA-mediated epithelial-mesenchymal transition (EMT) and metastasis in various carcinomas. (A) Twist promotes circCul2 expression by sponging miR-490-5p, and results in the upregulation of vimentin in hepatocellular carcinoma. (B) CircPRMT5 mediates the EMT in urothelial carcinoma of the bladder through the circPRMT5/miR-30c/SNAIL1 axis. (C) CicrRNA_0084043 increases metastasis in melanomas by regulating the miR-153-3p/Snail axis. (D) CircANKS1B facilitates tumor metastasis and invasion in triple-negative breast cancer through the circANKS1B/miR-148a-3p/miR-152-3p/USF1/TGF-β1 axis and a positive feedback loop of USF1/ESRP1/circANKS1B. (E) CircUHRF1 promotes migration, invasion, and the EMT in oral squamous cell carcinoma through a positive feedback loop of circUHRF1/miR-526b-5p/c-Myc/TGF-β1/ESRP1/circUHRF1. (F) CircRNA_100290 induces metastasis and the EMT in colorectal cancer (CRC) through the miR-516b/FZD4/Wnt/β-catenin signaling pathway. (G) CircAKT3 accelerates metastasis and the EMT in clear cell renal carcinoma by the circAKT3/miR-296-3p/E-cadherin axis. (H) CircAPC decreases the canonical Wnt/β-catenin signaling pathway in diffuse large B-cell lymphoma by the miR-888/APC axis in the cytoplasm and circAPC/TET1/APC in the nucleus. (I) CircP4HB promotes the EMT in non-small cell lung cancer (NSCLC) by the circP4HB/miR-133a-5p/vimentin axis. (J) CircSOX4 facilitates the EMT in lung adenocarcinoma (LUAD) by the circSOX4/miR-1270/ PLAGL2 axis. (K) CircPTK2(hsa_circ_0008305) enhances the EMT in NSCLC by the circPTK2/miR-429/miR-200b-3p/TIF1γ/snail axis. (L) CircPTK2 (hsa_circ_005273) stimulates the EMT in CRC via directly combining with vimentin. (M) Hsa_circ_0007059 inhibits the EMT, decreases Wnt/β-catenin, and ERK1/2 signaling pathways in A549 and H 1975 cells by suppressing miR-378. (N) Hsa_circ_0006427 represses the Wnt/β-catenin signaling pathway in LUAD via the miR-6783-3p/DKK1 axis. (O) cESRP1 suppresses the EMT in NSCLC via the cESRP1/miR-93-5p/Smad7/p21 axis.
The influence of EMT-related circRNAs on lung carcinoma
The EMT phenotype is commonly expressed in primary squamous cell carcinomas (SCCs) and lung adenocarcinomas (LUADs), and occurs early in the pathogenesis of SCC,suggesting a potential target for lung cancer chemoprevention and treatment44. In addition, the overexpression of forkhead box Q1 (FoxQ1) influences the poor prognosis in NSCLC and is associated with the EMT45. Further studies showed that the cancer stemness marker was associated with the EMT and predicted poor prognoses in patients with LUAD46. Thus,lung carcinoma has been shown to be linked to the EMT and metastasis47, and the EMT-related circRNAs have an effect on lung carcinomas (Table 2). Overall, the EMT-inducing and EMT-suppressive circRNAs play very important roles in the occurrence and development of lung carcinomas.
