连续双季碳螺环氧化吲哚拼接异噁唑类化合物的合成
周豪杰, 周 韦, 田民义, 刘雄利, 邓国栋
(贵州大学 西南药食两用资源开发利用技术国家地方联合工程研究中心,贵州 贵阳 550025)
双螺环氧化吲哚类化合物因其具有独特的结构和生物活性,吸引了药物化学工作者广泛的研究[1-3]。异噁唑基团普遍存在于天然产物和药物分子中。如Muscimol, Cloxacillin, Leflunomide和Isoxicam等[4-7],在生物制药领域有重要应用价值。因此,将异噁唑基团拼接到双螺环氧化吲哚骨架,合成一系列具备潜在活性的氧化吲哚衍生物,可以为生物活性筛选提供物质基础,对药物的筛选和制药行业具有重要的应用价值。最近,已经报道硝基异噁唑-3-烯氧化吲哚1作为优秀的2C合成子,在合成螺环氧化吲哚类化合物有广泛的应用[8-12]。
本文以硝基异噁唑-3-烯氧化吲哚1作为基于给体和受体的3C合成子,与叔丁基酯-3-烯氧化吲哚2,在碱性催化剂DABCO催化下发生Michael加成环化反应,获得10个新颖的螺环氧化吲哚拼接异噁唑类化合物3a~3j,产率为57%~70%,dr值为4/1~9/1, 其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征,并且经过单晶3f进一步确定了其结构,并且经过单晶3f进一步确定了其结构。所合成产物可为生物活性筛选提供物质基础。该化合物骨架含有连续两个螺环季碳中心,可以为生物活性筛选提供物质基础。
1 实验部分
1.1 仪器与试剂
WRS-1B型数字熔点仪;Bruker-400 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标); MicroTMQ-TOF型高分辨质谱仪。
所用试剂均为分析纯。
1.2 合成(以3a为例)
在反应管中依次加入1a1.4 mg(70.2 mmol),2a86.1 mg(0.3 mmol), 10 mol% DABCO 2.3 mg和2 mL二氯甲烷, 室温搅拌反应24 h(TLC检测)。直接经硅胶柱层析[洗脱剂:V(石油醚):V(乙酸乙酯)= 5/1]纯化得化合物3a79.8 mg。
用类似的方法合成3b~3j。
3a: 灰白色固体,m.p.87.9~88.5 ℃,产率62%, 8/1dr;1H NMRδ: 1.03(s, 9H), 1.14~1.18(m, 3H), 2.54(s, 3H), 2.79~2.83(m, 6H), 3.84~3.89(m, 2H), 5.07~5.12(m, 1H), 6.44(d,J=8.0 Hz, 1H), 6.81~6.85(m, 1H), 7.00~7.04(m, 1H), 7.07~7.11(m, 1H), 7.15~7.20(m, 2H), 7.40(d,J=8.0 Hz, 1H), 7.88(d,J=8.0 Hz, 1H);13C NMRδ: 9.8, 18.7, 24.9, 25.7, 26.1, 26.7, 41.7, 50.0, 60.6, 62.1, 80.6, 107.2, 114.5, 121.2, 123.0, 123.5, 125.9, 128.3, 129.1, 139.0, 143.1, 158.9, 167.3, 169.0, 170.1, 172.7, 178.7; HR-MS(ESI-TOF)m/z: Calcd for C34H36N4O9Na{[M+Na]+}667.2374, found 667.2371。
