Strategies to Improve the Stability of Perovskite-based Tandem Solar Cells
Wentao Zhou,Yihua Chen,Huanping Zhou
Beijing Key Laboratory for Theory and Technology of Advanced Battery Materials,Key Laboratory of Polymer Chemistry and Physics of Ministry of Education,BIC-ESAT,Department of Materials Science and Engineering,College of Engineering,Peking University,Beijing 100871,China.
Abstract:Organic-inorganic metal halide perovskite-based tandem solar cells have attracted significant research attention in recent years.The power conversion efficiency of perovskite-based tandem can efficiently meet the requirements of practical applications; however,their instability limits their commercialization.The most commonly used wide-bandgap perovskites suitable for top sub-cells,which are based on I/Br alloying at X site,often suffer from severe phase segregation.When exposed to light illumination,a smaller bandgap phase appears and acts as a carrier trap,leading to a reduction in the quasi-Fermi level splitting and large VOC deficit.The narrow-bandgap perovskites suitable for bottom sub-cells,which are based on Sn/Pb alloying at B sites,always face atmospheric instability.When exposed to air,Sn2+ is rapidly oxidized to Sn4+,which can shorten the carrier diffusion length and result in a drop in efficiency.Herein,we summarize the recent advances in perovskite-based tandem solar cells from the viewpoint of stability.We analyzed the stability data of highly efficient perovskite-based tandems reported so far,such as perovskite/silicon,perovskite/perovskite,and perovskite/copper indium gallium selenide (CIGS)tandems.We found that the key to improve the perovskite-based tandems is to improve the stability of the perovskite sub-cells.Then,we systematically analyzed the phase and atmospheric instability of wide- and narrow-bandgap perovskite,respectively,providing some reasonable strategies to tackle the instability.Compositional engineering,crystallinity optimization,and employing other perovskites with wide bandgaps are effective means to avoid phase instability of the I/Br alloying perovskite.Introducing the reducing additives,improving the film morphology,and forming a 2D/3D structure can help in improving the atmospheric stability of Sn-Pb narrow bandgap perovskites.Furthermore,we review the intrinsic instability of perovskite and corresponding improvement methods,which are inevitable in future tandem solar cells.By reducing the methylamine (MA)content in perovskite component and suppressing ion migration,the long-term operational stability is greatly enhanced.Finally,we briefly summarize the instability issues related to the interconnecting layer.In addition to the optimization of perovskite-based tandem devices,encapsulation also plays a crucial role in improving stability against environmental stressors.Studies based on improving the stability of perovskite-based tandems are still in the early stage.However,with a deeper understanding of the stability of perovskite sub-cells and the interconnecting layer,the commercialization of perovskite-based tandems,especially perovskite/silicon tandem devices,is promising to be achieved in the near future.
Key Words:Perovskite; Tandem solar cell; Stability; Wide bandgap; Narrow bandgap
1 Introduction
Solar cells collect sustainable and renewable solar radiation energy and convert it into electricity directly,which become potential alternatives for traditional fossil energy.In recent decades,photovoltaic technologies,mainly crystalline silicon solar cells,have experienced rapid development towards higher power conversion efficiency (PCE),lower manufacturing cost,and thus a gradually reduced levelized cost of electricity1.Nevertheless,photovoltaic (PV)technology only contributes a minor portion to the world electricity market share.Boosting the PCE of solar cells is indeed a dominant way to make PV more economically feasible.
According to Shockley-Queisser (S-Q)limit2,the maximum theoretical efficiency of a single-junction solar cell is about 33%.Multi-junction (e.g.tandem)solar cells can further mitigate thermal energy losses by combining two or more absorbers with different bandgaps.For a typical two-junction solar cell device,it consists of a wide bandgap top sub-cell absorbing high-energy photons,and a narrow bandgap bottom sub-cell absorbing remaining low-energy photons,which ultimately reduce energy loss due to thermalization.Based on detailed balance limit theory3,tandem solar cells could promote the PCEs to much higher level,depending on the number of absorbers employed.Currently,research efforts are focusing on increasing the number of active layers and optimizing them in desired ways.Specifically,tailoring their bandgaps and thicknesses with effective photon management4,5have shown effects to minimize spectral loss6,7.In addition,other functional layers within the device are carefully engineered to further increase the performance of tandem device.All these attempts lead to the highest PCE of the multijunction device with a PCE up to 47.1% with a concentrator and 39.2% without concentrator8.
In recently years,organic-inorganic hybrid perovskite materials,with a typical ABX3crystal structure,have attracted intensive attention when applied in thin-film solar cells due to its ideal optoelectronic properties,like high absorption coefficient and long carrier diffusion length.In this type perovskite,A site is usually occupied with CH3NH3+(MA+),HC(NH2)2+(FA+)or Cs+; B site is usually Pb2+or Sn2+; X site is halide ion such as I-,Br-and Cl-.Currently,PCE of perovskite-based single junction solar cells has reached up to 25.5%.Additionally,the ease of tuning its bandgap from 1.17 to ~2.8 eV via changing constituent components makes it suitable for absorbing layers in tandem solar cell9–11.On the bandgap regime of ~1.7–2.8 eV,it can be achieved by partial substitution of I-with Br-at X site12and/or replacing organic cations with inorganic cation Cs+at A site13.This makes perovskite suited for as absorbing layer in a wide bandgap top sub-cells to couple with other narrow bandgap absorber like silicon14,copper indium gallium selenide (CIGS)15organic photovoltaics (OPV)16,17,and narrow bandgap perovskites as well.