The EMT-inducing circRNAs in NSCLC
Among the most common histological subtypes of lung carcinoma, LUAD is responsible for a majority of cancer-relatedmortalities worldwide. Zhou et al.12reported that circENO1 and its host gene,ENO1were both amplified in LUAD cells to augment glycolysis and tumor growth, and silenced circENO1 impeded glycolysis, migration and the EMT, and induced cell apoptosisviathe circENO1/miR-223p/ENO1 axis. Similarly, circAGFG1 increased the EMT of NSCLC cellsviainvasion and migration by acting as a sponge for miR-203,which targeted ZNF28148. In addition, Wang et al.49reported that circP4HB was higher in NSCLC tissues than in healthy paired samples, and that circP4HB promoted higher vimentin expression and the EMTin vivoandin vitroviathe circP4HB/miR-133a-5p/vimentin axis (Figure 2I). Qi et al.50found that EMT-inducing circDDX42 (hsa_circ_0007534), a transcription product of DEAD-box helicase 42, decreased E-cadherin and increased the levels of Snail, N-cadherin, and vimentin.CircTwist1 (hsa_circ_0079530), which is 664 nt in length, was found to be upregulated in NSCLC. Knockdown of CircTwist1 resulted in the downregulation of mesenchymal marker proteins and the upregulation of epithelial marker proteins in A549 and H1299 cells51. Similarly, hsa_circ_0067934 led to an identical trend of changes of N-cadherin and vimentin, and an opposite effect for E-cadherin52. In LUAD, it was found that circCCDC66 and SUMO-activating enzyme subunit 2 (SAE2)were both highly expressed and associated with the EMT, lung cancer metastasis, and EGFR drug resistance. Hepatocyte growth factor (HGF) and c-Met upregulate SAE2 and circCCDC66 to enhance EMT and drug resistance; however,nicotinic acetylcholine receptor alpha 7 (nAChRα7) negatively modulates circCCDC66 expression53. LIM domain kinase 1(LIMK1) is a serine-threonine protein kinase, which affects
the actin cytoskeleton and participates in the EMT. Qin et al.54showed that hsa_circ_0012673 upregulated LIMK1viabinding to miR-320a to manipulate migration, invasion, proliferation, apoptosis, and the EMT in lung cancer cells. Because the EMT is closely correlated with the Wnt-associated pathways, many studies have focused on the effects of circRNAs on the expression levels of cyclin D1, β-catenin, and c-myc in the Wnt/β-catenin signaling pathway. Li et al.55reported that hsa_circ_000984 was highly expressed in NSCLC tissues and cells,and correlated with shorter disease-free survival and overall survival. Functionally, hsa_circ_000984 promoted EMT to a pro-oncogenic roleviaupregulating