3b: 灰白色固体,m.p.101.3~102.7 ℃,产率70%, 7/1dr;1H NMRδ: 1.03(s, 9H), 1.13~1.16(m, 3H), 2.21(s, 3H), 2.52(s, 3H), 2.78~2.83(m, 6H), 3.84~3.89(m, 2H), 5.09~5.12(m, 1H), 6.43(d,J=8.0 Hz, 1H), 6.80~6.83(m, 1H), 6.91~6.95(m, 1H), 7.05~7.10(m, 1H), 7.16~7.21(m, 2H), 7.75(d,J=8.4 Hz, 1H);13C NMRδ: 9.8, 18.7, 20.0, 24.8, 25.7, 26.6, 28.7, 41.6, 49.9, 60.6, 62.1, 80.4, 107.2, 114.3, 121.2, 122.1, 122.9, 123.0, 126.2, 128.7, 129.0, 133.0, 136.7, 143.1, 158.9, 167.3, 168.8, 170.2, 172.7, 178.9; HR-MS(ESI-TOF)m/z: Calcd for C35H38N4O9Na{[M+Na]+}681.2531, found 681.2535。
3c: 灰白色固体,m.p.143.3~144.5 ℃,产率62%, 6/1dr;1H NMRδ: 1.03(s, 9H), 1.15~1.18(m, 3H), 2.53(s, 3H), 2.79~2.83(m, 6H), 3.84~3.89(m, 2H), 5.09~5.14(m, 1H), 6.43(d,J=7.6 Hz, 1H), 6.80~6.84(m, 1H), 6.89~6.95(m, 2H), 7.05~7.10(m, 1H), 7.16~7.17(m, 1H), 7.75(d,J=8.4 Hz, 1H);13C NMRδ: 9.8, 18.7, 24.8, 25.7, 26.0, 26.6, 41.6, 49.9, 60.6, 62.1, 80.5, 107.2, 114.3, 121.2, 122.9(d,JCF=22.5 Hz), 126.2(d,JCF=24.1 Hz), 128.8, 129.0, 133.0, 136.7, 143.1, 158.9(d,JCF=244.0 Hz), 167.3, 168.9, 170.2, 172.7, 178.9; HR-MS(ESI-TOF)m/z: Calcd for C34H35N4O9FNa{[M+Na]+}685.2280, found 685.2284.
3d: 灰白色固体,m.p.99.9~100.9 ℃,产率61%, 5/1dr;1H NMRδ: 1.02(s, 9H),1.17~ 1.20(m, 3H), 2.25(s, 3H), 2.79~2.88(m, 2H), 2.93(s, 3H), 3.02~3.08(m, 1H), 3.45~3.58(m, 1H), 4.38~4.43(m, 1H), 4.98~5.02(m, 1H), 6.64~6.68(m, 1H), 6.85(d,J=8.0 Hz, 1H), 6.90~6.94(m, 1H), 7.20~7.24(m, 1H), 7.29~7.32(m, 1H), 7.56(s, 1H), 8.07(d,J=7.6 Hz, 1H);13C NMRδ: 11.3, 19.5, 26.1, 26.6, 27.2, 27.6, 43.7, 52.6, 63.6, 65.1, 82.3, 108.4, 116.8, 121.7, 126.8, 126.9, 129.3, 129.9, 130.2, 138.0, 143.3, 160.2, 168.1, 170.0, 171.7, 173.7, 174.9; HR-MS(ESI-TOF)m/z: Calcd for C34H35N4O9ClNa{[M+Na]+}701.1985, found 701.1987。