Two-terminal (2T)and four-terminal (4T)configurations are currently the mainstream of perovskite-based tandem photovoltaic devices.In the 2T configuration,the top sub-cell and bottom sub-cell are connected in series via an interconnecting layer,usually tunnel junction or recombination layer18.In the 4T configuration,top sub-cells and bottom subcells can be fabricated and optimized independently.In the past few years,the performance of perovskite/silicon tandem solar cell is rapidly improved since the first demonstration by Bailie et al.19,which mainly benefits from the solid foundation of silicon photovoltaic technology and rapid development of perovskite.In 2018,Oxford PV20developed a 1 cm2perovskite/silicon tandem device with certified efficiency of 28%.It is the first time that a perovskite/silicon hybrid device has edged out the best silicon single-junction device.Recently,McGehee et al.21reported a triple-halide perovskite with suppressed photoinduced phase segregation,which is combined with silicon bottom sub-cell achieving 27% efficiency in a twoterminal device.Although the great efficiency has been achieved by the perovskite-based tandem solar cells,the long-term stability issue still limited their further development.
Herein,we discussed and summarized progress and challenges on improving stability of perovskite-based tandem device,as illustrated in Fig.1.First,we summarized recent progress of stability of perovskite-based tandems.We focused on highly efficient perovskite/silicon tandems and perovskite/perovskite (all-perovskite)tandems reported in recent years,dedicated in reviewing their stability and corresponding test procedures,not the way to improve their efficiency.We then summarized the origin of critical instability problems of the perovskite sub-cells:phase instability of wide bandgap top subcell and atmospheric instability of narrow bandgap bottom subcell,and corresponding strategies to tackle them.What’s more,we briefly discussed the general intrinsic instability of perovskite materials,which could bring additional instability issue for perovskite-based tandems.We also briefly summarized the instability issues related with interconnecting layer.At last,we provided our prospects for the future development of stable perovskite-based tandem solar cells.

Fig.1 A schematic illustration of instability issues of perovskite-based tandem solar cell(Take perovskite/silicon and perovskite/perovskite tandems as examples).
2 Recent advances in stability of perovskitebased tandem solar cells
The key advantage of tandem solar cells is that they can theoretically achieve higher power conversion efficiency that single-junction cells cannot achieve.Recent research efforts are dedicated in improving efficiency of perovskite-based tandems in recent years through optical management,electrical loss reduction,etc.Although stability test for perovskite-based tandems is frequently conducted in most works that focus on improving efficiency,the study on improving stability of perovskite-based tandems are still in the early stage.Also,the progress on stability of perovskite-based tandems is lagging that of perovskite single-junction solar cells.In this section,we have summarized the recent advances of three mainstream perovskitebased tandem configurations:perovskite/silicon,perovskite/perovskite and perovskite/CIGS tandem solar cells,with more attention on their stability assessment and improvement.
2.1 Perovskite/silicon tandem solar cells
Perovskite/silicon tandem solar cell is the most attractive research direction among perovskite-based tandems,and its stability is continuously improving in recent years by enhancing the stability of wide bandgap perovskite top sub-cells.McGehee et al.22introduced a bilayer of SnO2and zinc tin oxide (ZTO)as interconnecting layer in perovskite/silicon tandem,realizing certified efficiency of 23.6% with 1-cm2area (Fig.2a–c).This method could effectively reduce parasitic absorption and improve carrier transport and also provide buffer function for wide bandgap Cs0.17FA0.83Pb(Br0.17I0.83)3perovskite.In addition,the dense and pin-hole free SnO2/ZTO bilayer was beneficial for improving stability of perovskite top sub-cell.The perovskite single-junction solar cell operated with minimal degradation in performance after ageing at maximum power point (MPP)for 1000 h without encapsulation.Then,perovskite sub-cells were encapsulated using sheets of glass with ethylene-vinyl acetate(EVA),by curing at a temperature of 140 °C for 20 min.The butyl rubber edge seal was also used to prevent device from moisture.After encapsulation,the devices were subjected to the damp heat test,according to the International Electrotechnical Commission (IEC)design qualification testing protocol 61215 for ‘Crystalline Silicon Terrestrial Photovoltaic (PV)Modules’.The perovskite devices passed damp heat test,which requires the performance of device drops within 10% in 1000 h when stored at 85 °C and 85% relative humidity.Recently,McGehee et al.21alloyed chlorine (Cl)into wide bandgap perovskite crystal lattice,achieving phase-stable triple halide (I/Br/Cl)perovskite.This method can suppress the light-induced phase segregation and increase photocarrier lifetime,enabling efficiency of 27% in 1-cm2two-terminal perovskite/silicon tandem.The semitransparent top sub-cell utilized triple-halide perovskite as absorber exhibited < 4% efficiency degradation after 1000 h MPP tracking at near 60 °C,as shown in Fig.2d,e.Ballif et al.23developed a deposition process that achieve the conformal growth of perovskite top sub-cell on micrometer-sized pyramids of textured monocrystalline silicon.The combination of conformal perovskite top sub-cell with textured silicon sub-cell can improve the light harvesting,exhibiting efficiency of 25.2%.The perovskite single-junction solar cell produced with the method presented in this work exhibited less than 10% efficiency loss after 1000 h damp heat tests at 85 °C and a RH of 85%.Kim et al.24introduced PEA(I0.25SCN0.75)as mixed anion additive in 1.68 eV bandgap perovskite,achieving a PCE of 20.7% in perovskite single-junction solar cell.They found that the addition of PEA(I0.25SCN0.75)could result in the formation of PbI2-based 2D phase located at surface and grain boundary.The wide bandgap perovskite solar cell with additive exhibited impressive light stability,which can remain 80% of initial efficiency after 1000 h of continuous illumination without capsulation.

