Suberin Biopolymer in Rice Root Exodermis Reinforces Preformed Barrier Against Meloidogyne graminicola Infection
Divya SINGH,Tushar K.DUTTA,Tagginahalli N.SHIVAKUMARA,Manoranjan DASH,Haritha BOLLINEDI,Uma RAO
(1Division of Nematology,ICAR-Indian Agricultural Research Institute,New Delhi 110012,India;2Division of Genetics,ICARIndian Agricultural Research Institute,New Delhi 110012,India)
Abstract:Exploration of novel genetic resources against root-knot nematode(RKN)is necessary to strengthen the resistance breeding program in cultivated rice,and investigations on the role of genotype-specific root anatomy in conferring a structural barrier against nematode invasion are largely underexplored.Here,we reported a highly-resistant rice germplasm Phule Radha that conferred remarkably lower RKN parasitic fitness in terms of reduced penetration and delayed development and reproduction when compared with susceptible cultivar PB1121.Using histological and biochemical analyses,we demonstrated that an enhanced suberin deposition in the exodermal root tip tissue of Phule Radha compared to PB1121 can effectively form a penetrative barrier against RKN infection,and this preformed barrier in the control tissue did not necessarily alter to a greater extent when challenged with RKN stress.Using qRT-PCR analysis,we showed that a number of suberin biosynthesis genes were greatly expressed in the exodermis of Phule Radha compared to PB1121.In sum,the present study established the role of rice exodermal barrier system in defense against an important soil-borne pathogen.
Key words:penetration;suberin lamellae;root-knot nematode;rice root exodermis
Rice is the staple food for more than 50% global population,particularly in Asian and African countries with 90% of its production area falls in Southeast Asia(Mantelin et al,2017;FAOSTAT,2019).Soilborne pathogens,such as plant-parasitic root-knot nematode(RKN,Meloidogynegraminicola),a sedentary endoparasite,are emerging as a major threat to rice agrosystems because of climate change and a major shift towards less water-intensive cultivation practices.As much as 80% yield loss can occur in upland as well as irrigated rice in simulated conditions(De Waele and Elsen,2007;Dutta et al,2012a,2014;Mantelin et al,2017).The second-stage juveniles(J2s)of RKN penetrate the root elongation zone,migrate intercellularly towards the root apex and using their pharyngeal gland secretion to induce a permanent feeding site [consisting of 3-5 giant cells(GCs)that provide nourishment to nematode during its entire life span] in the root vascular cylinder.Cells around GCs undergo numerous hyperplastic/neoplastic divisions to form galls(Kyndt et al,2014;Mantelin et al,2017).
In rice agriculture,M.graminicolahas been managed using synthetic nematicides such as Temik,Furadan and Fenamiphos(Huang et al,2015).Nevertheless,due to their ill effects on the environment,a complete ban or restricted use was imposed on these nematicides.It is therefore of utmost importance to identify novel sources of natural resistance to RKN in rice,for which more studies on rice-M.graminicolainteraction are necessary.Notably,this monocot plant is an excellent model system to study physiological and molecular interaction between plant and nematode(Kumari et al,2016,2017).
Studies on incompatible interaction between rice and RKN revealed that host plant resistance consists of both pre-penetration(reduced root invasion by J2s)and post-penetration(impeded J2s development to adult females,arrested GC development and degradation of GC)mechanisms(Cabasan et al,2014).A relatively less investigated aspect of host defense against nematodes is the protective function of preformed cell wall polymers,including suberin(a complex biopolyester)and lignin(encircle the cell as Casparian strip that reinforces radial and transverse cell walls)in the root endodermis that encloses central vascular cylinder(Holbein et al,2016,2019).Suberin and lignin confer the apoplastic transport barrier property(for toxin diffusion and nutrient loss)to the endodermis(Robbins et al,2014).Suberin can protect the host plant against pathogen infection by multiple means:reinforcing a mechanical barrier to pathogen invasion and growth,chemically fortifying the host cell walls to be more resistant to pathogen secreted cell wall,modifying enzymes and eliciting the production of free radicals that can arrest the further spread of the pathogen(Ride,1978;Balhadere and Evans 1995;Valette et al,1998;Holbein et al,2019).However,in rice-RKN pathosystem,the suberin-mediated plant defence is not yet investigated.
Although both cyst nematodes and RKN display sedentary endoparasitic lifestyle,they differ in their migration and feeding behavior inside the host tissue.The J2s of cyst nematodes can enter the root at any location by using their robust stylet,migrate intracellularly,invade cortex(including endodermis)and reach directly into the vascular cylinder to develop a feeding site.On the contrary,The J2s of RKN penetrate the root at elongation zone,migrate intercellularly(by secreting the pharyngeal gland enzymes via stylet orifice)towards the apical meristem and make a U-turn to reach the vascular cylinder bypassing the differentiated endodermis(Sijmons et al,1991;Wyss et al,1992;Holbein et al,2019).Therefore,the suberized plant root endodermis cannot prevent the RKN migration to root vascular tissue,in which GCs are initiated by post-parasitic J2s.Nevertheless,further expansion of GCs can be hindered by stronger suberin lamellae in the endodermis.This gives rise to the hypothesis of the present study(Fig.S1),which depicts that the exodermis with high suberin content in rice roots can limit the penetration ofM.graminicolaJ2s at very early stage of infection and even if penetrated,nematode-induced GCs may get degraded during later stage of infection,thus ultimately attenuating the RKN development to reproducing adult females.