3e: 灰白色固体,m.p.131.2~132.3 ℃,产率64%, 4/1dr;1H NMRδ: 0.92(s, 9H), 1.13~1.17(m, 3H), 2.17(s, 3H), 2.77~2.83(m, 2H), 2.86(s, 3H), 2.89~2.96(m, 1H), 3.37~3.46(m, 1H), 4.22~4.25(m, 1H), 4.32~4.37(m, 1H), 4.92~4.96(m, 1H), 6.61(d,J=7.6 Hz, 1H), 6.79(d,J=7.6 Hz, 1H), 6.85~6.89(m, 1H), 7.15~7.18(m, 2H), 7.44~7.47(m, 1H), 8.12(s, 1H);13C NMRδ: 10.2, 18.5, 25.1, 25.6, 26.2, 28.7, 42.6, 51.7, 62.4, 64.6, 81.2, 107.4, 115.2, 120.7, 123.3, 123.6, 125.9, 126.5, 127.8, 128.9, 129.9, 134.3, 139.3, 142.2, 159.1, 167.1, 169.0, 170.6, 172.6, 173.9; HR-MS(ESI-TOF)m/z: Calcd for C34H35N4O9ClNa{[M+Na]+}701.1985, found 701.1987。
3f: 灰白色固体,m.p.121.1~122.4 ℃,产率63%, 5/1dr;1H NMRδ: 1.02(s, 9H), 1.17~1.22(m, 3H), 2.25(s, 3H), 2.79~2.88(m, 2H), 2.93(s, 3H), 3.02~3.09(m, 1H), 3.45~3.54(m, 1H), 4.37~4.42(m, 1H), 4.97~5.01(m, 1H), 6.67(d,J=8.0 Hz, 1H), 6.81(d,J=7.2 Hz, 1H), 6.89~6.93(m, 1H), 7.21~7.23(m, 1H), 7.44~7.47(m, 1H), 7.70(s, 1H), 8.00(d,J=8.8 Hz, 1H);13C NMRδ: 11.3, 19.5, 26.1, 26.6, 27.2, 27.6, 43.7, 52.6, 63.6, 65.1, 82.3, 108.4, 117.2, 117.7, 121.7, 124.2, 126.7, 128.0, 129.6, 129.9, 132.3, 138.5, 143.4, 160.2, 168.1, 170.0, 171.7, 173.7, 174.8; HR-MS(ESI-TOF)m/z: Calcd for C34H35N4O9BrNa{[M+Na]+}745.1480, found 745.1477。
