Biological roles and potential clinical values of circular RNAs in gastrointestinal malignancies
Xueping Tao, Yongfu Shao, Jianing Yan, Liyang Yang, Qihua Ye, Qingling Wang, Rongdan Lu,Junming Guo
1Department of Biochemistry and Molecular Biology and Zhejiang Key Laboratory of Pathophysiology, Ningbo University School of Medicine, Ningbo 315211, China; 2Department of Gastroenterology, the Affiliated Hospital of Medical School of Ningbo University, Ningbo 315020, China
ABSTRACT Circular RNAs (circRNAs), a class of endogenous RNA molecules, are produced by alternative splicing of precursor RNA and are covalently linked at the 5′ and 3′ ends. Recent studies have revealed that dysregulated circRNAs are closely related to the occurrence and progression of gastrointestinal malignancies. Accumulating evidence indicates that circRNAs, including circPVT1, circLARP4, circ-SFMBT2, cir-ITCH, circRNA_100782, circ_100395, circ-DONSON, hsa_circ_0001368, circNRIP1,circFAT1(e2), circCCDC66, circSMARCA5, circ-ZNF652, and circ_0030235 play important roles in the proliferation,differentiation, invasion, and metastasis of cancer cells through a variety of mechanisms, such as acting as microRNA sponges,interacting with RNA-binding proteins, regulating gene transcription and alternative splicing, and being translated into proteins. With the characteristics of high abundance, high stability, extensive functions, and certain tissue-, time- and diseasespecific expressions, circRNAs are expected to provide novel perspectives for the diagnoses and treatments of gastrointestinal malignancies.
KEYWORDS Circular RNA; gastrointestinal malignancies; biological function; clinical value; digestive system
Introduction
Malignant cancers pose a serious threat to human health. The latest evidence indicates that the incidence of cancers of the digestive system is over 30%, with more than 50 million new cases worldwide every year, ranking first among cancers of different body systems, and posing a major threat to human health1. Due to their high incidence, insidious onset, high degree of malignancy, and propensity for metastasis, gastrointestinal malignancies [such as gastric cancer (GC), colorectal cancer (CRC), hepatocellular carcinoma (HCC), pancreatic cancer, and esophageal cancer] are difficult to diagnose and treat early2-5.
Circular RNAs (circRNAs), a class of endogenous circular RNA molecules, are produced by alternative splicing of precursor RNAs, during which the 5′ and 3′ ends are covalently linked6. Accumulating studies have demonstrated that circRNAs play critical roles in the pathological processes of gastrointestinal malignancies, including tumorigenesis, development,and metastasis6-8. CircRNAs are considered to be important gene expression regulators. At the epigenetic, transcriptional,and posttranscriptional levels, circRNAs are involved in cancer cell proliferation, differentiation, invasion, and metastasis by sponging microRNA (miRNA), interacting with RNA-binding proteins (RBPs), regulating gene transcription and alternative splicing, and being translated into proteins8-10. Moreover,with the characteristics of high abundance, high stability,extensive functions, and certain tissue, time, and disease specificities, circRNAs are expected to serve as novel diagnostic biomarkers and new treatment targets for gastrointestinal malignancies3,11,12.
Hence, the relationships between circRNAs and gastrointestinal malignancies are an important research field. Here,we focus on the biological roles and potential value of circRNAs to provide new ideas for a better understanding of the pathogenesis of gastrointestinal malignancies, as well as new perspectives for the study of prevention and treatment strategies.
Biogenesis
CircRNAs are mainly generated by the alternative splicing of a class of precursor RNAs and covalently linked to form a circular structure8. In eukaryotic cells, circRNAs can be classified into the following 6 main types based on their genomic origin:(1) exonic circular RNA (ecircRNA), which can be generated by intron-pairing-driven circularization (Figure 1A) and lariat-driven circularization (Figure 1B); (2) circular intronic RNA (ciRNA; Figure 1C); (3) exon-intron-derived circRNA(EIciRNA; Figure 1D); (4) RBP-driven circularization-derived circRNA (Figure 1E); (5) tRNA precursor derived circRNA(tricRNA; Figure 1F); and (6) circRNAs from other sources(Figure 1G).
Lariat-driven circularization and intron-pairingdriven circularization of exonic circRNAs
Lariat-driven circularization is also known as the exon skipping mechanism. During transcription, mRNA precursors can partially fold, causing some exons to jump with the folding of the RNA to form a lariat structure, after which the introns within the lariat structure are removed to form an ecircRNA(Figure 1A). Kelly et al.13confirmed that there were numerous circRNAs derived from lariat-driven circularization by first analyzing the relationship between the abundance of each exon in poly(A)+ mRNA and circularized mRNA, then plotting the relative retention rate of each exon in mature transcripts as a function of its circularization, and controlling for the decrease in exon abundance caused by the decrease in maternal gene expression.
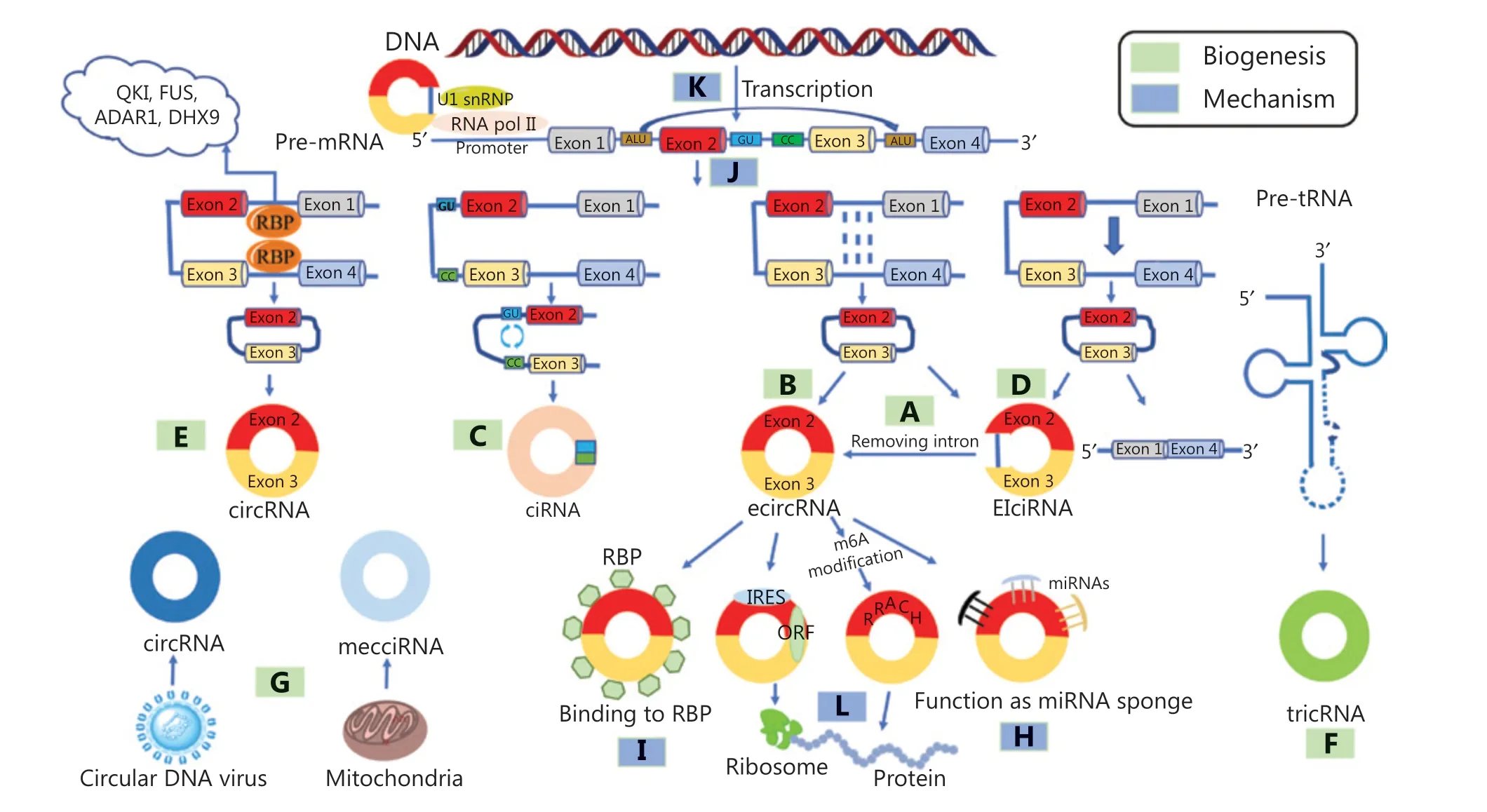
Figure 1 Biogenesis of circRNAs and the mechanisms of action of circRNAs. (A) Lariat-driven circularization (ecircRNA). (B) Intron-pairingdriven circularization (ecircRNA). (C) Circular intronic RNA. (D) Exon-intron-derived circRNA (EIciRNA). (E) RNA-binding protein (RBP)-driven circularization-derived circRNA. (F) The tRNA precursor-derived. (G) Other sources: fusion gene, circular DNA tumor virus, mitochondrial DNA-originated and mitochondria-encoded circular RNA (mecciRNA). (H) Functioning as miRNA sponges. (I) Interactions with RNA-binding proteins. (J) Involvement in alternative splicing regulation. (K) Regulation of parental gene transcription. (L) Protein-coding potential. QKI,Quaking; FUS, fused in sarcoma; ADAR1, adenosine to inosine acting on RNA enzyme 1; DHX9, DEAH-box helicase 9. IRES, internal ribosome entry site; ORF, open reading frame.