Fig.2 (a)A schematic illustration of wide bandgap perovskite single-junctional semitransparent solar cell.(b)Efficiency (black),current density(JMPP,red)and voltage (VMPP,blue)of perovskite single-junction device with no encapsulation during 1000 h of continuous MPP tracking.(c)Efficiency (black),JMPP (red)and VMPP (blue)of encapsulated with EVA,glass,and a butyl rubber edge seal during damp heat testing.(d)A schematic illustration of top-illuminated semitransparent perovskite device.(e)Normalized PCE during MPP tracking under accelerated conditions (0.77-sun illumination and 60 °C)of 1-cm2 semitransparent (red,tested in N2,averaged from two devices in the same batch)and opaque devices (blue,tested in air with relative humidity of ~30%).(f)A schematic illustration of solution-processed perovskite/textured silicon tandem device.a-Si:H(n),n-doped hydrogenated amorphous silicon; a-Si:H(i),intrinsic hydrogenated amorphous silicon; a-Si:H(p),p-doped hydrogenated amorphous silicon.(g)J–V curves of tandem device without encapsulation at the beginning and the end of the MPP tracking test.(h)J–V parameters measured over >400 h of MPP tracking at 40 °C.
The operational stability test for tandem devices is undoubtedly stricter than that of wide bandgap perovskite top sub-cells.Relatively less research work have reported the stability of the whole tandem device.In the work by Ballif et al.23,operational stability for perovskite/silicon tandem device at MPP conditions under continuous AM 1.5G illumination without any ultraviolet-blocking filter was conducted.The tandem device can retain MPP output efficiency of 22% after 61 h.Sargent et al.25combined solution-processed perovskite top sub-cells with fully textured silicon heterojunction bottom subcells,realizing certified efficiency of 25.7% for perovskite silicon tandem.The highly efficient tandem device also exhibited impressive operational stability.The tandem devices encapsulated using glass and polyolefin encapsulate (POE)retained their original efficiency after ageing at MPP for 400 h,as shown in Fig.2f–h.The operational stability test was set at 40 °C and ~40% to 50% relative humidity using 1-sunequivalent illumination.The authors attributed this excellent stability to the replacement of organic hole transport layer with inorganic NiOX.Huang et al.14developed a blade-coating method for conformal deposition of hole transport layer and planarizing perovskite layer on textured silicon sub-cell.The unencapsulated perovskite/silicon tandem device remained 92% of its initial efficiency after constant illumination for 100 h in ambient atmosphere.
2.2 Perovskite/perovskite tandem solar cells
Improving stability of narrow bandgap perovskite sub-cell is crucial for perovskite/perovskite tandem solar cell.Alloying Sn and Pb at B site of perovskite is the commonly used method to achieve bandgap suited for bottom perovskite sub-cell.The facile oxidation of Sn2+is the major obstacle for Sn-Pb perovskite,which will be discussed in detail later in this review.By optimizing the narrow bandgap perovskite sub-cell,stability of all-perovskite tandem is gradually improved.Yan et al.26incorporated PbCl2as additive to enlarge the grain size and reduce the electronic disorder of Sn-Pb perovskite.The 2T allperovskite tandem was constructed by combining 1.75 eV perovskite top sub-cell with 2.5% Cl-incorporated 1.25 eV perovskite bottom sub-cell.The tandem device could retain 85% of its initial efficiency after MPP tracking in air for 80 h.The VOCof tandem device remained steady under a continuous 1 sun illumination for over 200 s.In addition,an encapsulated tandem device retained 94% of its initial efficiency after storage in ambient air for 45 days.Later in 2019,Yan et al.27found that the addition of guanidinium thiocyanate (GuaSCN)in Sn-Pb perovskite can improve the efficiency and stability of Sn-Pb perovskite sub-cell and corresponding all-perovskite tandem device,as shown in Fig.3a,b.Introducing GuaSCN can result in the formation of 2D structure at grain boundaries,reducing defects states in grains and grain boundary/surface regions.The operational stability tests were conducted for 1.25 eV narrow bandgap perovskite and corresponding 4T and 2T tandem device.The narrow bandgap bottom sub-cell was encapsulated with cover glass and ultraviolet-curable epoxy before testing.It showed approximately a linear degradation over time at the first 115 h of MPP tracking:~0.09% per hour; and ~0.05% per hour for the 4T tandem device.The 2T device can retain more than 88% of initial efficiency after 100 h of continuous MPP tracking.Huang et al.28added cadmium ions (Cd2+)in Sn-Pb perovskite precursor.The addition of Cd2+can de-dope narrow bandgap Sn-Pb perovskite and reduce electron trap density,which play a positive role in improving stability of