3g: 灰白色固体,m.p.100.3~101.2 ℃,产率64%, 9/1dr;1H NMRδ: 1.03(s, 9H), 1.17~1.21(m, 3H), 2.59(s, 3H), 2.81~2.86(m, 3H), 3.79~3.90(m, 3H), 4.97~5.01(m, 1H), 6.39(d,J=7.6 Hz, 1H), 7.00~7.09(m, 2H), 7.15~7.20(m, 2H), 7.36~7.38(m, 1H), 7.91(d,J=7.6 Hz, 1H);13C NMRδ: 9.8, 18.8, 25.0, 25.6, 26.1, 26.7, 42.0, 49.9, 60.6, 61.7, 80.7, 108.2, 114.7, 123.5, 123.6, 125.8, 126.7, 128.6, 129.0, 139.0, 141.7, 159.1, 167.1, 169.0, 169.9, 172.5, 178.6; HR-MS(ESI-TOF)m/z: Calcd for C34H35N4O9ClNa{[M+Na]+}701.1985, found 701.1985。
3h: 灰白色固体,m.p.134.1~135.7 ℃,产率57%, 4/1dr;1H NMRδ: 1.01(s, 9H), 1.15~1.19(m, 3H), 2.51(s, 3H), 2.74~2.82(m, 3H), 3.88~3.96(m, 2H), 4.46(d,J=16.0 Hz, 1H), 4.65(d,J=16.0 Hz, 1H), 5.06~5.11(m, 1H), 6.26(d,J=8.0 Hz, 1H), 6.66~6.68(m, 1H), 6.79~6.83(m, 1H), 6.94~6.98(m, 2H), 7.08~7.11(m, 3H), 7.18~7.25(m, 2H), 7.46(d,J=8.0 Hz, 1H), 7.97(d,J=8.0 Hz, 1H);13C NMRδ: 9.8, 18.7, 25.7, 26.6, 28.7, 42.6, 50.5, 60.5, 62.2, 80.6, 108.5, 114.6, 121.3, 122.4, 123.5, 124.0, 125.8, 126.3, 126.4, 127.7, 128.4, 129.1, 133.9, 139.2, 142.5, 158.9, 167.2, 169.1, 169.8, 172.8, 178.5; HR-MS(ESI-TOF)m/z: Calcd for C38H36N4O8Na{[M+Na]+}699.2425, Found: 699.2427。
3i: 灰白色固体,m.p.122.1~123.9 ℃,产率62%, 6/1dr;1H NMRδ: 1.02(s, 9H), 1.12~1.16(m, 3H), 2.05(s, 3H), 2.52(s, 3H), 2.52~2.83(m, 3H), 3.89~3.96(m, 2H), 4.41(d,J=16.0 Hz, 1H), 4.80(d,J=16.0 Hz, 1H), 5.09~5.13(m, 1H), 6.25(d,J=8.0 Hz, 1H), 6.62(d,J=7.2 Hz, 2H), 6.79~6.83(m, 1H), 6.94~7.01(m, 2H), 7.04~7.11(m, 3H), 7.24(d,J=7.2 Hz, 2H), 7.85(d,J=8.4 Hz, 1H);13C NMRδ: 9.6, 18.5, 19.8, 25.4, 26.4, 26.5, 28.4, 42.3, 42.4, 50.2, 