In intron-pairing-driven circularization, two flanking introns have reverse complementary structures (RCMs),such as Alu repeats, and the pairing of flanking introns bring splice junction sites close to each other, forming a secondary structure that mediates the back-splicing process to form an ecircRNA (Figure 1B). For example, in the flanking regions of hsa_circ_POLR2A, there are several relatively short introns with an upstream reverse Alu sequence and two downstream forward Alu sequences, which form inverted repeated Alu pairs (IRAlus) with reverse complementary structures that mediate the circularization of hsa_circ_POLR2A14.
Circular intronic RNAs
The circularization process of intronic RNA is driven by several short sequences located in specific introns, including the GU-enriched sequence near the 5′ end of the splice site and the C-rich sequence near the branch point (Figure 1C).The reverse complementary interactions between these short sequences ensure that they bind to form a lariat structure;then, the lariat is spliced by the spliceosome, which facilitates the formation of ciRNAs15.
Exon-intron-derived circRNAs
In the process of reverse splicing of a class of circRNAs related to RNA polymerase II (pol II), while exons are circularized,some intron sequences are not removed but retained, forming EIciRNAs (Figure 1D). For example, circEIF3J is an atypical EIciRNA. It can bind to the U1 snRNP binding site in the retained introns to accelerate the transcription of the parental genes16.
RBP-driven circularization-derived circRNAs
RBPs are involved in the circularization process. Some RBPs specifically bind to the flanking intron sequences of the transcript, bringing the donor sites and the acceptor sites close to each other and inducing exon circularization to form circRNAs (Figure 1E). For example, quaking-5 (QKI-5), an RBP,was initially recognized as a tumor suppressor. The latest evidence suggests that during the epithelial-mesenchymal transition (EMT), QKI-5 promotes exon circularization to form circRNA by binding to flanking intron sequences17.
The tRNA precursor-derived circRNAs
In the process of metazoan pre-tRNA self-splicing, tRNA splicing endonuclease (TSEN) recognizes and cleaves the canonical bulge-helix-bulge (BHB) motif of the anticodon loop18. Then,the generated 2′,3′-cyclic phosphate groups and 5′-OH are linked by the RNA ligase RtcB to form tricRNA (Figure 1F).In a similar manner, eukaryotic pre-tRNA was discovered to form tricRNAs, which are mostly located in the cytoplasm and are highly conserved19.
Other sources
Apart from the above mechanisms, there are other sources of circRNAs (Figure 1G). The flanking introns of the reverse splicing site have been confirmed to promote the circularization of a fusion gene to form a new type of circRNA. For example, F-circSR1 and F-circSR2 are derived from the SLC34A2-ROS1 fusion gene20. F-circEA is derived from the EML4-ALK fusion gene21. Additionally, circular DNA tumor virus and mitochondrial DNA produce circRNAs22,23.
Biological characteristics
Generally, circRNAs are widely expressed and abundant in diverse cell types and organisms, with over 10-fold higher abundance than that of linear RNA in some tissues24. Due to the lack of free 3′ and 5′ ends, circRNAs are resistant to degradation by debranching enzymes and RNA exonucleases, and stably exist in the cytoplasm25. The half-life of exon-derived circRNAs is more than 48 hours, which is significantly longer than the 10 h half-life of most mRNAs24,26. In particular, circRNAs are expressed in certain tissue-, time- and disease-specific patterns, making them potential diagnostic markers and therapeutic targets for diseases3,11,12.
Clearance of circRNAs
In eukaryotes, circRNAs are highly conserved, and their clearance mechanism has been revealed in recent years, which mainly occurs by the following three processes.
N6-methyladenosine-mediated degradation
N6-methyladenosine (m6A) modification, widely found in eukaryotes, is a chemical modification that adds a methyl group to the sixth (N) position of adenine in RNA molecules27. The m6A recognition protein contains YTH domain- containing family 2 (YTHDF2), which can bind the target molecules28.By recruiting heat-responsive protein 12 (HRSP12), YTHDF2 then mediates the cleavage of target molecules by the RNase P/MRP complex (Figure 2A). In addition, ribonuclease L(RNase L) can also mediate circRNA degradation after viral infection (Figure 2B).
Argonaute protein 2-mediated degradation
Argonaute protein 2 (AGO2), a member of the widely expressed Argonaute protein family, has a conserved structure and plays an important role in RNA interference29. Some studies have indicated that miR-671 can trigger AGO2 to mediate CDR1 involving degradation by binding to a highly conserved miRNA binding site in CDR1as30,31. Moreover,miR-7 can recruit the miR-671 RNA-induced silencing complex (RISC) or be retained in ciRS-7 (CDR1as) through other unknown mechanisms, thereby enhancing the clearance process (Figure 2C).
Extracellular vesicle output
Extracellular vesicles, membrane structures surrounded by a lipid bilayer and secreted by living cells, contain components such as RNA and proteins. Lasda et al.32found that cultured cells secreted exosomes that contained circRNAs. Moreover,they found that circRNAs were enriched far more than linear RNAs32. CircRNAs can be removed from cells by secreted vesicles (such as exosomes or microvesicles) (Figure 2D).
Mechanisms of action of circRNAs
CircRNAs were once regarded as byproducts and did not receive much attention. Recently, the biological mechanisms of action of circRNAs have been extensively revealed.CircRNAs have been found to exert their biological effects by functioning as microRNA (miRNA) sponges (Figure 1H),binding with RBPs (Figure 1I), regulating the alternative splicing (Figure 1J) and transcription of parent genes (Figure 1K),and being translated into proteins (Figure 1L).
Function as miRNA sponges
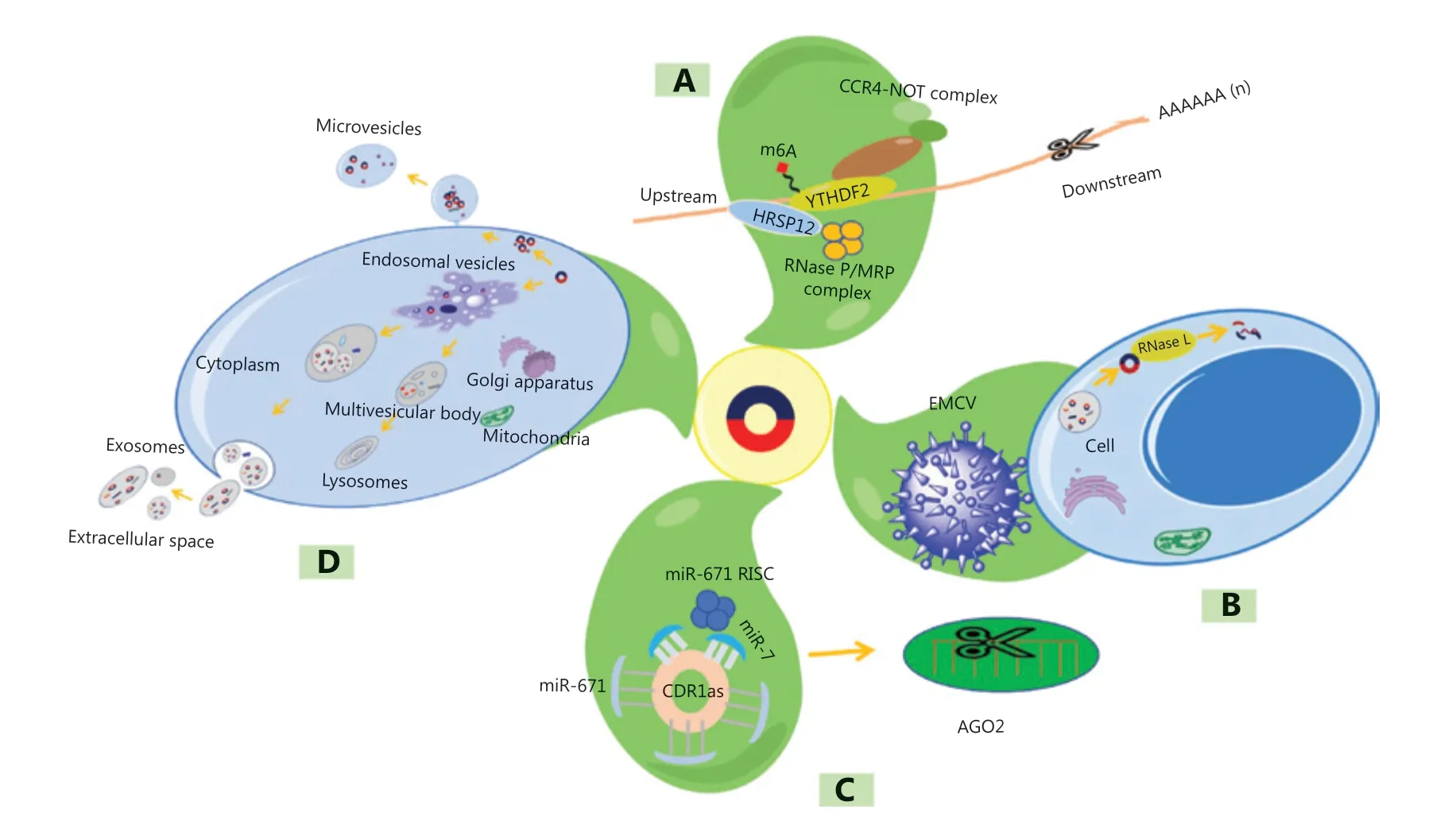
Figure 2 Clearance of circRNAs. (A) N6-methyladenosine-mediated degradation. (B) RNase L-mediated circRNA degradation after viral infection. (C) Argonaute protein 2 (AGO 2)-mediated degradation. (D) CircRNAs released in the form of extracellular vesicles. YTHDF2, YTH domain-containing family 2; HRSP12, heat-responsive protein 12; CCR4, carbon catabolite repression 4; MRP, multidrug resistance-associated protein; EMCV, encephalomyocarditis virus; RISC, RNA-induced silencing complex.