corresponding 2T monolithic all-perovskite tandem device.The tandem device can maintain 91.8% of their initial efficiency under 1 sun illuminetion for 200 h.Tan et al.29introduced metallic Sn powder in Sn-Pb perovskite precursor and optimize interconnecting layer,achieving the highest efficiency of 2T all-perovskite tandem device reported so far.The tandem solar cell also exhibited promising operational stability,maintaining 90% of their initial efficiency after 463 h of MPP tracking under full 1-sun illumination,as shown in Fig.3c,d.Recently,Tan et al.30found that introducing bulky organic cation phenethylammonium(PEA)to passivate Sn-Pb perovskite is beneficial for improving operational stability of perovskite bottom sub-cell.The PEA-passivated Sn-Pb perovskite exhibited T90(the time at which PCE degrades to 90% of the initial value)of 8.6 h.The corresponding 2T tandem device retained 95% of their efficiency after 65 h of operation at MPP point.

Fig.3 (a)MPP tracking of wide bandgap top perovskite sub-cell,the filtered bottom sub-cell,and 4T tandem device under simulated AM 1.5G one-sun illumination.(b)SPO efficiency and MPP tracking (inset)of the 2T tandem cell under one-sun illumination.(c)A schematic illustration of monolithic 2T all-perovskite device.(d)MPP tracking of 2T tandem device under full AM 1.5G illumination.(e)A schematic illustration of tandem devices based on typical structured ICLs of C60/SnO2−x/ITO/PEDOT:PSS and simplified ICLs of C60/SnO2−x (BCP,bathocuproine; PTAA,poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine]).(f)Long-term photostability of tandem device based on C60/SnO1.76 ICLs measured under continuous simulated AM1.5G illumination for 1000 h at room temperature.
Optimizing interconnection layer in 2T tandem device is also helpful to ensure both high efficiency and stability.Huang et al.31demonstrated a simplified interconnection layer only composed of C60and SnO2−x,ensuring the high efficiency and photostability of 2T all-perovskite tandem under illumination.Compared to a complicated interconnection layer (ICL)in most existing all-perovskite tandems,the simplified interconnection structure C60/SnO2−xcan reduce the sputtering process damage for underlying functional layers and improve stability of tandem device (Fig.3e).The 2T tandem device was constructed by combining 1.78 eV perovskite Cs0.4FA0.6PbI1.95Br1.05with 1.21 eV perovskite Cs0.05MA0.45FA0.5Pb0.5Sn0.5I3.The tandem device remained 94% of initial efficiency under 1-sun illumination after 1000 h,as shown in Fig.3f.The improved stability could be attributed to multiple factors:more Cs in 1.78 eV perovskite for suppressed phase segregation; less Sn in 1.21 eV perovskite for suppressed oxidation; more stable buffer layer SnO1.76compared to poly (3,4-ethylenedioxythiophene)-poly(styrenesulfonate)(PEDOT:PSS)for bottom sub-cell.The phase instability of wide bandgap perovskite and atmospheric instability of narrow bandgap perovskite will be discussed later.
2.3 Perovskite/CIGS tandem solar cells
Perovskite and CIGS photoactive materials both possess tunable bandgaps,which provides the potential of combining two sub-cells into a tandem solar cell with respectable efficiency.In recent years,through optimization of the perovskite sub-cells,stability of perovskite/CIGS solar cells has been gradually improved.Yang et al.32deposited a transparent metal oxide layer composed of zinc oxide (ZnO)nanoparticles and indium tin oxide (ITO)on the top of perovskite/CIGS tandem device,allowing for sufficient light transmission while improving operational stability.They proposed that the deposited metal oxide layer is dense to be resistant against moisture.The unencapsulated perovskite/CIGS tandem device remained >88% of its initial efficiency (started with 22.0% efficiency)after 500 h of continuous MPP tracking at 30 °C ambient environment.Amran et al.33replaced poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA),a hole transport layer in perovskite top sub-cell,with self-assembled monolayers (SAM)based on carbazole bodies with phosphonic acid anchoring groups.They argued that SAM layer could provide a stronger hole-selectivity and can be conformally deposited on rough surface of the as-deposited CIGSe bottom sub-cell.The SAM-based perovskite single single-junction perovskite device showed better operational stability under MPP tracking at simulated 1 sun.The time evolving VOCof single-junction perovskite device was also tracking at open-circuit condition under light soaking at 1 sun illumination.SAM-based device rendered virtually stable VOC,while VOCof PTAA-based device showed substantial drop after 2 h.It is inferred that SAM was chemically robust with virtually no volume,which could avoid structural damage caused by ion migration from perovskite to the SAM/perovskite interface.