In the present study,comparative attraction,penetration,development and reproduction ofM.graminicolawere examined in two genetically diverseOryza sativacultivars,PB1121(improved basmati rice that shows susceptibility to RKN)(Kumari et al,2016,2017)and Phule Radha(resistance to RKN)(Kumbhar et al,2015).Using histochemical and enzymatic analyses,comparatively greater suberin deposition was observed in the root exodermis of Phule Radha than PB1121.Additionally,using gene expression analyses,a greater accumulation of suberin was documented in Phule Radha than PB1121,implicating that suberin may contribute to the passive defense of the resistant rice genotype againstM.graminicolainfection.
RESULTS
Phule Radha exhibited resistance against M.graminicola infection in PF-127 medium
Initially,to assess the degree of nematode resistance in diverse rice accessions,six candidate genotypes[PB1121(semi-dwarf,improved basmati),Vandana(improved,drought tolerant,tall,upland,disease resistant),Suraksha(improved,irrigated,tall,insect resistant),EK70(landrace),Khalibagh(landrace)and Phule Radha(improved derivative of dwarf TN-1)]were inoculated with 30 J2s in PF-127 medium that harbored a soil-free,axenic environment.At 16 d after inoculation(DAI),PB1121 and Phule Radha were found to be the most susceptible and resistant genotypes,respectively(Fig.S2).When compared directly,the mean numbers of galls,egg masses,eggs per egg mass and multiplication factor(MF)ratio per plant were significantly lower in Phule Radha than in PB1121.Intermediate developmental stages such as J2 and J3/J4 mixed stages were documented in Phule Radha,indicating the delayed or retarded nematode development in that genotype(Fig.1).
Additionally,a comparativeM.graminicolalife cycle progression was investigated in PB1121 and Phule Radha with primary inoculum of 100 J2s per plant in PF-127 medium.Significantly more J2s had penetrated the roots of PB1121 than Phule Radha at 1,2,3 and 4 DAI(Table S1).Accordingly,more J2s were developed to J3s/J4s in PB1121 than in Phule Radha at 5 and 7 DAI.Faster nematode development and reproduction was observed in PB1121,in which young female stage was attained at 10 DAI,adult female at 12 DAI and reproducing females at 16 DAI in considerably greater numbers than in Phule Radha.By contrast,a delayed nematode development was recorded in Phule Radha(Fig.2 and Table S1).Swellings at the root tip,indicative of gall formation,were observable in PB1121 at 2 DAI.Consequently,the growth of the infected roots was ceased and galls were formed in the lateral roots due to the progression
of RKN damage.At 16 DAI,more lateral root emergences was visible in Phule Radha compared to PB1121,suggesting the reinforcement of structural barriers in Phule Radha root system to prevent RKN further invasion(Fig.2).

Fig.1.Meloidogyne graminicola infected plantlets of PB1121 and Phule Radha in Petri plates containing PF-127 medium at 16 d after inoculation.
Phule Radha can prevent M.graminicola penetration,but cannot affect M.graminicola attraction
PB1121 and Phule Radha were inoculated with about 100 J2s at 1.5 cm behind the root tip in the PF-127 medium.Nematode attraction towards the root tips of either genotype was not significantly different at 2,4,6,8 and 10 h after inoculation(Fig.3-A and -B),suggesting that RKN can locate both the hosts alike.However,a dramatic reduction in RKN penetration was documented at 24,48 and 72 h after inoculation in Phule Radha compared to PB1121(Fig.3-C),indicating that unlike PB1121,Phule Radha may contain a preformed barrier in the root exodermis that hinders nematode invasion.According to the histological observation,a differential architecture of GC,induced by the post-parasitic juveniles of RKN,was observed at the vascular cylinder of PB1121 and Phule Radha at 7 DAI.Head of the young female was localized among the 3-5 well developed GCs with dense and granular cytoplasm containing several small nuclei in PB1121.On the contrary,in Phule Radha,although the post-parasitic J2s reached vascular cylinder and initiated GC formation,GCs were later disintegrated(vacuolated and degraded cytoplasm devoid of nucleus)and unnourished nematode cannot develop to young female stage(Fig.3-D).The possibility of a physical barrier in Phule Radha endodermis cannot be ruled out this case.
To further validate that differential penetration of RKN towards the root tips of PB1121 and Phule Radha is only because of genotype-specific differential exodermal deposition of the physical barrier,a chemotaxis assay(in a Petri dish containing both agar and PF-127 medium)was conducted,in which RKN chemotaxis index(CI)was assessed towards the root exudates of PB1121 and Phule Radha.As expected,J2s ofM.graminicolaattracted alike towards the root exudates of either genotype(CI in Phule Radha was 0.78,and CI in PB1121 was 0.69;P> 0.01)(Fig.4).
Deposition of suberin lamellae in root exodermis of Phule Radha can protect against M.graminicola invasion

Fig.3.Comparative attraction to and penetration of PB1121 and Phule Radha root tips by M.graminicola.

Fig.4.Chemotaxis of M.graminicola towards root exudates of PB1121 and Phule Radha.