Circular RNA sponge for miR-7 (ciRs-7), also known as antisense to the cerebellar degeneration-related protein 1 transcript (CDR1as), is a typical circRNA33. Because ciRs-7 was discovered as acting as a sponge of miRNAs, increasing attention has been directed to the interaction between circRNAs and miRNAs. The structural basis for circRNAs in regulating downstream miRNAs involves the existence of seed sequences that target miRNAs by incomplete complementary base pairing. When a circRNA adsorbs a functional miRNA, the activity of the miRNA is reduced, and its regulatory effect on downstream target genes is inhibited. Most of the circRNAs found so far can act as miRNA sponges to exert their biological effects. For example, CDR1as, which contains over 70 miR-7 binding sites, can absorb a large amount of miR-7 by functioning as a miR-7 sponge, thus decreasing the regulatory function of miR-7 and indirectly inhibiting tumor progression, cell proliferation, and invasiveness in multiple cancers (including GC and CRC)33,34.
CircRNAs can directly regulate downstream target genes by acting as miRNA sponges, and can also directly or indirectly participate in signal transduction pathways in the same manner. For example, Zhang et al.35found that by adsorbing miR-149-5p, upregulated circNRIP1 in GC increased the expression level of mammalian target of rapamycin (mTOR) to activate the AKT1/mTOR signaling pathway, thus accelerating energy anabolism and inhibiting autophagy-related catabolism of GC cells.
Binding to RBPs
CircRNAs can bind to proteins and either directly function as protein sponges or indirectly regulate RNA. The resulting RNA-protein complex then regulates the interaction between RNA and RBPs36. In addition, RBPs involved in the process of RNA alternative splicing affect protein functions and posttranscriptional gene expressions36,37. For example, circSMARCA5 has been confirmed to regulate the mRNA splicing of vascular endothelial growth factor-A (VEGFA) by affecting the expression and function of serine/arginine rich splicing factor 1 (SRSF1), subsequently influencing the progress of glioblastoma37. By interacting with DEAD-box polypeptide 3 (DDX3),circ-CTNNB1 has been shown to transactivate transcription factor Yin Yang 1 (YY1), promoting the expression of genes related to the activation of β-catenin protein and affecting tumor progression38.
Alternative splicing regulation
As a common process in eukaryotic organisms, alternative splicing is a major source of biological protein diversity. The latest evidence confirmed that circRNAs participate in the process of alternative splicing39. For example, it was reported that circMbL affected linear RNA synthesis and regulated the expression of related genes by competing with the linear splicing of classical pre-mRNA40. In addition, circ-UBR5 has been shown to participate in the RNA splicing process by binding to splicing regulatory factor quaking (QKI), NOVA alternative splicing regulatory factor1, and U1 small nuclear RNA39.
Transcriptional regulation of parental genes
One of the most prominent biological roles of RNAs is the regulation of parental gene expression. Similarly, some circRNAs have been confirmed to have similar regulatory functions.These circRNAs act as transcriptional regulators to participate in the transcription of parental genes and exert indirect biological effects. For example, circ-ITCH is transcribed from its parental geneITCHand then regulatesITCHgene transcription by acting as a sponge for miR-7, miR-17, and miR-214,thereby inhibiting the Wnt/β-catenin pathway and downregulating the expression of the proto-oncogenec-mycthrough the ubiquitin-mediated degradation of phosphorylated Dvl241.Similarly, circITGA7 has been shown to upregulate the transcription of its host gene integrin alpha 7 (ITGA7) by inhibiting the transcription factor RAS-responsive element binding protein 1viathe Ras pathway42.
Translation into proteins
CircRNAs containing internal ribosomal entry sites can be translated into proteins
CircRNAs do not have a free 5′ cap or 3′ poly (A) tail. Thus,they lack an effective initial structure to guide protein translation. However, by inserting a synthetic internal ribosome entry site (IRES) upstream of the initial codon of protein synthesis, circRNAs can be translated into proteins43. For example, circ-ZNF609 contains an open reading frame(ORF) sequence bounded by a start codon and a stop codon,and can be translated into proteins in a splicing-dependent manner44. In another study, Van Heesch et al.45systematically analyzed the mechanism and pattern of RNA translation in cardiac tissues and successfully found many translation products of circRNAs and 40 translatable circRNAs produced from 39 genes, including the well-known CDR1as and the newly detected circCFLAR, circSLC8A1, circMYBPC3,and circRYR2.
N6-methyladenine modification promotes circRNA translation
The m6A modification, an internal RNA modification widely existing in eukaryotes, has been demonstrated to affect multiple stages of RNA metabolism, including mRNA localization, splicing, translation, and degradation, and is related to various pathophysiological processes. Yang et al.46indicated that RRm6ACH (R = G or A; H = A, C, or U), a recognized m6A motif enriched in circRNAs, which promoted the initiation of protein translation from circRNAs and also directly affected the translation efficiency. It is worth noting that m6Adriven translation requires the involvement of the initiating factor, eukaryotic translation initiation factor 4 gamma 2, and m6A reader YTH N6-methyladenosine RNA-binding protein 3, which can be enhanced by methyl transferase METTL3/14,inhibited by demethylase FTO, and upregulated during heat shock46.
Relationship of circRNAs with gastrointestinal malignancies
CircRNAs participate in gene regulation and cancer-related signaling pathways. Dysregulated circRNAs are only involved in pathological processes such as cancer cell proliferation, differentiation, apoptosis, invasion, metastasis and angiogenesis,and also affect cell metabolism and drug sensitivity. In Table 1,we summarize the function and clinical significance of cancer-related circRNAs in gastrointestinal malignancies.
Cell proliferation, differentiation, and cell cycle regulation
Excessive proliferation, impaired differentiation and maturation, and an imbalance in cell cycle regulation are features of cancer cells and important mechanisms of cancer cell hyperproliferation47. CircRNAs play crucial roles in the proliferation, differentiation, and cell cycle regulation of gastrointestinal cancer cells (Figure 3A). CircRNAs affect cell proliferation and the cell cycle mainly by regulating downstream miRNAs and directly or indirectly participating in signal transduction pathways or interactions with proteins. For example, in GC, overexpression of ciRS-7 blocked the inhibitory effect of miR-7 by antagonizing the miR-7-mediated PTEN/PI3K/AKT signaling pathway, which ultimately promoted the proliferation of cancer cells48. In pancreatic cancer, circ-PDE8A was demonstrated to stimulate cell growth by functioning as a competitive endogenous RNA (ceRNA) to absorb miR-338 and regulate the miR-338/MACC1/MET pathway, whereas circ-FOXK2 was found to be involved in the cell cycle and proliferation by interacting with RBPs and sponging miR-942 to promote cancer progression49,50. Similarly, in CRC, circ_0136666 increases SH2B1 levels by competitively binding miR-136 to participate in the cell cycle and facilitate cancer cell proliferation51. Moreover, some circRNAs, such as circSMARCA5 and circ_100395 and hsa_circ_0007142, regulate cell proliferation and cell cycle regulation, and also affect cell differentiation,which contributes to the cancer burden and poor prognoses of patients37,52,53. Consistent with these observations, many other circRNAs, such as circ_0008035 sponging miR-599 and circPDSS1 sponging miR-186-5p in GC, circ_101280 sponging miR-375, circSLC3A2 sponging miR-490-3p and circABCB10 sponging miR-670-3p in HCC, hsa_circ_001653 sponging miR-377 in pancreatic cancer, and hsa_circ_0006168 sponging miR-384 in esophageal cancer have been shown to affect cell proliferation and cell cycle regulation and differentiation by regulating cancer-related signaling pathways or interactions with proteins54-60. Overall, circRNAs regulate cell growth and differentiation and directly affect patient clinical characteristics, such as cancer diameter, and cancer growth rate and differentiation during cancer progression. Targeting specific circRNAs that are associated with cell proliferation, differentiation, or the cell cycle is therefore a potential novel approach to inhibit cancer growth.