3 Phase instability of wide bandgap perovskite
As discussed above,tandem solar cells usually consist of two sub-cells with different but suitable bandgaps.This allows tandems to mitigate energy losses compared to single-junctional device and have potential to exceed S-Q efficiency limit.The partial replacement of constituent components at X sites in perovskite absorber from I‒to Br‒/Cl‒will bring about an enlargement in the bandgap,which have been widely investigated in numerous literatures9,34–36.However,the common wide bandgap perovskite absorber,which based on the I/Br component,often suffers from the severe phase segregation and followed huge VOCdeficit,limiting the commercial development of perovskite-based tandem solar cells.In this section,we discussed the origin of phase instability in wide bandgap perovskite,and comprehensively reviewed the possible improvement strategies,including compositional engineering,crystallinity optimization and employing other perovskite with wide bandgap.
3.1 Phase segregation and underlying mechanism
Alloying iodide and bromide at X site makes wide bandgap perovskite vulnerable in phase stability,reflecting in the serious phase segregation under illumination36,37.Hoke et al.38firstly observed the light-induced phase segregation in MAPb(I1−xBrx)3(Fig.4a–c),which could cause the formation of smaller bandgap phase acting as traps.The smaller bandgap phase was consistent with the iodine-rich phase.Photoinduced carriers will be trapped into low bandgap phase,leading to the reduction in quasi-Fermi level splitting and large VOCdeficit.Phase segregation phenomenon in mixed halide perovskite,also called “Hoke effect”,was subsequently confirmed by other groups39.The mechanism behind phase segregation has attracted intensive research interests.Brivio et al.40carried out density functional theory (DFT)simulation to understand thermodynamic origin of light-induced instability of MAPb(I1−xBrx)3.They calculated the thermodynamic total energy and entropy of the mixing composition and built the phase diagram based on the calculated Helmholtz free energy.They suggested that the phase diagram could explain the miscibility gap at 300 K.Bischak et al.41employed cathodoluminescence (CL)imaging combined with molecular simulations to understand the dynamics of halide phase segregation,as shown in Fig.4d–e.It was observed that photoinduced electron-hole pairs would rapidly dissociate and create free electrons and holes,which can deform the surrounding lattice via electron–phonon coupling.Local strain caused by the spatial overlap between the deformed lattice and a single-charge density could result in local phase segregation.This finding suggests that the substantial photoinduced carriers play an important role on phase instability of mixed halide perovskite.In addition,Barker et al.42believed that halide defects act as low-energy migration pathways for halide ions and promote halide segregation.Halide migration process is driven by the internal gradient of photoinduced carrier generation rate via strain gradient in lattice.

Fig.4 (a)PL spectra of an MAPb(BrxI1−x)3,x=0.4 perovskite film over 45 s in 5 s increments under 457 nm,15 mW·cm−2 light at 300 K.Inset:temperature dependence of initial PL growth rate.(b)Normalized PL spectra of MAPb(BrxI1−x)3 perovskite films after illuminating for 5–10 min with 10–100 mW·cm−2,457 nm light.(c)PL spectra of x = 0.6 perovskite film after sequential cycles of illumination for 2 min(457 nm,15 mW·cm−2)followed by 5 min in the dark.(d,e)Duty cycle for CL image series for perovskite film.The scale bars are 2 μm.
3.2 Compositional engineering
Compositional engineering is a simple but effective method to improve the phase stability of wide bandgap perovskite.Snaith et al.43demonstrated the photostable mixed halide perovskite FA0.83Cs0.17Pb(I0.6Br0.4)3with 1.74 eV bandgap via partially replacing FA+with Cs+in thermally stable FAPbI3perovskite.As shown in Fig.5a,the composition-optimized perovskite showed no significant red shift in photoluminescence (PL)emission after light exposure,which indicated an enhanced phase stability compared to MAPb(I1−xBrx)3reported by Hoke and coworkers38.The semitransparent solar cell using FA0.83Cs0.17Pb(I0.6Br0.4)3as absorber delivered stabilized power output of 12.5%.Duong et al.44have shown that employing four cations (Rb,Cs,FA and MA)in perovskite is beneficial for improving photostability.Perovskite with quadruple cation exhibited 95% efficiency after 12 h of continuous one-sun illumination.It is worth noting that this report investigated the cell performance under working condition instead of the red shift in PL emission which has milder illumination intensity.McGehee et al.45believed that raising Cs fraction is beneficial for reducing the reliance on incorporating high fraction of Br to obtain bandgap required for top sub-cell.The reduced Br fraction results in better resistance against halide segregation.As shown in Fig.5b,1.68 eV (25Cs/20Br)and 1.75 eV (40Cs/30Br)bandgap perovskites with more Cs and lower Br only showed slightly peak shift in PL measurement after excitation.Recently,McGehee et al.23incorporated MAPbCl3in crystal lattice to achieve stable triple-halide perovskite (I,Br,Cl).Different from other reports using MACl or PbCl2as additive46,47,they found that FA0.75Cs0.25-perovskite with higher Br fraction can accommodate higher content of Cl uniformly distributed in crystal lattice.Incorporating of Cl could tune the bandgap of triple-halide perovskite to 1.67 eV with Br fraction limited to 15%.Optimized triple-halide perovskite is more photostable upon illumination which have proved via PL measurement,as shown in Fig.5c.Through further optimization,a large-area semitransparent device (active area = 1 cm2)exhibited stabilized 16.83% efficiency in an 11-point stabilized power output (SPO)test measured in National Renewable Energy Laboratory PV Device Performance laboratory.Apart from Cs and Cl,DMA(dimethylammonium)was capable of reducing the reliance of increasing bandgap on increasing Br fraction48.