the expression of cyclin D1, β-catenin, and c-myc in the Wnt/β-catenin pathway. It was also reported that hsa_circ_001569 predicted a poor prognosis and enhanced the expressions of crucial members of the Wnt/β-catenin pathway, including Wnt1, β-catenin, and transcription factor 4 (TF4)56. In the same manner, upregulated circ-SOX4 (hsa_circ_0131457) activates the Wnt signaling pathway and promotes the EMTviathe circSOX4/miR-1270/ PLAGL2 axis in LUAD57(Figure 2J). Taken together, it is clear that the EMT-inducing circRNAs promote EMT-mediated metastasis,mainly by affecting the major members of the EMT-related signaling pathways in NSCLC.
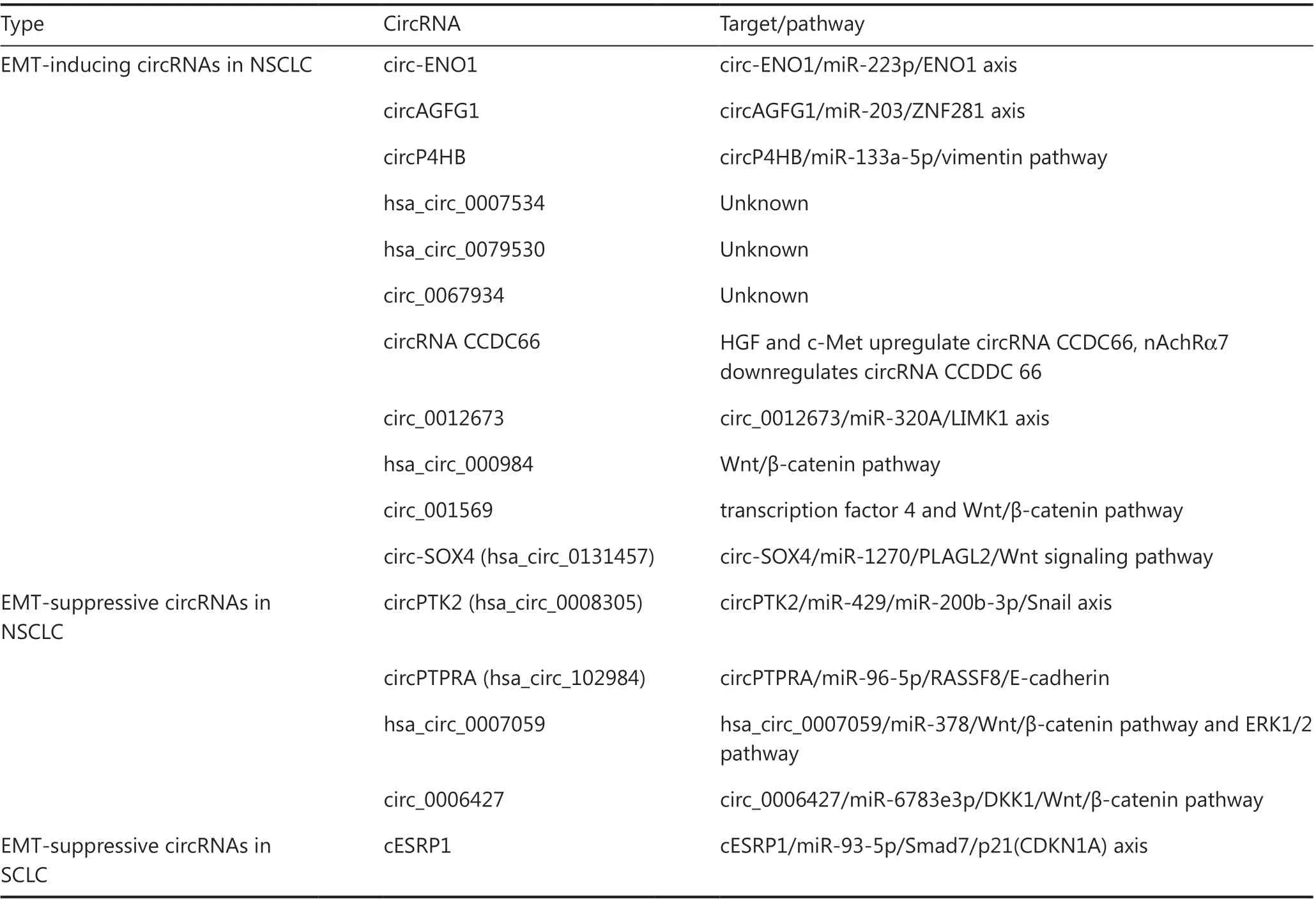
Table 2 The epithelial-mesenchymal transition (EMT)-related circRNAs in lung carcinoma
The EMT-suppressive circRNAs in NSCLC
In contrast to EMT-inducing circRNAs, EMT-suppressive circRNAs usually negatively modulate EMT and inhibit certain cell programs such as migration, metastasis, and invasion.As mentioned above, Snail is one of the classical EMT-TFs.In NSCLC, circPTK2 (hsa_circ_0008305) binds to miR-429/miR-200b-3p to enhance transcriptional intermediary factor 1γ (TIF1γ), which suppresses the function of Snail in the nucleus58(Figure 2K). Notably, circPTK2 (hsa_circ_0003221),derived from thePTK2, enhances migration in bladder cancer cells59. Recently, Yang et al.60reported that circPTK2(hsa_circ_0005273), unlike hsa_circ_0008305 in NSCLC,stimulated the EMTviabinding to vimentin protein at Ser38, Ser55, and Ser82 in CRC (Figure 2L). Ras association domain-containing protein 8 (RASSF8) is an acknowledged tumor suppressor of lung cancer61. Wei et al.62showed that circPTPRA (hsa_circ_102984) derived from protein tyrosine phosphatase receptor type A gene (PTPRA) inhibited tumor metastasis and the EMT by sponging miR-96-5p and releasing RASSF8 and E-cadherin. In A549 and H1975 cells, overexpression of hsa_circ_0007059 restrains cell proliferation, decreases the EMT, and hinders the Wnt/β-catenin and ERK1/2 signaling pathwaysviasuppressing miR-37863(Figure 2M). In addition, Yao et al.64have shown that the cytoplasmic-located has_circ_0006427 sponges miR-6783-3p to release Dickkopf WNT Signaling Pathway Inhibitor 1 (DKK1) and inhibit the Wnt/β-catenin pathway, and thereby repress cell proliferation,migration, invasion and EMT in LUAD cells (Figure 2N). In summary, the EMT-suppressive circRNAs play important roles in suppressing EMT-mediated metastasis in NSCLCviaacting as sponges of miRNAs to influence EMT-TFs, EMTrelated signaling, and EMT markers.