60.2, 61.9, 80.3, 108.2, 114.2, 121.0, 122.0, 123.1, 123.2, 125.2, 126.0, 126.6, 127.3, 127.4, 128.6, 128.8, 133.4, 133.7, 136.6, 142.4, 158.6, 167.1, 168.7, 169.7, 172.5, 178.5; HR-MS(ESI-TOF)m/z: Calcd for C38H35N4O8FNa{[M+Na]+}717.2331, found 717.2334。
3j: 灰白色固体,m.p.103.1~104.5o℃,产率60%, 5/1dr;1H NMRδ: 1.11(s, 9H), 1.16~1.19(m, 3H), 2.54(s, 3H), 2.73~2.82(m, 3H), 3.88~3.92(m, 2H), 4.56(d,J=16.0 Hz, 1H), 4.68(d,J=7.6 Hz, 1H), 5.12~5.19(m, 1H), 6.32(d,J=8.0 Hz, 1H), 6.79~6.83(m, 2H), 6.95~7.00(m, 1H), 7.12~7.21(m, 6H), 7.46(s, 1H), 7.91(d,J=8.8 Hz, 1H);13C NMRδ: 9.0, 17.9, 24.8, 25.9, 27.9, 28.5, 41.5, 49.5, 59.4, 61.4, 80.3, 107.8, 115.1, 120.7, 121.0, 122.5, 124.6, 125.0, 125.7, 126.9, 127.6, 128.5, 128.8, 133.0, 136.8, 141.7, 158.1, 166.2, 168.0, 168.8, 171.8, 177.3; HR-MS(ESI-TOF)m/z: Calcd for C38H35N4O8ClNa{[M+Na]+}733.2036, found 733.2037。
2 结果与讨论
2. 1 合成
首先对催化剂进行筛选(表1),发现该反应在催化剂DABCO作用下,溶剂二氯甲烷中, 24 h内能反应完全,达到62%的产率,伴随少量副产物产生。在其它碱性催化剂作用下(DMAP, DBU, TEA或K2CO3),未见明显的产物生成[13-17]。
然后通过对底物扩展,发现该反应的活性普遍较高,在最优反应条件下, 24 h内基本能反应完全(TLC检测), 产率为57%~70%,dr值为4/1~9/1。

表1 反应条件的优化
2.2 化合物3f的单晶
在无水乙醇溶剂中对化合物3f进行了单晶培养,经XRD分析确证白色晶体3f(CCDC: 2045482)的结构。图1 为化合物3f的单晶结构图。由图1 分析可知,化合物3f属monoclinic晶系,P21/c空间群,晶胞参数a=9.8588(10) Å,b=16.3136(12) Å,c=19.8603(14) Å,α=90°,β=99.707(8)°,γ=90°。
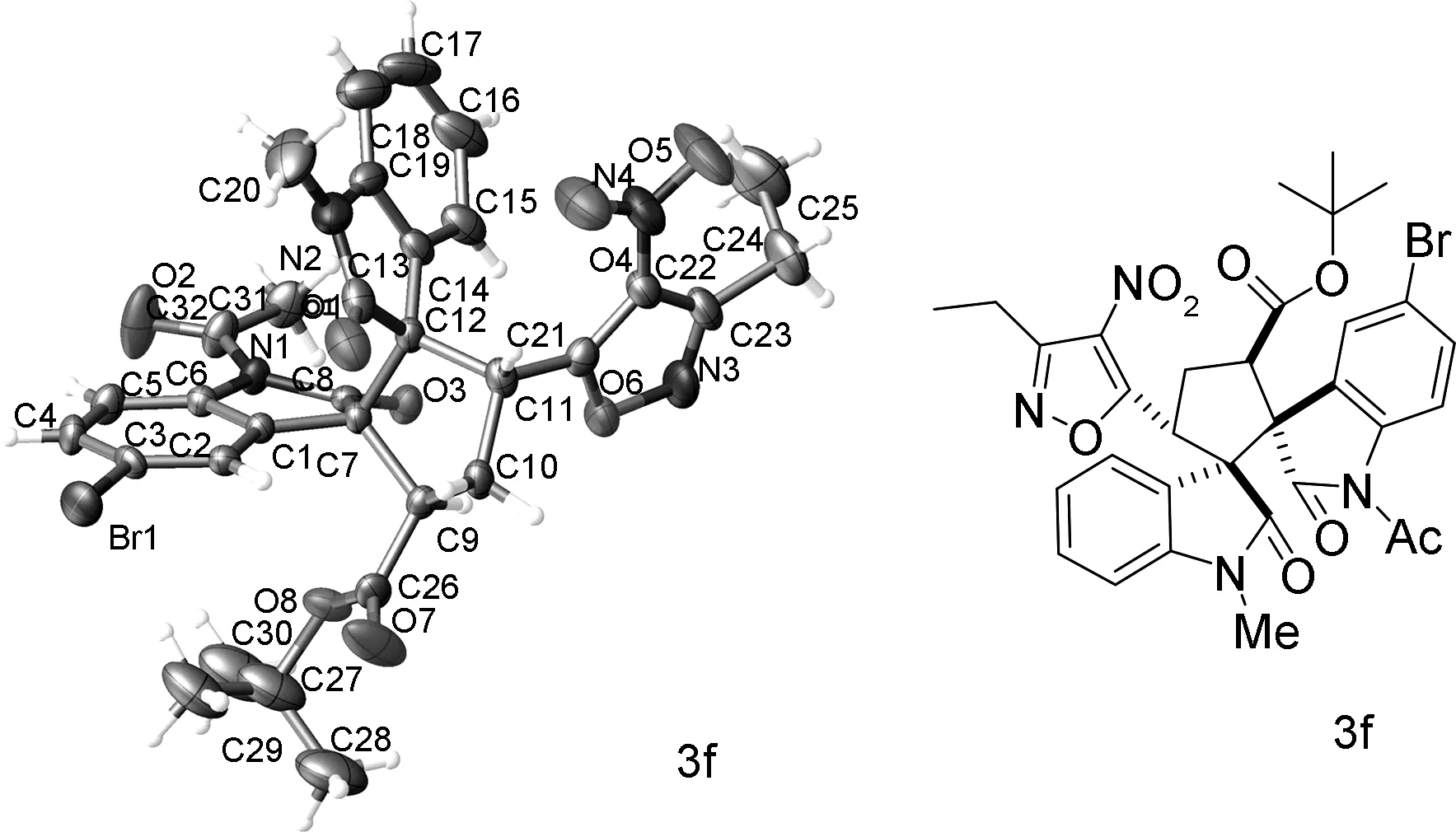
图1化合物3f的单晶结构
以硝基异噁唑-3-烯氧化吲哚1作为基于给体和受体的3C合成子,在碱性催化剂DABCO催化下,与叔丁基酯-3-烯氧化吲哚2发生Michael加成环化反应,获得10个新颖的螺环氧化吲哚拼接异噁唑类化合物3a~3j,产率为57%~70%,dr值为4/1~9/1,该化合物骨架含有连续两个螺环季碳中心,可以为生物活性筛选提供物质基础。