Fig.5 (a)PL spectral measured after 0,5,15,30 and 60 min of light exposure of MAPb(I0.6Br0.4)and FA0.83Cs0.17Pb(I0.6Br0.4).(b)PL spectra of 1.68 eV bandgap perovskite (25Cs/20Br)and 1.75 eV bandgap perovskite (40Cs/30Br)after excitation.The yellow line:initial PL;the blue line:0.1 sun for 10 min; the green line:1 sun for 10 min; the red line:10 sun for 10 min.(c)The shift of the PL spectral centroids of triple-halide perovskites (I-,Br- and Cl-)over time under 1-sun,10-sun and 100-sun.
3.3 Crystallinity optimization
In addition,improving crystallinity of mixed-halide perovskite film is also beneficial for improving their phase stability.Hu et al.49found that MAPb(Br0.27I0.73)3perovskite films deposited on non-wetting PTAA hole transport layer owned better phase stability,as compared to those of perovskite films deposited on PEDOT:PSS layer.They attributed the better photostability of perovskite films to better crystallinity and larger grains due to the hydrophobic nature of PTAA surface.According to other works,the reduced density of grain boundaries is beneficial for suppressing halide segregation39,41,50,which could explain why increasing crystallinity is effective.Hillhouse et al.51found that improving crystallinity and grain size of (FA0.83Cs0.17)Pb(I0.66Br0.34)3by tailoring annealing process can improve its resistance to light-induced phase separation.Perovskite annealed at 160 °C for 50 min is more stable against light illumination compared to perovskite annealed at 75 °C for 10 min.After illuminated for 5 min under illumination with Newport AAA Solar Simulator,perovskite annealed at higher temperature showed no peak shift in PL spectra.
3.4 Employing other perovskite with wide bandgap
Although I/Br hybrid perovskite absorber is the most popular component to fabricate wide bandgap sub-cell and construct tandem solar cells.It’s not the only appropriate component.The easiest strategy to solve the phase segregation issue is replacing I/Br hybrid perovskite absorber with other type of perovskite materials which also have wide bandgap.
The first option worth considering for top sub-cell in tandem configuration is all-inorganic halide perovskite.Cesium-based perovskites:CsPbI3,CsPbIxBr1−x,etc.,have been emerging as absorbing layer in all-inorganic perovskite photovoltaics.It has been reported that cesium-based perovskites exhibit better resistance against high temperature and light soaking52–55.Especially,the phase segregation is absent in the CsPbI3perovskite.In addition,α-CsPbI3has a proper bandgap of 1.73 eV with gradually improved efficiency56,57.This provides a promising pathway for tandem device combined with silicon or other small bandgap semiconductors.However,the photoactive phase α-CsPbI3can spontaneously convert into non-photoactive δ-phase (also called yellow phase)in ambient air,especially with moisture58.The δ-phase is thermodynamically stable at room temperature,while black phases (α,β and γ)are metastable59,60.Although the moisture induced phase transition issue bring about the difficulties for the employment of all-inorganic halide perovskite in tandem solar cells.Numerous works with regard to all-inorganic perovskite single junction devices have focused on addressing this concern and achieved the remarkable results61–69,which could guide the fast development of tandem solar cells based on the all-inorganic halide perovskite with wide bandgap.
The second potential option to take the place of I/Br hybrid perovskite absorber is double perovskite.Apart from alloying I with Br at X site and replacing organic cations with Cs+,replacing Pb at B site with Sb or Bi to construct Sb- or Bi-based perovskites can also enlarge the bandgap.The chemical formula of perovskite with incorporating Sb or Bi at B site is A3B2X9,named double perovskite,which contains a monovalent and a trivalent metal ion at B site to maintain the charge neutrality.Slavney et al.70demonstrated Cs2AgBiBr6as light absorber with direct bandgap of 1.95 eV and superior thermal and light stability.However,due to the existence of deep-level defects and corresponding carrier recombination,solar cells based on these types of perovskite exhibited poor photovoltaic performance71,72,which severely limit further development of high-efficiency and stable double perovskite-based tandem solar cells.