Cell apoptosis regulation
The growth rate of cancer is mainly determined by the ratio of cell proliferation to cell death, and inhibition of apoptosis is another important factor for the excessive growth of cancer cells47. CircRNAs participate in the regulation of cell apoptosis (Figure 3B). Likewise, “miRNA sponging” is considered the main mechanism by which circRNAs regulate cell apoptosis. For example, circ-NOTCH1 has been shown to reduce GC cell apoptosis by sponging miR-637 and then upregulating the expression of its target gene,Apelin61.Similarly, circ_0000267 has been shown to sponge miR-646 to attenuate cell apoptosis in HCC cells62. Consistent with this observation, circHIPK3, circZMYM2, circ_0006215,circAKT3, circ_0101432, and hsa_circ_0000523 also act as“miRNA sponges” to regulate the apoptosis of various cancer cells, including pancreatic cancer, CRC, HCC, and GCcells63-68. Importantly, some circRNAs are also involved in regulating the expression of apoptosis-related proteins and/or related signaling pathways. For example, circ-IGF1R activates a variety of apoptotic effectors, including the apoptotic inhibitors B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein (Bax), thereby exerting anti-apoptotic effects by activating the PI3K/AKT signaling pathway, subsequently influencing HCC cell proliferation, apoptosis, and cell cycle progression69. Similarly, circZNF609 has been shown to induce cell apoptosis by activating the expression of theBaxandp53genes and reducing Bcl-2 expression in CRC cells70.Thus, circRNAs are important players in apoptosis regulation. The development of apoptosis-inducing targets might therefore provide new perspectives for cancer research.
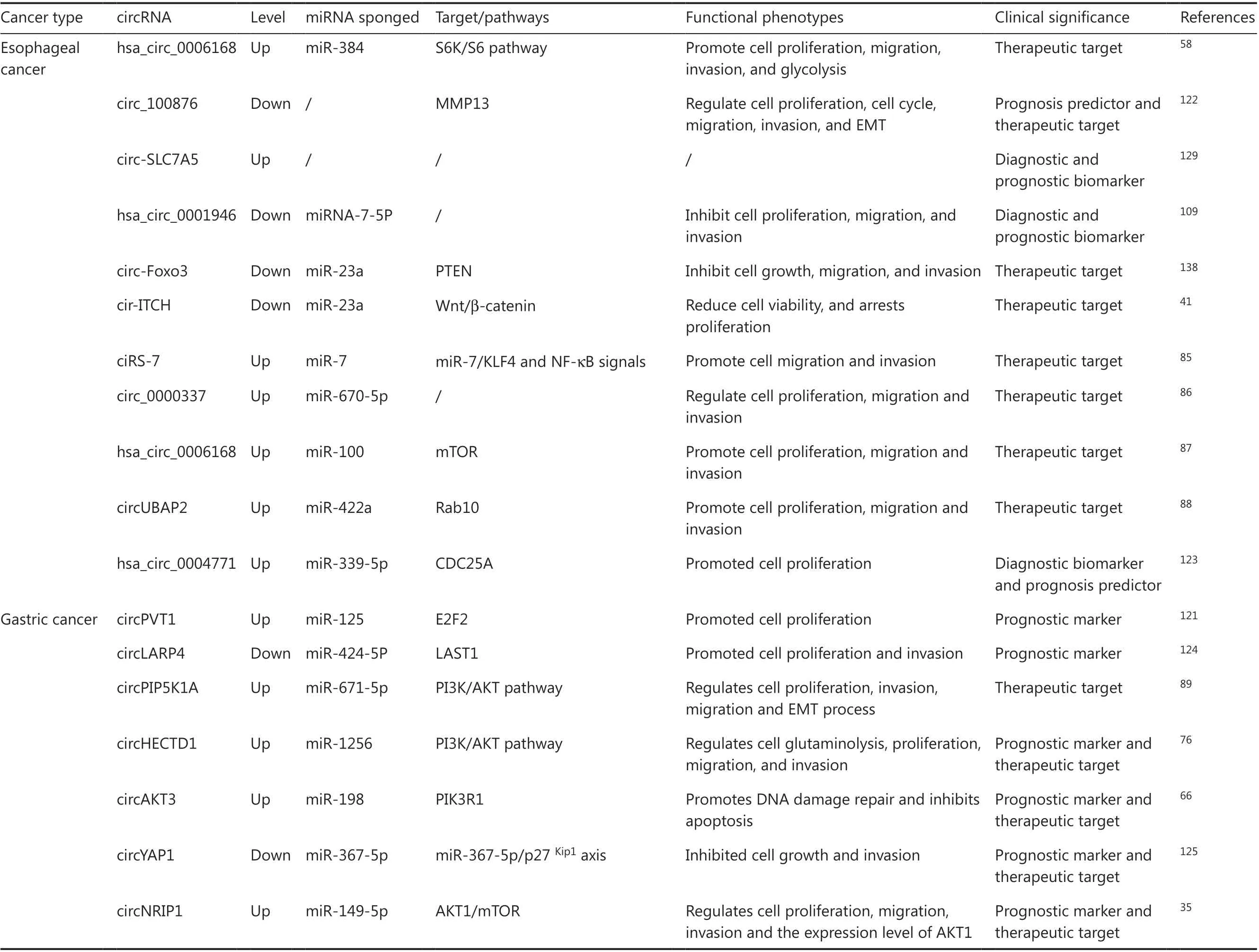
Table 1 Relationship between circRNAs and gastrointestinal malignancies