The chemical compositions of aliphatic and aromatic suberin from the exodermis of PB1121 and Phule Radha semi-thin root sections were analyzed via gas chromatography-mass spectrometry(GC-MS).The aromatic suberin was mainly composed of coumaric and ferulic acids,while aliphatic suberin was composed of various substance classes including fatty acids,alcohols,α,ω-diacids,ω-hydroxy acids and 2-hydroxy acids(Fig.5).Total suberin content increased by 2-fold in Phule Radha(12-15 μg/mg)compared to PB1121(6-9 μg/mg)including both control and RKN-stressed roots.In corroboration,significantly greater deposition of specific suberin monomers(fatty acids,ω-hydroxy acids,coumaric and ferulic acids)was observed in the Phule Radha control roots than in the PB1121 control roots.However,except ω-hydroxy acids and ferulic acids in PB1121,no significant difference in aromatic or aliphatic suberin monomers between control and nematode infected roots of both rice genotypes was observed(Fig.5).Taken together,
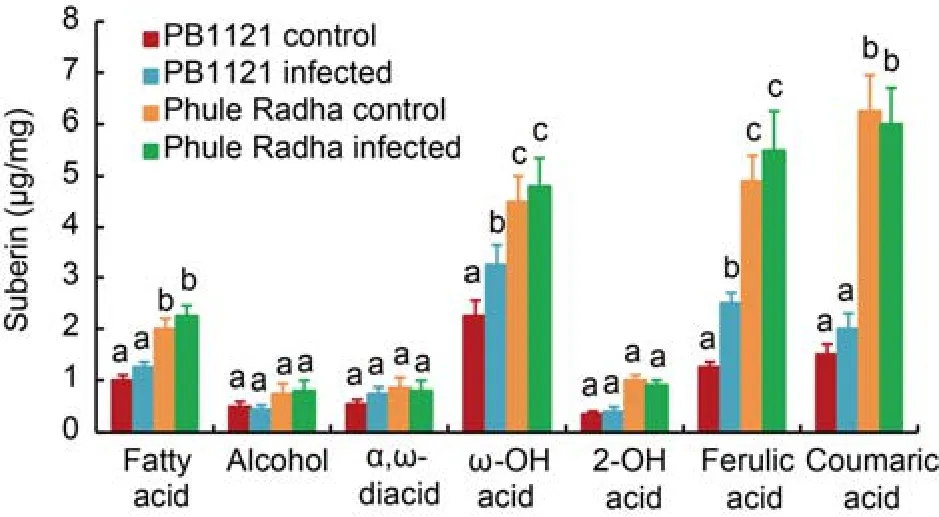
Fig.5.Suberin content in root tips of PB1121 and Phule Radha.
Aliphatic and aromatic suberin released from the exodermis of control and nematode infected [5-day-old plantlets were inoculated with 100 J2s(the second stage juveniles)ofM.graminicolain the PF-127 medium and plantlets harvested at 2 d after inoculation] roots after transesterification with BF3-methanol.Total aliphatic suberin amount and monomer compositions were performed via gas chromatographymass spectrometry analysis.Bars represent Mean ± SE(n= 3)and bars with unshared letters(within each substance class)indicate significant difference atP<0.01 by thepost-hocTukey’s test.this is indicative of the possibility that as a preformed physical barrier to RKN invasion,suberin was deposited in greater amount in the exodermis of Phule Radha than PB1121 and it did not significantly alter under nematode stress at 2 DAI.

Fig.6.Comparative suberin lamellae deposition in roots of PB1121 and Phule Radha.
In coherence,an intense yellowish green fluorescence in the exodermis(in the root elongation zone,about 1 cm from the root apex)of the control and nematodeinfected Phule Radha was documented.Comparatively faint yellowish green fluorescence was observed in the control and nematode-infected roots of PB1121(Fig.6).Sections of both genotypes were taken at the identical distance from root tip.Underlying the suberin lamellae,Casparian strips(CS)can function as a protective barrier to nematode infection(Holbein et al,2019).In view of this,uninfected root tips of PB1121 and Phule Radha were stained with berberine and aniline blue.As indicated by intense green fluorescence,a thicker CS lining was observed in the exo- and endo-dermal tissues of Phule Radha compared to non-existent or thinner CS lining in the exodermis and endodermis of PB1121(Fig.7).
Suberin biosynthesis genes are highly expressed in roots of Phule Radha compared to PB1121
qRT-PCR analysis on total RNA extracted from exodermal tissues of the control andM.graminicolainfected(2 DAI)PB1121 and Phule Radha was carried out for different candidate suberin biosynthesis genes.Transcripts ofCYP86A1,CYP86B1andMYB107were markedly upregulated by 11.0-,11.5- and 9.5-fold in the control Phule Radha than in the control PB1121,respectively(Fig.8).Abundance ofFAR1,GPAT5,KCS2,ASFT,GDSLandHOTHEADwere significantly increased by 8.0-,6.0-,7.5-,5.0-,4.0-and 2.2-fold in the control Phule Radha than in the control PB1121,respectively.Nevertheless,transcripts ofABCG2andLACS4were not significantly altered in Phule Radha compared to PB1121.Interestingly,although the expression levels ofCYP86A1,CYP86B1,FAR1,GPAT5,KCS2,GDSLandMYB107were significantly upregulated in the RKN-infected PB1121 than in the control PB1121,the transcript levels of these genes in the RKN-infected PB1121 were far lower compared to those in the control Phule Radha.Except forCYP86A1,FAR1andMYB107,all the suberin biosynthesis genes were not significantly altered in the RKN-infected Phule Radha than in the control Phule Radha(Fig.8).Therefore,a considerable differential expression of these genes in uninfected Phule Radha and uninfected PB1121 is suggestive of the possibility of preformed stronger suberization in Phule Radha compared to weak suberization in PB1121.