Thirdly,the low dimensional perovskite absorber also presented the wide bandgap characteristic by introducing the bulky organic cations,like butylammonium (BA),phenethylammonium (PEA)or allylammonium (ALA).Their formula of R2An‒1BnX3n‒1is mainly determined by the stoichiometry of organic cations in perovskites,wherein the n represents the layer number of the [PbI6]2‒octahedral lattice.The quantum well and dielectric confinement effects,which was originated from the bulky organic cations,significantly influence their optoelectronic properties.Especially,the bandgap could be adjusted from 1.65 to ~2 eV continuously by altering the stoichiometry and n value in the low dimensional perovskite.Meanwhile,different from I/Br hybrid perovskite absorbers,low dimensional perovskites do not suffer from the phase segregation under illumination.Also they always exhibited improved moisture resistance due to the hydrophobicity of bulky organic cations.Thus,it’s promising to employ low dimensional wide bandgap perovskite into tandem solar cells and accelerate the realization of high-efficiency and stable photovoltaic tandem devices.
4 Atmospheric instability of narrow bandgap perovskite
The unique advantage of hybrid halide perovskite materials is not only to obtain wide bandgap perovskite by alloying iodine and bromide at X site,but also to realize narrow bandgap perovskite by alloying lead (Pb)and tin (Sn)at B site.The proper narrow bandgap of Sn-Pb mixed perovskite and rapidly growing efficiency of corresponding solar cell enable the success of allperovskite tandem photovoltaic device6,26,73with both high efficiency and low levelized cost of electricity74.However,atmospheric stability of Sn-Pb perovskite is always concerned and hampers their further development.In this section,we discussed the in-depth mechanism of atmospheric instability in narrow bandgap perovskite and systematically reviewed the optimization strategies,including introducing reducing additives,improving film morphology and forming 2D/3D structure.
4.1 Atmospheric instability and underlying mechanism
The major obstacle of narrow bandgap Sn-Pb perovskites is their atmospheric instability.Kanatzidis et al.75firstly demonstrated the synthesis of a series of Sn-Pb mixed perovskites and conducted study on their structural,electrical and optical properties.The UV-Vis spectra showed that MASn1–xPbxI3possessed anomalous narrowing bandgap,even as low as 1.1 eV.Sn/Pb alloying enables perovskite exhibit bandgap bowing effect instead of Vegard’ law.Take MASn1–xPbxI3as example,it possesses narrower bandgap with specific composition compared to neat Sn (1.3 eV)or neat Pb (1.55 eV)perovskite76,77.A similar trend was found by Liao et al.77in (FASnI3)1−x(MAPbI3)xperovskite composition.When x = 0.4,(FASnI3)0.6(MAPbI3)0.4exhibit bandgap value of 1.25 eV,which is derived from the PL emission peak of 1000 nm.They also fabricated solar cell using this type as absorber with champion efficiency of 15.08%.However,when exposed to air,the performance of Sn-Pb mixed perovskite solar cells rapidly decline.The main reason is that Sn2+is inevitably oxidized to Sn4+when composed to oxygen,both in raw materials and in Sn-Pb perovskite.Oxidation of Sn2+would result in the formation of VSnand corresponding high background hole density,which may shorten carrier lifetime and carrier diffusion length75,78.In addition,VSnis regarded as the most popular intrinsic defect in CsSnI3due to its low formation energy,even in the absence of oxygen,as calculated by Kanatzidis et al.79.Thus,the major obstacle towards stability of Sn-Pb mixed perovskite is suppressing the formation of VSnduring device fabrication and operation.
4.2 Introducing reducing additives
Reducing additives are universally employed in Sn-Pb perovskite to improve atmospheric stability through reducing intrinsic VSntrap density and suppressing the oxidation of Sn2+in initial film fabrication and the following working operation.The improvement of atmospheric stability is often accompanied by the improvement of the PCE.Many researchers focused on how to reduce the concentration of VSnin Sn-Pb perovskite absorber.Kumar et al.80demonstrated SnF2as additive to reduce the background carrier density in CsSnI3.According to their thermodynamic analysis,SnF2increased the chemical potential of Sn (µSn)and the corresponding formation energy of VSn,thus decreasing trap density.The reduced density of VSnwas beneficial for improving both atmospheric stability and operational stability29.In recent years,SnF2is commonly used in achieving highly efficient and stable low bandgap Sn-Pb perovskite solar cells81.Lin et al.29used Sn powder as additive to reduce Sn4+and corresponding VSnin Sn-Pb perovskite precursor,as shown in Fig.6a,b.By using this method,they obtained 24.8% efficient all-perovskite tandem solar cell with impressive operation stability.Ascorbic acid was also reported by Xu et al.82to improve the efficiency and stability of Sn-Pb perovskite.They found that ascorbic acid (AA)can effectively retard the oxidation of Sn2+in perovskite precursor when exposed to air and form adduct with SnI2via Lewis acid-base interaction,which reduced the trap density and improve the film morphology.The Sn-Pb perovskite solar cells,which was processed with AA additive,exhibited better storage stability in N2-filled glovebox.Some additives were reported to improve atmospheric stability of neat-Sn perovskite such as FASnI383,84via coordination interaction with Sn2+,antioxidant function,etc.We speculate that these additives are also effective to achieve low bandgap Sn-Pb perovskite with better atmospheric stability.