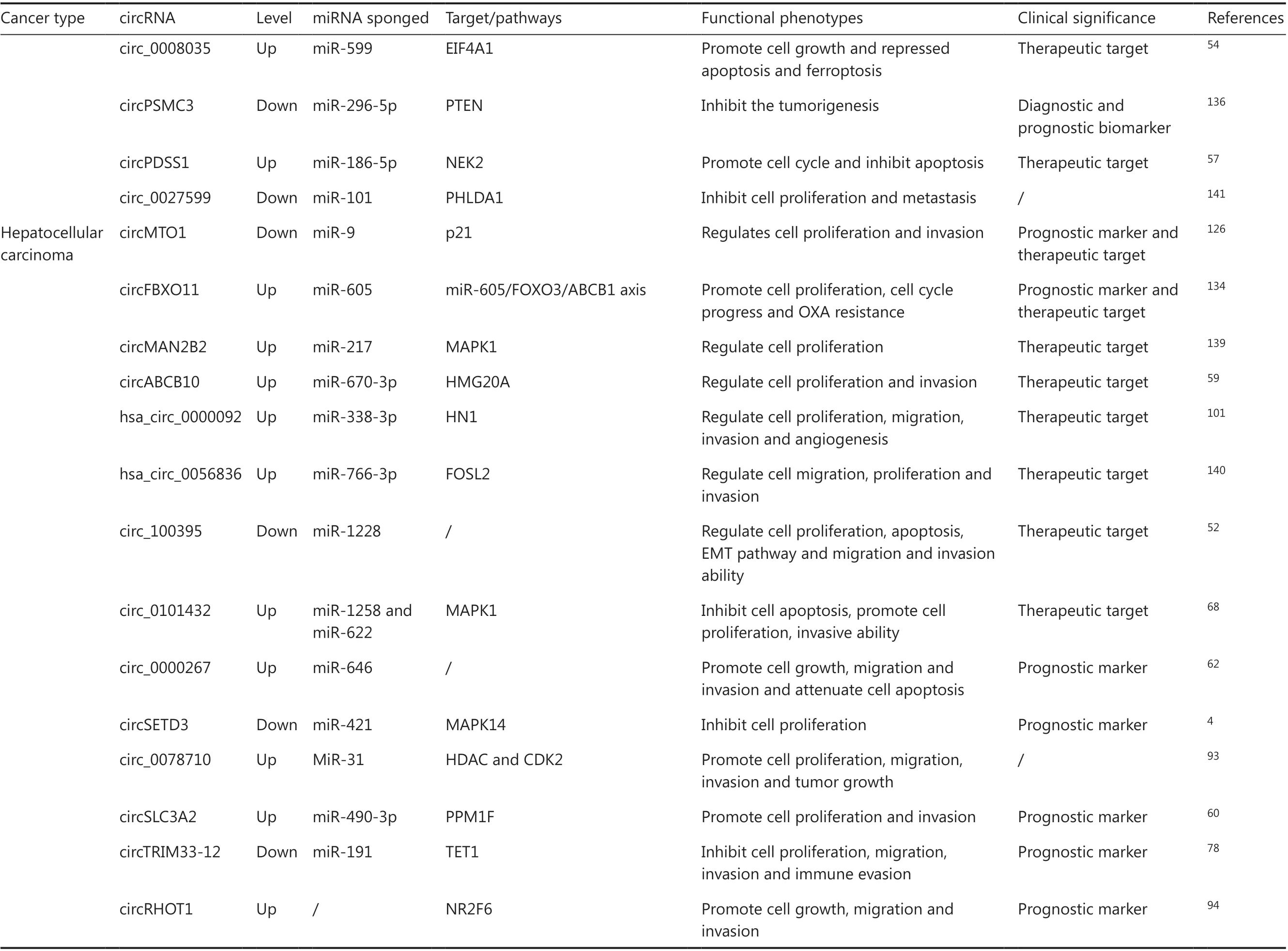
Table 1 Continued
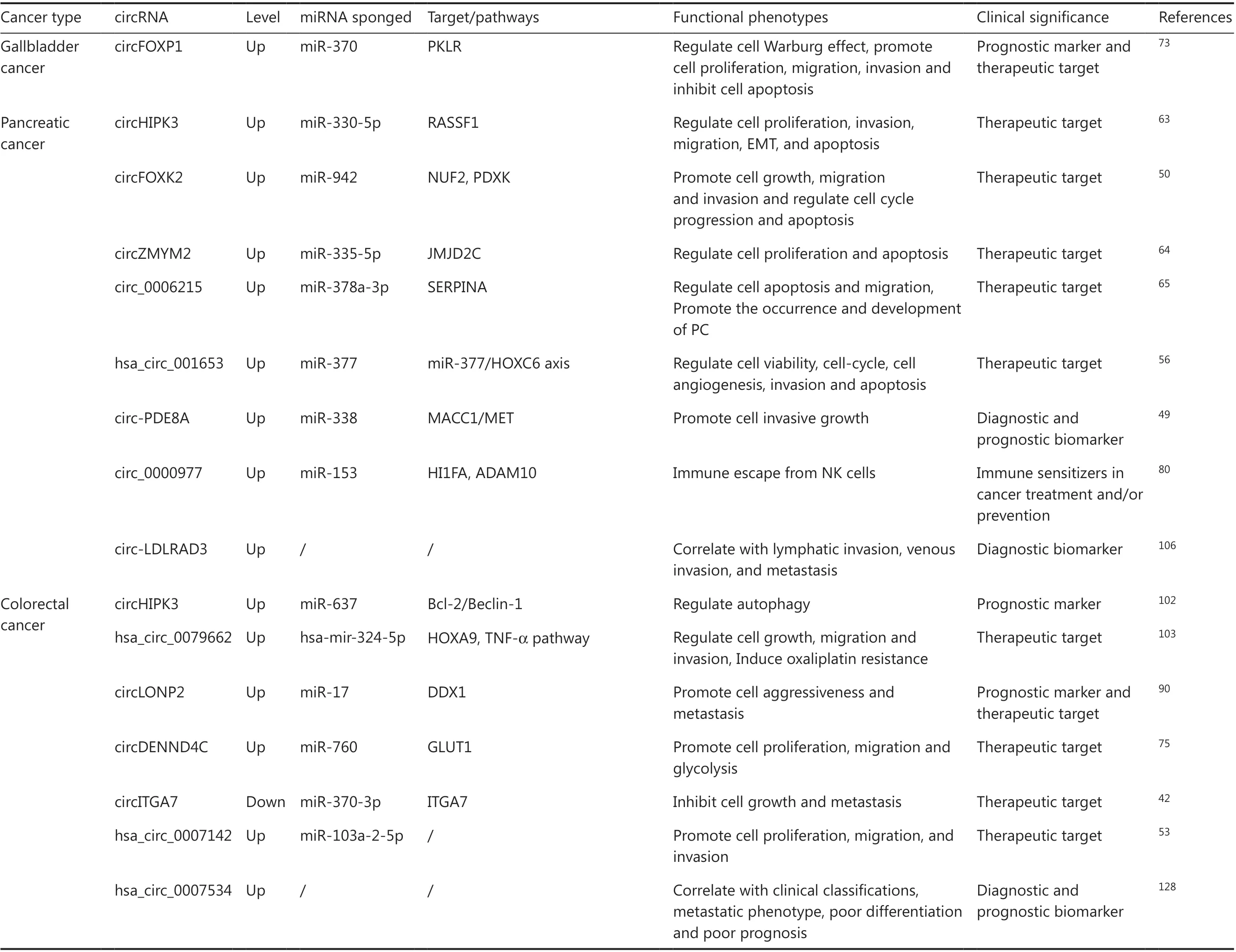
Table 1 Continued

Table 1 Continued
Cell metabolism regulation
Cancer cell energy metabolism is mainly mediated through the glycolytic pathway, that is, the Warburg effect, which enables cancer cells to maintain high intensity metabolic activities even in hypoxic environments71. CircRNAs have been shown to participate in the regulation of cellular metabolism-related factors by functioning as miRNA sponges or being involved in signaling pathways. For example, hsa_circ_0006168 regulates glycolysis in esophageal cancer cells by competitively binding miR-384 to enhance the expression of retinoblastoma-binding protein 7 (RBBP7), subsequently activating the S6K/S6 pathway58. In addition, circRNAs were also found to be involved in glycolysis or glutamine catabolism by regulating transporters, enzymes, or transcription regulators72. In gallbladder cancer, circFOXP1 stimulated the cell Warburg effect by interacting with the RBP polypyrimidine Tract-binding protein 1 (PTBP1), which significantly increased the expression of pyruvate kinase PKLR and protected PKLR mRNA from degradation73. Likewise, in CRC,circDENND4C promoted cancer cell glycolysis through the increased expression of glucose transporter type 1 (GLUT1)by sponging miR-760, whereas circVAPA participated in glycolysisviaits effect on cAMP response element-binding 5 (CREB5) by sponging miR-125a74,75. Moreover, a recent study revealed that circHECTD1 facilitated glutaminolysis in GC progression by inhibiting miR-1256 and activating the β-catenin/c-Myc signaling pathway76. Together,these studies indicate that circRNAs are closely related to the regulation of gastrointestinal cancer cell metabolism(Figure 3C).