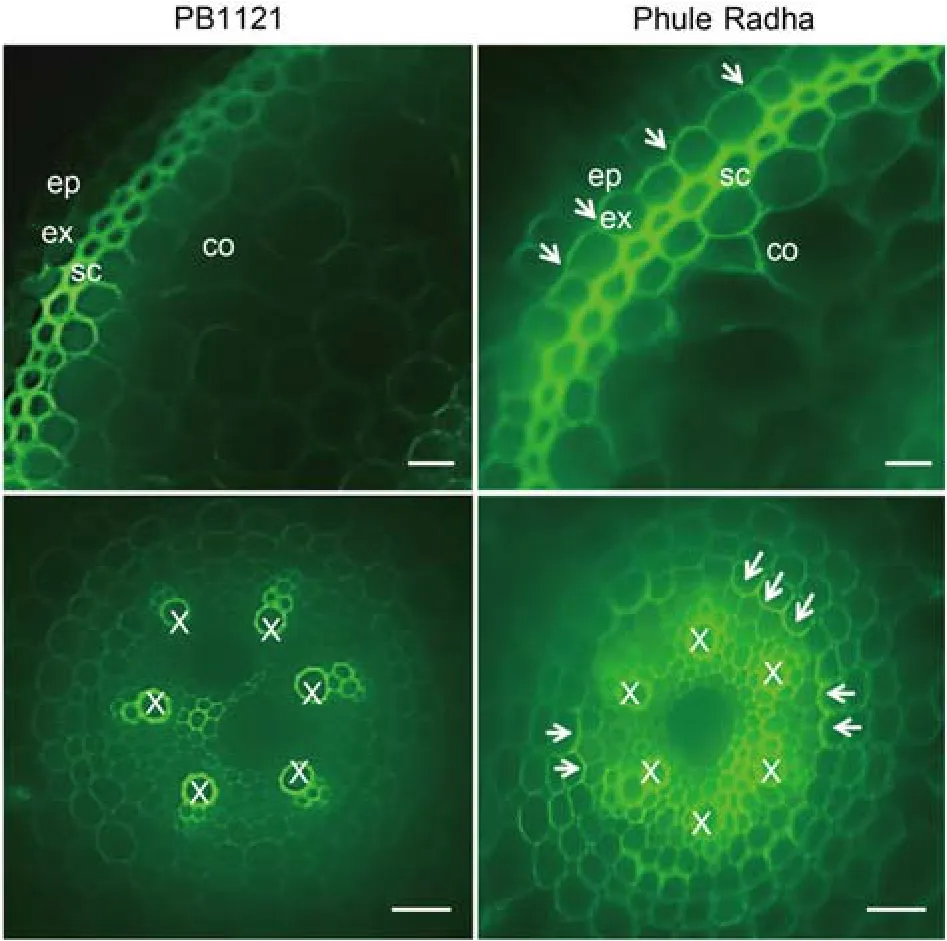
Fig.7.Comparative Casparian strip(CS)deposition in roots of PB1121 and Phule Radha.
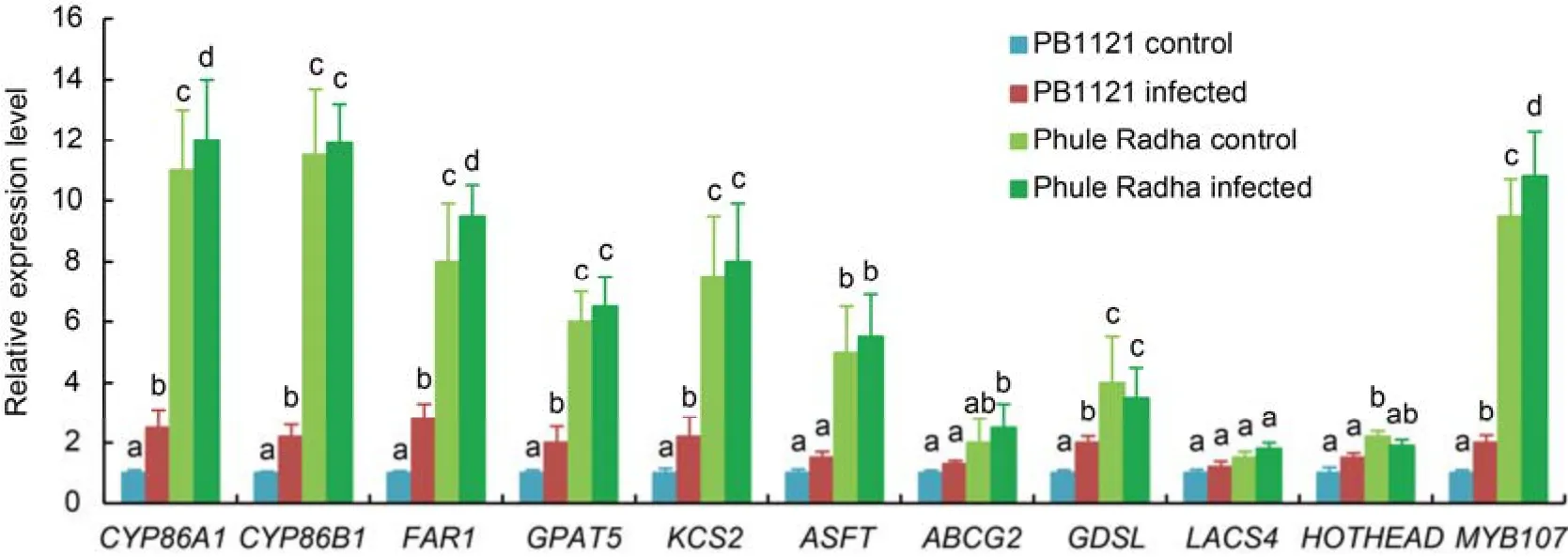
Fig.8.Relative expression levels of suberin biosynthesis genes in root tip exodermis of PB1121 and Phule Radha.