The EMT-suppressive circRNAs in SCLC
The effects of EMT-related circRNAs on SCLC presently remain largely unknown. Huang et al.65showed that cESRP1(circular RNA epithelial splicing regulatory protein 1) located in the cytoplasm sequestered miR-93-5p to free Smad7/p21(CDKN1A) and further regulate the TGF-β-induced EMT(Figure 2O). Moreover, the inhibition of the TGF-β pathway and cESRP1 overexpression improved the responsiveness to chemotherapy in a patient-derived xenograft model suffering acquired chemoresistance.
Conclusions
Although numerous studies have verified that circRNAs are more stable than their cognate mRNAs, the effectiveness of back splicing is lower than canonical splicing from certain expression vectors66. Recent advances have shown that circ RNAs can act as minimally-invasive or noninvasive biomarkers for various diseases including cancers, although the detection techniques in blood and other body fluids still need to be improved15,67.More importantly, circRNAs may work together as a group,with complicated crosstalk with other regulators68. The EMT is a chief element of the metastatic cascade, which is important in tumor deterioration. Additionally, the EMT is responsible for chemoresistance and immune resistance69,70. Although the correlations between miRNAs and EMT-TFs have been wellestablished71, as sponges for miRNAs, the roles of circRNAs in the EMT remain to be discovered. The levels of certain circRNAs could be distinctly changed during the EMT due to splicing programs stimulated by a set of splicing factors such as RBFOX2,SRSF2, and QKI8. CircRNAs function as parts of a noncoding RNA adjustment net by separating proteins and miRNAs during the EMT. A quantitative EMT scoring system based on gene expression profiles has recently been established to grade the status of the EMT47. This system has been used to emphasize the developmental lineage of each cancer subtype from microscopic and macroscopic EMT gradients, and each cancer subtype has a unique tendency to exhibit different EMT states.Lung carcinoma displays higher EMT scores, which always forecast a poor prognosis47. Investigators of EMT-related circRNAs can therefore provide novel therapeutic approaches and tactics for patients with lung carcinoma. Specifically, a large range of small molecule agents for targeting characteristics with EMT in lung carcinoma have been developed in preclinical phases72.For example, bufalin, a Chinese medicine, impedes the migratory activity of A549 human lung cancer cells and the TGF-βinduced EMT73. However, there is controversy concerning the oligonucleotide chemistry that targets circRNAs, but further studies may bring new hope to patients with drug resistance.
Grant support
This work was supported in part by research grants from the Non-profit Technology Research Program of Zhejiang (Grant No. LGF18H160006), the Non-profit Technology Research Program of Ningbo (Grant No. 2019C50040), the Natural Science Foundation of Ningbo (Grant No. 2018A610204),the Scientific Innovation Team Project of Ningbo (Grant No.2017C110019) and the K.C. Wong Magna Fund at Ningbo University.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2021年2期
Cancer Biology & Medicine2021年2期
- Cancer Biology & Medicine的其它文章
- Circular RNAs: new biomarkers of chemoresistance in cancer
- Biological roles and potential clinical values of circular RNAs in gastrointestinal malignancies
- Biomaterial-based platforms for cancer stem cell enrichment and study
- sLex expression in invasive micropapillary breast carcinoma is associated with poor prognosis and can be combined with MUC1/EMA as a supplementary diagnostic indicator
- Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development
- Targeting FGFR in non-small cell lung cancer: implications from the landscape of clinically actionable aberrations of FGFR kinases