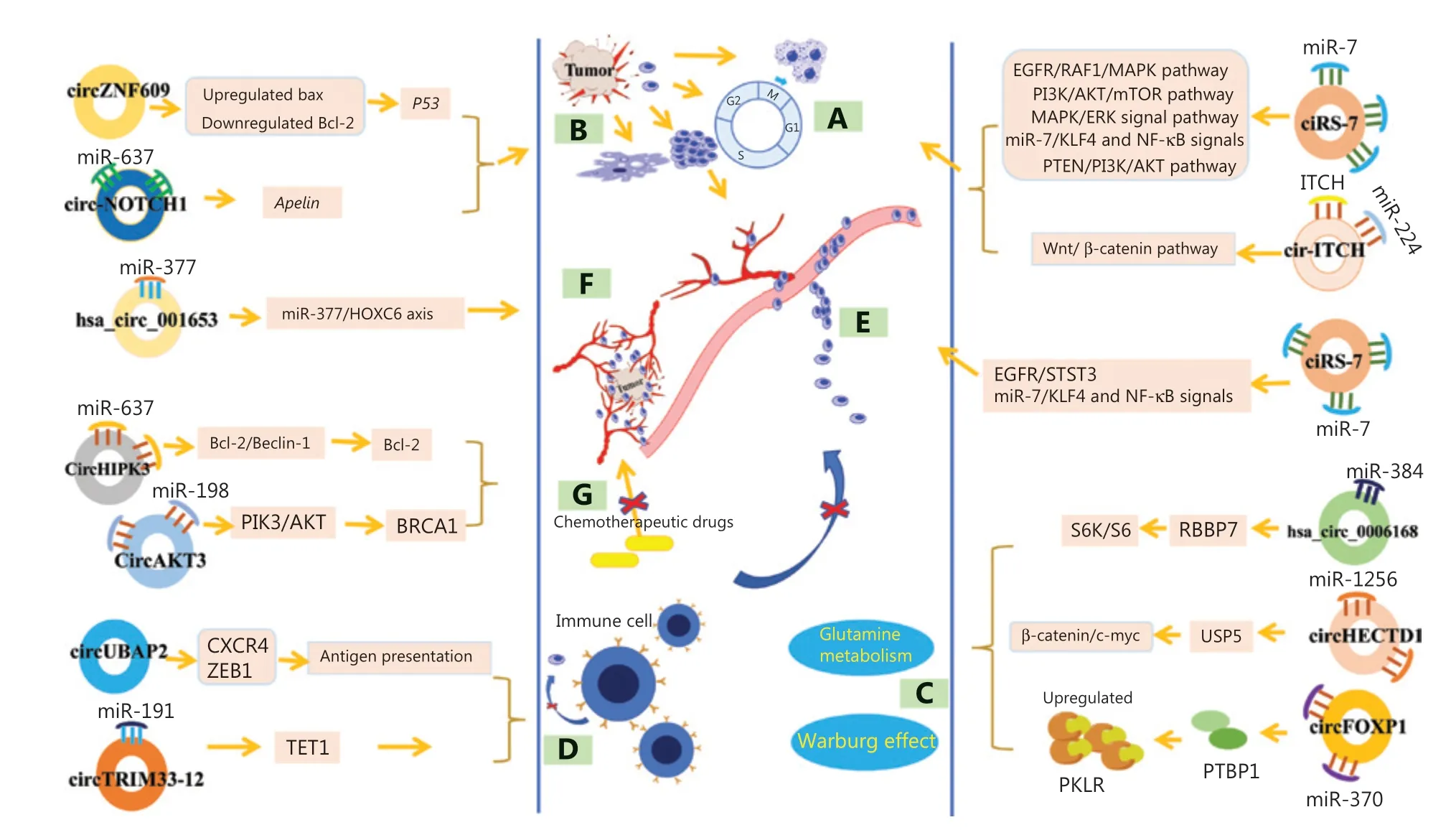
Figure 3 The relationships of circRNAs with gastrointestinal malignancies. (A) Cell proliferation, differentiation and cell cycle regulation.(B) Cell apoptosis regulation. (C) Cell metabolism regulation. (D) Immune escape. (E) Invasion and metastasis regulation. (F) Angiogenesis regulation. (G) Drug resistance regulation. EGFR, epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; MAPK, mitogen-activated protein kinase; ERK, extracellular regulated protein kinases; KLF4, Krüppel-like factor4; NF-κB, nuclear transcription factor-κB; PTEN, phosphatase and tensin homology deleted on chromosome ten; CXCR4, C-X-C chemokine receptor type 4; ZEB1, zinc finger E-box binding protein 1; PTBP1, polypyrimidine tract-binding protein 1; PKLR, L-type pyruvate kinase; USP5, ubiquitin-specific peptidase 5; BRCA1, breast cancer type 1; bcl-2, B-cell lymphoma-2; HOXC6,homeobox C6; TET1, tet methylcytosine dioxygenases 1; S6K, S6 kinase; RBBP7, retinoblastoma-binding protein 7.
Immune escape
Through immune surveillance mechanisms, immune cells can recognize and eliminate cancer or cancer-transformed cells,exerting anticancer effects. Cancer cells can escape immune surveillance by reducing antigen expression47. Preliminary evidence has shown that some circRNAs, such as circUBAP2 and circTRIM33-12, participate in cancer immune escape by directly or indirectly reducing the expression of surface antigens. In pancreatic cancer, circUBAP2 regulates the expression of chemokine receptor 4 (CXCR4) and zinc finger E-boxbinding homeobox1 (ZEB1) and inhibits the antigen presentation of cancer cells (Figure 3D), leading to tumor infiltration by immune cells and immune escape through a competitive endogenous circRNA network77. Similarly, circTRIM33-12 was also found to affect immune evasion of cancer cells by sponging miR-191, subsequently affecting the expression of tet methylcytosine dioxygenases 1 (TET1), which is the target gene of miR-191 in HCC78. The abnormal expression of both circUBAP2 and circTRIM33-12 ultimately leads to immune escape of cancer cells, which is associated with poor clinical outcomes. However, other circRNAs participate in cancer immune escapeviadysfunctional activation of natural killer(NK) cells. For instance, in HCC, circARSP91 was shown to upregulate the expression of UL16-binding protein-1 (ULBP1),a killer cell lectin, to increase the susceptibility of cancer cells to NK cell cytotoxicity, thus enhancing the cytotoxicity of NK cells and promoting immune surveillance79. In pancreatic cancer, circ_0000977 was revealed to regulate the miR-153 downstream targets of hypoxia-inducible factor 1-alpha (HIF1A),a disintegrin, and Metalloproteinase Domain 10 to modulate HIF1A-mediated immune escape of pancreatic cancer cells from NK cells80. Moreover, the latest evidence also shows that some circRNAs, such as hsa_circ_0020397, can enhance the expression of programmed death-ligand 1 (PD-L1), which is closely related to cancer escape from immune control81.Notably, the escape of cancer cells from immune surveillance often contributes to excessive cancer cell growth and metastasis, leading to adverse clinical prognoses of patients82.Although limited circRNAs correlated with immune escape have been found, there is considerable evidence that circRNAs play important roles in cancer immune escape. More immune escape-related circRNAs need to be discovered in the future,and the specific mechanisms require further in-depth studies.
Invasion and metastasis regulation
Malignancies can grow abnormally at the primary site and adjacent tissues, and can also spread to distant organs. Some dysregulated circRNAs were discovered to exert regulatory effects on the invasion and metastasis of malignancies(Figure 3E), which seriously affected the clinicopathological features and prognoses of cancer patients. The “miRNA sponge” function is their main mechanism. In colon polyps and colon cancer tissues, a high level of circCCDC66 has been found to be related to pathological processes including cell proliferation, migration, invasion, and anchorage-independent growth, which contributes to cancer development and poor prognoses of patients83. In pancreatic cancer, tissue ciRS-7 levels are related to several malignant tendencies, such as venous invasion and lymph node metastasis84.Mechanistically, upregulated ciRS-7 was found to inhibit the expression of miR-7 and upregulate the expression of epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 3 (STAT3), to affect the proliferation and invasion of pancreatic cancer cells84.Conversely, in esophageal cancer, increased ciRS-7 has been shown to function as a ceRNA to activate miR-7/KLF4 and NF-κB signaling, further inducing the migration and invasion of cancer cells85. Likewise, other circRNAs, such as circ_0000337, hsa_circ_0006168, circUBAP2, circPIP5K1A,circNRIP1, circ_0006215, circLONP2, circ_0009361, circRNA_001569, circ_0078710, and circRHOT1 have also been shown to regulate cell invasion and metastasis in gastrointestinal malignancies by acting as miRNA sponges or ceRNAs35,65,86-94.
CircRNAs also participate in the regulation of invasion and metastasis by recruiting protein factors or promoting the EMT.Sun et al.95found that circ-ADD3 enhanced the interaction between cyclin-dependent kinase 1 (CDK1) and histone-lysine N-methyltransferase (EZH2), resulting in ubiquitination and degradation of EZH2 through phosphorylation at Thr-345 and Thr-487 during the progression of HCC. The subsequent decrease in EZH2 levels significantly increased the levels of a number of anti-metastatic genes, includingcirc-ADD3, by reducing the level of H3K27me3 on their promoter regions,thus forming a regulatory circuit to inhibit HCC metastasis95.Chen et al.52demonstrated that circ_100395 participated in the EMT process to regulate HCC cell metastasis. Mechanistically,downregulated circ_100395 suppresses the EMT by directly binding to the downstream factor miR-1228, while EMT pathway dysfunction involves the antimetastatic effect in HCC cells52. In addition, the roles of other circRNAs, such as hsa_circ_0012563 in esophageal cancer, circRNA_0023642 in GC, and circFNDC3B and circRNA_101951 in CRC, in invasion and metastasis by regulating EMT pathways, have been reported96-99. Together, these findings provide new evidence that circRNAs are directly involved in the invasion and metastasis of gastrointestinal malignancies, which might be responsible for the degree of cell malignancy, distant metastasis, postoperative recurrence, and even the poor clinical outcomes of patients.