DISCUSSION
A majority of popularly grownO.sativa(Asian rice)cultivars and landraces are known to be vulnerable toM.graminicolainfection,while only true RKN resistance has been reported in non-cultivated African rice,O.glaberrimaandO.longistaminata(De Waele et al,2013;Kyndt et al,2014;Mantelin et al,2017).Efforts to introgress RKN resistance genes fromO.glaberrimaintoO.sativais not yet successful because the interspecific progenies did not confer the same degree of nematode resistance documented inO.glaberrima(Cabasan et al,2018).In order to meet the burgeoning food demand due to explosive global population growth,improvedO.sativacultivars with higher yield potential and biotic/abiotic stress tolerance are being developed using resistance breeding approaches.In view of this,O.sativacultivars that conferM.graminicolaresistance can improve the current germplasm repository,which may become amenable for exploitation in varietal improvement program.In the present study,we reported anO.sativacultivar Phule Radha(Kolamba 540 × TN-1)as a potential resistant source toM.graminicolaparasitism.To avert the error-prone,labor-intensive and non-axenic pot-based screening,we adopted a soil-freein vitroscreening system using the PF-127 medium that mimics the natural three-dimensional soil environment(Kumari et al,2016,2017).A drastic reduction inM.graminicolaparasitic fitness(95.1%,85.9%,89.3% and 99.0% in terms of the numbers of galls,egg masses,eggs per egg mass and MF ratio,respectively,at 16 DAI;30 J2s were inoculated per root tip)was observed in Phule Radha when directly compared with PB1121 as the susceptible source(Fig.1-B).Notably,M.graminicolacan complete its life cycle within 15 and 19 DAI in the PF-127 medium and soil,respectively(Dutta et al,2011;Kumari et al,2016).When compared with other nematode resistantO.sativagermplasm(such as Vandana,Suraksha,EK70 and Khalibagh)(Kumari et al,2016,2017;Hatzade et al,2020),Phule Radha conferred the greatest degree of resistance toM.graminicola.
Next,Phule Radha and PB1121 were subjected to nematode development studies for comparative analysis of RKN life cycle progression in resistant versus susceptible genotypes(Fig.2).Results indicated thatM.graminicolapenetrated Phule Radha in considerably lesser number than PB1121 during 1 to 4 DAI even with higher initial inoculum densities(100 J2s per root tip).Consequently,a delayed nematode development and extremely reduced reproductive potential was documented in Phule Radha compared to PB1121.It was assumed that a genotype-dependent resistance mechanism might contribute to differential penetration barrier in Phule Radha and PB1121.
Plant root exudates contain discrete bioactive compounds including ions,enzymes,primary and secondary metabolites that are liberated from root border cells and secreted into the rhizosphere(Bais et al,2006).These chemical compounds can regulate nematode chemotactic behavior in terms of attraction,repellence,motility inhibition or even death(Curtis,2008;Dutta et al,2012b;Shivakumara et al,2019).This prompted us to investigate whether there is any differential pre-penetration RKN behavior towards the root exudates of Phule Radha and PB1121.Surprisingly,M.graminicolaJ2s did not exhibit any significant difference in attraction towards the root tip of Phule Radha and PB1121 at 2,4,6,8 and 10 h after inoculation(Fig.3-A).In addition,the J2s of RKN also chemotaxed alike towards the root exudates of either cultivar in an agar-PF-127 based assay system(Fig.4).These findings reinforced the previous hypothesis that a genotype-dependent penetration barrier may exist in Phule Radha and PB1121.
Preformed complex biopolymers such as suberin and lignin form an apoplastic transport barrier that are deposited in the exoderma and endodermal cell walls of rice roots as suberin lamellae and Casparian strips(Ranathunge et al,2011;Kreszies et al,2018).Suberized cell walls constitute poly aliphatic(hydrophobic)and poly aromatic moieties and together they embody a protective barrier to pathogen invasion(Krishnamurthy et al,2009;Kreszies et al,2018).More importantly,the total suberin content in rice roots is species as well as genotype dependent,e.g.lowland and upland rice varies in total suberin content(Ranathunge et al,2016;Kreszies et al,2018).In this direction,we analyzed the relative suberin content in Phule Radha and PB1121 using both biochemical and histological analyses(Figs.5-7).Intriguingly,cross sectioning of rice roots at 7 DAI indicated underdeveloped and disintegrated GCs in Phule Radha vascular tissue in contrast to fully functional GCs in PB1121 vascular cylinder(Fig.6).Our result is in accordance with the finding of Petitot et al(2017)who demonstrated highly vacuolated and degenerated GCs inO.glaberrimaaccession TOG5681 compared to functional GCs inO.sativacv.Nipponbare infected withM.graminicola.We speculated that a stronger suberization in Phule Radha endodermis might arrest the further development ofM.graminicolafrom post-parasitic J2 stage even after successfully establishing a feeding site in the vascular cylinder of Phule Radha.The role of rice defense response genes(salicylic acid,jasmonic acid,ethylene,pathogenesis-related proteins)in limiting the RKN disease progression(Holbein et al,2016;Kumari et al,2016,2017;Mantelin et al,2017)cannot be eliminated in this case.However,considering that suberized exodermis primarily poses a penetrative barrier to RKN infection because post penetration RKN can make a U-turn to reach vascular cylinder bypassing the suberized endodermis,analysis of suberin content was performed selectively in the exodermal tissue.