Angiogenesis regulation
Neovascularization plays a key role in cancer growth, providing necessary nutrients for cell growth and tissue metabolism. Angiogenesis is an important factor for continuous tumor growth and metastasis47. A growing number of circRNAs have been discovered that regulated angiogenesis and increased the malignant degree of gastrointestinal cancers (Figure 3F). For example, circRNA-100338 levels were greatly increased in both highly metastatic HCC cells and their secreted exosomes, and enhanced the proliferation, invasive abilities, angiogenesis, permeability, and the formation of vasculogenic mimicry (VM) of human umbilical vein endothelial cells100. Circ_0000092 was also increased in HCC tissues and cell lines and promoted the progression and angiogenesis of HCC by regulating the miR-338-3p/HN1 axis101. Moreover,in pancreatic cancer, hsa_circ_001653 was demonstrated to regulate cancer angiogenesis by acting as a miR-377 sponge and activating the miR-377/HOXC6 axis56. Thus, circRNAs are also involved in the regulation of cancer angiogenesis by acting as miRNA sponges. The development of circRNA-targeted antiangiogenic drugs is a potential therapeutic strategy for cancer, but it is mainly dependent on the identification of more vascular proliferative RNAs and the characterization of their molecular mechanisms.
Drug resistance regulation
Comprehensive treatment based on surgery and chemotherapy is currently the main treatment method for gastrointestinal malignancies. Recently, some circRNAs related to chemotherapeutic drug resistance have been identified (Figure 3G). Dysregulated circRNAs in gastrointestinal cancer tissues have important impacts on the efficacy of clinical chemotherapy in patients, often leading to treatment failure or cancer recurrence and metastasis. For example, in recurrent CRC and oxaliplatin (OXA) resistant patients, the upregulated circHIPK3 in tissues promotes oxaliplatin resistance and cancer progression by sponging miR-637, increasing the expression of STAT3, which subsequently activates the downstream Bcl-2/beclin1 signaling pathway102. Importantly, in patients with postoperative OXA-based adjuvant chemotherapy, the circHIPK3 level was found to be negatively correlated with 5-year disease-free survival and overall survival rates, suggesting that circHIPK3 has important impacts on the efficacy of clinical OXA chemotherapy in patients102. In another study,the upregulation of hsa_circ_0079662 was demonstrated to promote CRC migration and invasion and induce oxaliplatin resistanceviathe TNF-α pathway103. Similarly, in GC patients,circAKT3 was significantly overexpressed in cisplatin-resistant GC tissues and cells. The dysregulated circAKT3 acts as a miR-198 sponge to activate the PI3K/AKT signaling pathway and upregulate the DNA damage repair molecule breast cancer 1(BRCA1), which inhibits cancer cell apoptosis and also leads to resistance in DNA-damaging CDDP-based chemotherapy66. Likewise, the decreased circRNA_101505 can function as a ceRNA by sponging miR-103 to target and upregulate the expression of the downstream oxidored-nitro domain-containing protein 1, which confers cisplatin resistance to cancer cells, resulting in poor overall survival of HCC patients104.This evidence suggests that targeting drug resistance-related circRNAs may increase the sensitivity of patients to chemotherapeutic drugs, and reduce or reverse drug resistance and improve the prognoses.
Potential clinical applications of circRNAs in gastrointestinal malignancies
CircRNAs are characterized by their abundance, high stability, extensive functions, and certain tissue, time, and disease specificities, suggesting their potential clinical applications in the diagnoses and treatments of gastrointestinal tumors.
Biomarkers for gastrointestinal malignancies
Plasma circRNAs
In cancer screening, plasma biomarkers are particularly important because of their unique advantages, such as convenience,repeatable sampling, and minimal trauma. Unfortunately, the current biomarkers used in the clinic have suboptimal sensitivity and specificity, which limits further clinical application,especially for early screening.
Recent studies have shown that some circulating plasma circRNAs have better diagnostic potentials than traditional markers such as CEA, CA19-9, and AFP, and the combined use of these markers significantly improves the diagnostic efficacy. Plasma hsa_circ_0003998 levels in HCC were significantly higher than those in hepatitis B patients and healthy individuals, and decreased rapidly after surgery105. A study of the ability of hsa_circ_0003998 as a plasma marker to distinguish HCC patients from hepatitis B patients and healthy individuals reported that the sensitivity and specificity of plasma hsa_circ_0003998 were 0.83 and 0.7 and 0.8 and 0.84, respectively105. Moreover, the combined use of hsa_circ_0003998 and AFP further improved the diagnosis efficacy with the highest sensitivity and specificity of 0.88 and 0.92, respectively105.In pancreatic cancer, circ-LDLRAD3 is an ideal marker of diagnosis and invasion capacity. The sensitivity of plasma circ-LDLRAD3 combined with CA19-9 in the diagnosis of pancreatic cancer was 80.33%, and the specificity was up to 93.55%, which was significantly higher than that of CA19-9 or circ-LDLRAD3 alone106. Similarly, in early GC identification,plasma hsa_circ_0006848 combined with CEA, CA19-9, and CA72-4 also improved the diagnostic value, with a sensitivity and specificity of 73.3% and 90.0%, respectively107.
Although combined detection significantly improves efficiency, single plasma circRNA markers still have excellent performance. When used alone, circSMARCA5 has the potential to be an ideal biomarker for HCC screening, especially in patients with low AFP levels. Plasma circSMARCA5 has unique diagnostic value in HCC with a sensitivity and specificity of 86.67% and 89.32%, respectively, and also displays good predictive value for distinguishing HCC from hepatitis or cirrhosis patients with AFP levels below 200 ng/mL108. In esophageal cancer screening, the sensitivity and specificity of plasma hsa_circ_0001946 were up to 92% and 80%, respectively, and those of hsa_circ_0043603 were 64% and 92%, respectively, which were both significantly higher than those of CEA109. In GC screening, the sensitivity of plasma hsa_circ_0000181 reached 99.0% when the cutoff value was 7.27110. Likewise, more circRNAs, such as hsa_circ_0000976, hsa_circ_0007750, and hsa_circ_0139897 in HCC and circ-ABCC1, circ-CCDC66,and circ-ABCC1 in CRC, have good clinical diagnostic values, whether in combination or used alone111,112. Although an increasing number of circRNAs in cancer tissues have been identified, the diagnostic value of most circRNAs is still largely unknown and needs further study.
Exosomal circRNAs
Exosomes play important roles in various pathophysiological processes such as intercellular signal transmission113.Exosomal circRNAs are involved in multiple pathological processes, including the EMT, tumor cell proliferation, and tumor angiogenesis. In addition, exosome circRNAs are considered indicators for the early diagnosis and prognostic evaluation of multiple cancers114. For example, circHIPK3, was significantly upregulated in a cholangiocarcinoma (CCA) cell line, tissues,and plasma exosomes, and was found to promote the proliferation, invasion, and migration of CCA cells115. Moreover,exosome-mediated circHIPK3 had a cancer-promoting effect on adjacent normal cells116. Thus, circHIPK3, which is enriched and stably expressed in exosomes, was considered a noninvasive biomarker for CCA diagnosis. Dysregulated circ-PDE8A in pancreatic cancer was shown to be associated with cancer invasion, progression, and low survival times49. Tumor cell-excreted circ-PDE8A could enter the peripheral circulation through exosomes and was easy to detect49. Therefore,circ-PDE8A is predicted to be a diagnostic indicator for cancer invasion evaluation and prognosis of pancreatic cancer. In GC, plasma exosomal hsa_circ_0065149 is thought to be an important indicator for early screening. The sensitivity and specificity of exosomal hsa_circ_0065149 were 48.7% and 90.2%, respectively116. Regarding sensitivity, exosomal hsa_circ_0065149 sensitivity was significantly higher than that of traditional biomarkers such as CEA (4.3%), CA19-9 (4.8%),and CA-125 (1.9%)116.
The study of exosomal circRNAs as tumor indicators of the digestive system is still in its infancy, and only a few circRNAs have been discovered. Moreover, research on exosomal circRNAs as biomarkers still faces many challenges, of which the low concentration of exosomes in body fluids, low abundance of circRNAs in exosomes, relatively complicated detection processes of exosomes in fluids, and the need for a larger fluid sample have greatly restricted the in-depth study of exosomal circRNAs. Future improvements in the above 4 aspects are expected to help further reveal the value of exosomal circRNAs as biomarkers.
Gastric juice circRNAs
Gastric juice, a gastric endocrine product secreted by gastric mucosal epithelial cells, fundus glandular cells, and cervical mucous cells, has an obvious advantage for its use in GC due to its organ specificity. Some circRNAs, such as hsa_circ_0014717 and hsa_circ_0065149, were successfully extracted and detected in gastric juice by RT-PCR and DNA sequencing116,117. A preliminary study showed that hsa_circ_0014717 in gastric juice was stable enough to meet the needs of clinical diagnoses, and the change in its levels could indicate some specific pathological changes in gastric mucosa117. At present, research on circRNAs in gastric juice is still in its infancy, and most related clinically significant and functional mechanisms are unclear.However, the stability of gastric juice circRNAs indicates that they may have great potential in the future.