According to a GC-MS-based precise quantitative method,the total suberin content was increased by 2-fold in Phule Radha compared with both the control and RKN-infected PB1121.Barring the levels of alcohols,α,ω-diacids and 2-OH acids,significantly higher levels of both aliphatic(fatty acids and ω-OH acids)and aromatic(ferulic and coumaric acids)suberin monomers were detected in Phule Radha than PB1121.Interestingly,none of the suberin monomer levels were significantly differed in the control and RKN-stressed Phule Radha.Except the levels of ω-OH acids and ferulic acids,a similar trend was observed in PB1121(Fig.5).A 5-6-fold increase in ferulic and coumaric acids content was observed in Phule Radha compared to PB1121.This was in line with the previous finding that rice(Azucena vs IR64)has the greater amount of aromatic suberin than the aliphatic one and this property is species or genotype dependent(Schreiber et al,2005;Ranathunge et al,2016).
Taken together,our results exemplified that the preformed stronger suberization in Phule Radha(than in PB1121)can embody a protective barrier that hindersM.graminicolapenetration of host tissue,and this preformed barrier does not significantly alter even under nematode stress.In corroboration,histological studies demonstrated an intense yellowish green fluorescence in the exodermal suberin lamellae of Phule Radha root tip compared to weak fluorescence in the control and RKN-stressed PB1121 root tips.Additionally,an intense green fluorescence in the exodermal and endodermal CS of the control Phule Radha compared to weak fluorescence in the control PB1121 was observed(Figs.6 and 7).A genotype specific root anatomical change in the CS lining ofO.sativaroot tip has been reported earlier(Cai et al,2011).Also,simultaneous accumulation of lignin and suberin as the structural barrier during the CS formation process in maize roots has been described by Man et al(2018).
A number of enzymes are involved in the suberin biosynthetic pathway.Primarily,cytochrome P450-dependent fatty acid oxidases(CYP86A1 and CYP86B1).GPAT acyltransferases and acyl-CoA synthetases(LACS)catalyze the polyester biosynthesis.β-ketoacyl-CoA synthases are involved in suberin monomer elongation.R2R3 MYB transcription factors regulate the polyester synthesis(Pollard et al,2008;Lashbrooke et al,2016).Acylreductases(FAR)and acyltransferases(GDSL)are other important enzymes involved in the pathway(Kreszies et al,2018).HOTHEAD,a putative oxidoreductase catalyzes the conversion of ω-OH fatty acids to ω-oxo fatty acids(Pollard et al,2008).ABC transporters aid in apoplastic polyester synthesis by facilitating the export of acylglycerols(Pollard et al,2008).ASFT,an acyltransferase from BAHD family,esterifies ferulate as a linker between aliphatic and aromatic domain(Molina et al,2009).Many of these suberin biosynthesis genes showed significantly increased expression in the Phule Radha root exodermis in comparison to those in the PB1121 root exodermis(Fig.8).For example,steady-state mRNA levels ofCYP86A1,CYP86B1,FAR1,GPAT5,KCS2,ASFT,GDSL,HOTHEADandMYB107were strongly upregulated in control Phule Radha compared to control PB1121.However,the transcripts ofABCG2andLACS4were not differentially expressed between uninfected Phule Radha and PB1121.Although the transcripts ofCYP86A1,CYP86B1,FAR1,GPAT5,KCS2,GDSLandMYB107were significantly upregulated in infected PB1121 compared to its control tissue,the fold change values of these genes in infected PB1121 were substantially lower than the uninfected Phule Radha exodermal tissues.Interestingly,barring the expression ofCYP86A1,FAR1andMYB107,suberin biosynthesis genes were not differentially expressed between the control and infected tissues of Phule Radha.This supported our assumption that the stronger suberization in Phule Radha than PB1121 is indeed a part of preformed host defense mechanism againstM.graminicolapenetration,and even upon nematode stress,no overall change occurs to this inherent anatomical property.
To date,only a handful of studies have described the role of endodermal suberin and lignin in conferring resistance to nematode infection(Balhadere and Evans,1995;Valette et al,1998).Most recently,Holbein et al(2019)demonstrated the role of endodermal suberin and lignin in arresting theM.incognitaandHeterodera schachtiiinfectivity by rendering the degradation of feeding sites inArabidopsis.However,exodermis is absent inArabidopsis(Kreszies et al,2018)and the role of exodermal suberin as a penetrative barrier to nematode infection was not yet investigated.Herein,we reported that the preformed suberization in rice root exodermis can pose a barrier toM.graminicolaentry inside the host tissue and this specific root anatomical property varied in different genotypes ofO.sativa.This presented hypothesis can be further validated in future by generating rice mutant lines targeting the suberin biosynthesis genes.
METHODS
Nematode culturing,rice seed germination and collection of root exudates
A pure culture ofM.graminicolaGolden &Birchfield was maintained inO.sativacv.PB1121 in a greenhouse.Egg masses were isolated from the galled roots of infected plants using sterilized forceps,and hatched in sterile water using a modified Baermann assembly(Southey,1986).Freshly-hatched J2s were used for further experiments.
Seeds of PB1121 and Phule Radha were soaked overnight in sterile water and surface-sterilized with 70% ethanol for 30 s followed by rinsing three times in distilled water.Sterilized seeds were germinated in wet filter paper in a Petri plate incubated at 28 °C in a growth chamber.Four- to five-day-old seedlings were used for further experiments.
Root exudates of PB1121 and Phule Radha were collected from hydroponically grown seedlings in Hoagland solution for 15 d and vacuum concentrated to 1 mL of suspension(from 50 mL initial volume for each genotype)using a referred method(Shivakumara et al,2018,2019).
Screening of rice for RKN resistance in Pluronic gel medium(PF-127)
Rice seedlings were initially screened forM.graminicolaresistance in the PF-127 medium as described earlier(Kumari et al,2016,2017).Briefly,60 mL of 23% PF-127 was poured into 150 mm × 20 mm Petri plate containing 7-9 uniformly distributed seedlings of the same genotype at<15 °C and approximately 30 J2s were inoculated near the root tip of each seedling by pipetting.The gel was solidified at room temperature(RT)and Petri dishes were incubated in a growth chamber at 28 °C with a 12 h light /12 h dark photoperiod at 350 μmol/(m2·s).At 16 DAI,plantlets were harvested from PF-127 by placing the dishes in an ice bath.Roots were stained with acid fuchsin as depicted previously(Dutta et al,2011),galls were dissected under a microscope,and the number and stage of penetrated nematodes were recorded.A nematode MF ratio was calculated as [(number of egg masses × number of eggs per egg mass)/ nematode inoculum level] to determine the reproductive potential ofM.graminicolain PB1121 and Phule Radha.For each accession,three plates were included and the experiment was repeated at least thrice.Photomicrographs were obtained in an Axiocam MRm microscope(Carl Zeiss,Germany).To assess the attraction of RKN to the root tips of PB1121 and Phule Radha,approximately 100 J2s were inoculated at 1.5 cm posterior from the plant root tip in the PF-127 medium in a 50 mm × 10 mm Petri plate(Dash et al,2017).J2s touching the root tip were counted under a microscope at 2,4,6,8 and 10 h after inoculation.Additionally,to examine the comparative penetration and development ofM.graminicolain PB1121 and Phule Radha,seedlings were harvested at different time points till 16 DAI.The number of endoparasites were microscopically examined in acid fuchsin-stained roots.The experiment was performed with at least three biological and three technical replicates.
Chemotaxis assay of M.graminicola against rice root exudates
RKN chemotaxis towards the root exudates of PB1121 and Phule Radha was performed in a 50 mm × 10 mm Petri dish containing both PF-127(at the central area)and agar(at the periphery of the dish on both sides).The preparation of this experimental assembly is detailed in Shivakumara et al(2018,2019).A well of 1.5 mm diameter was made at agar-PF-127 junction on each side of the plate.About 10 μL of root exudates was applied on one well and identical amount of sterile water(as control)was applied in the opposite well.After 40 min of gradient establishment,nearly 100 J2s were inoculated at the center of the dish.After another 1 h at RT,J2s accumulating at agar-PF-127 boundary were collected by pipetting and observed under a microscope.CI was calculated as the number of J2s at the root exudate side minus the number of J2s at the control side divided by the total number of J2s inoculated,in which CI ranges from 1.0(perfect attraction)to-1.0(perfect repulsion)(Shivakumara et al,2018,2019).The root exudate of marigold(cv.Arpit;known to repel RKNs)was used as another control.The experiment was carried out with at least three biological and three technical replicates.
Histological analysis of RKN infected rice root
To understand the compatible/incompatible interaction ofM.graminicolawith different rice genotypes,histopathology studies were conducted during the initial stages of plant-nematode interaction.Nematode infected roots(at least 10 plants were inoculated with 30 J2s per root)of PB1121 and Phule Radha were harvested from the PF-127 medium at 7 DAI.Galled segments of each root system(0.5-1.0 cm long)were excised and fixed in formalin-aceto-alcohol.Dehydration of the root segments was done in a graded series of ethanol(30%-100%,30 min at each concentration)followed by infiltration with xylene and embedding in paraffin wax(Ruzin,1999).Transverse sections(10 μm)of the segments were cut with a motorized Leica RM2165 microtome(Leica Biosystems,Germany).Sections were then transferred to glass slides at 60 °C on a hot plate and the slides were kept to dry at RT for 72 h.Removal of paraffin was done by soaking the sections in xylene followed by rinsing with ethanol and distilled water.Sections were stained with safranin and fast green(Johansen,1940)and observed in an Axiocam MRm microscope(Carl Zeiss,Germany).In each genotype,a minimum of 10 sections were examined.