Prognostic indicators of gastrointestinal malignancies
Some dysregulated circRNAs related to the clinicopathological characteristics, malignant biological behavior (such as tumor growth, differentiation, and metastasis), and prognosis of gastrointestinal cancers are important regulators of tumorigenesis and progression, and also provide key indicators for evaluating the prognoses of patients with malignancies.For example, CDR1as, a classical circRNA overexpressed in multiple cancers, including HCC, CRC, and CCA, was found to affect patient clinical prognoses by acting as a ceRNA to sponge miRNAs118-120. CDR1as levels were shown to be positively correlated with multiple clinicopathological features and malignant biological behaviors, including cancer size and tumor-node-metastasis (TNM) stage118-120. Furthermore,patients with higher CDR1as levels in both CRC and CCA had a worse overall survival than patients with lower CDR1as expressions118,120. In addition, CCA patients with elevated CDR1as levels had a higher risk of metastasis and postoperative recurrence rate118. Likewise, in GC, patients with lower circPVT1 levels were shown to have a worse overall survival(median survival of 20 monthsvs. 46 months) and disease-free survival (median survival of 17 monthsvs. 36 months) than those with higher circPVT1 levels121. In HCC, the upregulation of hsa_circ_0003998 was shown to be associated with higher serum AFP levels, larger cancer size, poorer differentiation,microvascular invasion, and lower overall survival rate, which were prognostic factors for HCC patients105. Similarly, other dysregulated circRNAs associated with poor clinical outcome of malignancies, such as circ_100876 and hsa_circ_0004771 in esophageal cancer, circLARP4 and circYAP1 in GC, circ-MTO1 and circSETD3 in HCC and circRNA_103809 and hsa_circ_0007534 in CRC, have also been identified as prognostic indicators, providing clues for the prognostic evaluation of gastrointestinal malignancies4,122-128.
Notably, circRNAs related to cancer prognosis in certain tissues are also present in plasma. These fluid circRNAs can be used as cancer diagnostic markers and also as references for prognostic evaluations. For example, in pancreatic cancer,overexpression of circ-PDE8A in tissues was correlated with lymphatic invasion, TNM stage, and poor survival rate, whereas plasma exosomal circ-PDE8A levels were also closely related to cancer progression and patient overall survival, providing evidence for cancer diagnosis or progression49. Furthermore,hsa_circ_0007534 was found to be significantly upregulated in both CRC tissues and plasma. Importantly, hsa_circ_0007534 in CRC plasma was associated with clinical classification, metastatic phenotype, and poor differentiation, and was positively correlated with poor survival of CRC patients, providing an indicator for prognostic evaluation128. Likewise, other plasma circRNAs, such as hsa_circ_0000419 and hsa_circ_0065149 in GC, circ-LDLRAD3 and circ-PDE8A in pancreatic cancer, and hsa_circ_0001946 and circ-SLC7A5 in esophageal cancer, have also been associated with clinicopathological features, malignant biological behaviors, and prognoses of cancers, and were shown to serve as prognostic indicators5,49,106,109,116,129.
Overall, circRNAs are widely involved in the malignancy of tumors, and circRNAs in tissues and body fluids are considered independent biomarkers, providing clues for the prognostic evaluation of gastrointestinal malignancies. Specific detection of these circRNAs may improve the current dilemma of prognostic evaluations in patients.
Therapeutic targets of gastrointestinal malignancies
Specific targeted upregulation or silencing of dysregulated circRNA expression can suppress cancer cell growth, proliferation and invasion, and also improve differentiation, apoptosis,and multidrug resistance. For this reason, circRNAs represent potential targets for the treatment of gastrointestinal malignancies, which may largely reverse the difficulties in future cancer treatments. For example, circHIPK3, a well-known multifunctional circRNA, acts as an oncogene that is significantly overexpressed in esophageal cancer, GC, CRC, and pancreatic cancer, and widely promotes the occurrence and development of multiple cancers by acting as a “miRNA sponge” or modulating cancer-related signaling pathways63,102,130-133. Patients with high levels of circHIPK3 have been found to be associated with worse clinicopathological features and prognoses, such as larger tumor size, worse cell differentiation, and higher risk of recurrence and metastasis, while silencing of circHIPK3 inhibited cancer cell proliferation, migration, and invasion,induced apoptosis, and even sensitized cells to oxaliplatin and gemcitabine in these malignancies63,102,130-133. Similarly,in HCC, reversing the overexpression of circFBXO11 in cells significantly repressed carcinoma progression and oxaliplatin resistance by sponging miR-605, subsequently affecting the expression of FOXO3 and ABCB1, which are target genes of miR-605, suggesting that circFBXO11 has the potential to improve patient prognosis and survival134. Consistent with these observations, other low-expressed circRNAs act as tumor suppressor genes, such as circMTO1 and circHIAT1 in HCC,circITGA7 and hsa_circ_0000523 in CRC, circPSMC3 and circ_0027599 in GC, hsa_circ_0001649 and hsa_circ_001653 in pancreatic cancer, circ-Foxo3 and cir-ITCH in esophageal cancer, and circMAN2B2 and hsa_circ_0056836 in HCC also showed similar characteristics when their expression levels were restored41,42,56,67,126,135-142. Overall, growing evidence indicates that circRNAs provide potential therapeutic targets for the treatment of gastrointestinal malignancies. Therefore,future studies aimed at tumor-specific circRNAs and designing and implementing the transfection of vectors with precise targeted overexpression or silencing of circRNAs will provide a new scheme in cancer treatment.
Perspectives
Over the past several years, much progress has been made in understanding the biological functions of circRNAs and the mechanisms responsible for their contributions to carcinogenesis of the digestive system. These investigations have shown that circRNAs play key roles in the development and progression of gastrointestinal malignancies, and are associated with patient prognoses and clinical outcomes143,144. Research in this field therefore holds much promise in identifying novel diagnostic biomarkers and new treatment targets for gastrointestinal malignancies.
However, several outstanding limitations and challenges remain. First, investigation of more dysregulated circRNAs in carcinogenesis is still in its infancy, and the mechanisms responsible for their contributions to cancer biology still remain to be identified in gastrointestinal malignancies. In the future, more dysregulated circRNAs need to be identified by developing high-throughput RNA sequencing technology.Second, there are no uniform naming rules for circRNAs to date, which will certainly increase some unnecessary contradictions and problems in research work. Therefore, considering the large number of circRNAs, it is urgent to establish a uniform naming convention to unify the data. Third,although there are many circRNA databases available to meet different research needs, they still lack relevance. In addition, these databases generally lack clinical data of specific circRNA-related diseases, and the data need to be constantly updated to meet new requirements. Fourth, the understanding of the roles of circRNAs in cancer is not comprehensive enough. Current studies on the function and mechanism of circRNAs in cancer are mostly limited to a single or a few circRNAs, which lack systematic approaches, comprehensiveness, and integration. In addition, mechanistic studies have mainly focused on downstream targets, such as miRNA sponges, binding with RBPs, regulating alternative splicing and transcription of parental genes, and translation into proteins. Other mechanisms, such as transcriptional regulation, and transport and degradation of circRNAs, are poorly understood and need to be characterized in the future. Fifth,current clinical transformation studies of circRNAs are insufficient. Most circRNA studies are still theoretical. The clinical significance and potential applications in gastrointestinal malignancies are still poorly understood. More attention should be paid to clinical transformation.
In conclusion, recent studies have confirmed that circRNAs influence the occurrence and evolution of gastrointestinal tumors through various regulatory mechanisms.To date, circRNAs are expected to be ideal biomarkers for clinical screening, prognostic evaluation and gene therapy targets of gastrointestinal malignancies. Because tumorigenesis and development are complex processes involving multiple factors and multiple stages, the molecular mechanisms of circRNAs in gastrointestinal malignancies need further study.
Grant support
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81702367 and 81772279),the Medical and Health Research Project of Zhejiang Province (Grant No. 2018KY159), the Affiliated Hospital of Medical School of Ningbo University Youth Talent Cultivation Program (Grant No. FYQMKY202001), and the Scientific Innovation Team Project of Ningbo (Grant No.2017C110019).
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2021年2期
Cancer Biology & Medicine2021年2期
- Cancer Biology & Medicine的其它文章
- Circular RNAs: new biomarkers of chemoresistance in cancer
- Biomaterial-based platforms for cancer stem cell enrichment and study
- sLex expression in invasive micropapillary breast carcinoma is associated with poor prognosis and can be combined with MUC1/EMA as a supplementary diagnostic indicator
- Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development
- Targeting FGFR in non-small cell lung cancer: implications from the landscape of clinically actionable aberrations of FGFR kinases
- Three-dimensional collagen-based scaffold model to study the microenvironment and drug-resistance mechanisms of oropharyngeal squamous cell carcinomas