Qualitative estimation of suberin lamellae in rice root exodermis
About 1 cm long apical part of the root was cut using a sterilized blade,rinsed with 70% ethanol and embedded in 5%agar.Cross sections of 100 μm thickness were cut in a Leica RM2165 microtome(Leica Biosystems,Germany).To remove the adherent agar,sections were cleared in solution of 85%lactic acid saturated with chloral hydrate at 70 °C for 1 h,followed by washing several times in deionized water.To visualize cell wall suberization,sections were stained with 0.01% Fluorol Yellow 088 in polyethylene glycol-glycerol for 1 h(Brundrett et al,1991)and viewed under UV light with a Zeiss Axiocam MRm microscope(Carl Zeiss,Germany)as yellowish green fluorescence(excitation filter is 489 nm and emission filter is 509 nm).To visualize CS,transparent sections were stained with 0.1% berberine hemisulfate and 0.5% aniline blue(Man et al,2018)and viewed under an epifluorescence microscope(Carl Zeiss,Germany)as bright green fluorescence(excitation filter is 490 nm and emission filter is 520 nm).
Quantitative estimation of suberin in rice root exodermis
Primary root segments of 1 cm length were cut as described above,and nearly 100 segments from identical genotype were pooled together.GC-MS-based estimation of suberin monomers was performed as described by Kreszies et al(2018).In brief,vacuum-infiltrated root segments were enzymatically digested with 1% cellulase(Sigma-Aldrich,USA)and 1% pectinase(Sigma-Aldrich,USA)in 50 mmol/L sodium acetate buffer(pH 4.0)at RT for 2-4 weeks in a shaker.When tissues were correctly digested,central cylinder with endodermis was separated from the outer root cell layers containing exodermis and sclerenchyma using sterilized needle and forceps under a microscope.Isolated cell walls were washed in borate buffer and transferred to 1 :1 of chloroform :methanol at RT in a shaker for 2 weeks to extract soluble lipids.Samples were dried in a desiccator,weighed and trans-esterified with 300 μL of 10% BF3-methanol for 24 h at 70 °C to release aliphatic and aromatic suberin monomers.Samples were injected to HP5MS column on a Thermo Fisher Scientific DSQII gas chromatograph combined with a mass selective detector for monomer identification followed by quantitative estimation based on an internal standard in a similar gas chromatograph system coupled with an electron ionization detector.Suberin amounts were presented as the μg per mg dry weight of root exodermis.The experiment was conducted with three biological replicates.
Expression analysis of suberin biosynthesis genes in rice root
Quantitative reverse-transcription PCR(qRT-PCR)analysis of suberin biosynthesis genes(primers are listed in Table S2)was performed on the infected root samples at 48 h after inoculation in PB1121 and Phule Radha.Total RNA was extracted from the mechanically separated(under the microscope using sterilized forceps)exodermal tissue of root tips(100 plants of identical genotype were pooled together)using NucleoSpin Total RNA Kit(Macherey-Nagel,Germany)following the manufacturer’s protocol.RNA quantity and quality were assessed with a NanoDrop spectrophotometer(ND-1000,Thermo Fisher Scientific,USA).First-strand cDNA was synthesized from the total RNA using SuperScript VILO cDNA Synthesis Kit(Invitrogen,USA)following the manufacturer’s instructions.qRT-PCR was performed in a Realplex2 thermal cycler(Eppendorf,Canada)with a reaction mixture of 10 μL containing 5 μL of SYBR Green PCR Master mix(Eurogentec,Belgium),750 nmol/L of each primer and 1.5 ng of cDNA.As an internal reference,the expression of18S rRNAgene ofO.sativawas recorded.Specificity of qRT-PCR reaction was assessed using a melt curve program described previously(Kumari et al,2016,2017).Cycle threshold(Ct)values were imported from the Realplex2 software(Eppendorf,USA)and fold changes in expression of suberin biosynthesis genes were determined via the augmented comparative Ct method(Livak and Schmittgen,2001).RNA isolated from uninfected rice was used as a control.At least three biological and three technical replicates were applied for each sample.
Statistical analysis
Data of the experiments were normalized using a generalized linear model and subjected to one-way analysis of variance(ANOVA)in the SAS software(version 14.1).Statistical comparisons were made between different treatments or compared individually to controls viapost-hocTukey’s honest significant difference test atP<0.01.
ACKNOWLEDGEMENT
This study was supported by the grant from the Department of Biotechnology,Ministry of Science and Technology,India(Grant No.BT/PR18924/COE/34/48/2017).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science;http://www.ricescience.org.
Fig.S1.Hypothesis of present study schematically represented.
Fig.S2.Screening of rice varieties for nematode resistance.
Table S1.Comparison ofM.graminicolainvasion,development and reproduction in PB1121 and Phule Radha.
Table S2.List of oligonucleotides employed for qRT-PCR analysis of suberin biosynthesis genes in rice.
- Rice Science的其它文章
- Editing of Rice Endosperm Plastidial Phosphorylase Gene OsPho1 Advances Its Function in Starch Synthesis
- Valuation of Rice Postharvest Losses in Sub-Saharan Africa and Its Mitigation Strategies
- Rice Bran Oil:Emerging Trends in Extraction,Health Benefit,and Its Industrial Application
- Combined Drought and Heat Stress in Rice:Responses,Phenotyping and Strategies to Improve Tolerance
- SB1 Encoding RING-Like Zinc-Finger Protein Regulates Branch Development as a Transcription Repressor
- OsbZIP72 Is Involved in Transcriptional Gene-Regulation Pathway of Abscisic Acid Signal Transduction by Activating Rice High-Affinity Potassium Transporter OsHKT1;1