Fig.6 (a)A photograph of Sn-Pb precursor with and without Sn powders as additives when exposed in air.The color change from brightyellow to orange indicates the oxidation of Sn2+ to Sn4+.(b)A schematic illustration of the mechanism of reduced Sn vacancies introducing Sn powders.(c)The normalized d.c.conductance of FA0.75Cs0.25Sn0.4Pb0.6I3 films of different film compactness and size over time heated at 85 °C in air.The initial values of the conductance are denoted as σ0 in the legend.Cross-sectional SEM images of small-grained (d)and large-grained(e)perovskite devices (on bare ITO without hole transport layer)after ageing in air at 85 °C for 500 h.
4.3 Improving film morphology
Improving film morphology is also effective for improving atmospheric stability.Sn-Pb perovskites with better compactness and larger grain size own better resistance to oxygen and thus better atmospheric stability.Prasanna and coworkers85compared the stability of FA0.75Cs0.25Sn0.4Pb0.6I3perovskite films with small-grained and large-grained domains.The largegrained perovskite film exhibited larger grain size in average and reduced boundary density.They conducted lateral d.c.conductance measurement of perovskite heated at 85 °C in air.The conductance of both films first increased and then dropped at long ageing times.The small-grained perovskite film showed a greater decreased rate in conductivity compared to that of large-grained film.They attributed this phenomenon to different oxidation rates of Sn2+in perovskite film.At the beginning,partial oxidation of perovskite will lead the formation of high density of compensating holes,which could cause the increase in conductivity75,79.As time goes on,more and more tin dioxide(SnO2)appeared at grain boundary according to their observation from x-ray photoelectron spectra (XPS)spectrum.The larger amounts of insulating SnO2,attributed to the faster oxidation at wider and more frequent boundaries in smallgrained film,resulted in a faster drop in conductance (Fig.6c–e).This suggested that better atmospheric stability could be achieved in Sn-Pb perovskite with improved film morphology.
4.4 Forming 2D/3D structure
In addition,the combination of two-dimensional (2D)and three-dimensional (3D)perovskite to form 2D/3D structure is beneficial for improving atmospheric stability of Sn-Pb perovskite solar cells.Layered perovskite at grain boundaries could effectively hinder the diffusion of oxygen into crystal lattice,suggesting the better atmospheric and operation stability86.Tong et al.27used guanidinium thiocyanate (GuaSCN)as additive to improve the efficiency and stability of Sn-Pb perovskite solar cells.They found that 2D perovskite structure was formed at grain boundaries with the addition of GuaSCN,which could hinder the oxygen diffusion into crystal lattice to suppress the oxidation of Sn2+and block the diffusion of Sn out of grains.The carrier lifetime of perovskite film was increased from ~139 to ~1232 ns after adding 7% GuaSCN,as shown in Fig.7a,which indicated the suppressed carrier recombination.The GuaSCN-modified Sn-Pb perovskite solar cells and corresponding 4T tandem solar cell exhibited impressive operation stability at MPP tracking.Recently,Wei et al.30introduced the low concentration of phenethylammonium iodide(PEAI)in antisolvent to fabricate 1.25 eV Sn-Pb perovskite solar cells with good operation stability.The addition of PEAI formed ultrathin layered perovskite at film surface,which inhibited the oxidation of Sn,as proved by the XPS of Sn 3d shown in Fig.7b.The Sn4+signal was obviously reduced.The TRPL characterization,as shown in Fig.7c,indicated that PEAI-modified perovskite exhibited enhanced carrier lifetime.The PCE of Sn-Pb perovskite solar cells with the addition of PEAI dropped to 90% after 8.6 h at MPP tracking,which exhibited 200-fold enhancement compared to reference device.The 2T tandem device remained 95% of initial efficiency after 65 h at MPP tracking.
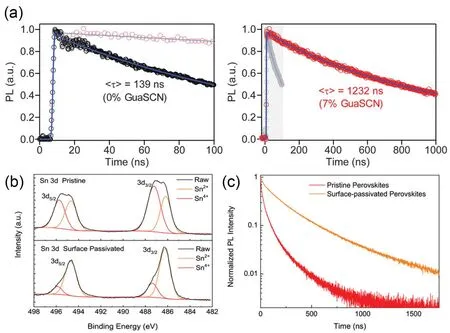
Fig.7 (a)Carrier lifetime measured by TRPL of perovskite films without and with GuaSCN.(b)The XPS spectra of Sn 3d of pristine and surface passivated perovskite film.(c)The TRPL decay of perovskite films probed from top side of samples.
5 Intrinsic instability of perovskite
Due to the strict requirements of tandem solar cells,the most popular composition of perovskite sub-cells in tandem solar cells is I-Br alloying perovskite for wide bandgap,and Sn-Pb alloying perovskite for narrow bandgap.Researchers are thus focusing on the most concerning stability issue of these sub-cells,namely the phase instability and atmospheric instability.In addition to these apparent issues,owing to relatively soft ionic crystal structure,the hybrid halide perovskite exhibited intrinsic instability,which is also one of the main obstacles lying at the approach towards the commercialization of perovskite-based tandem solar cells87.In this section,we discussed the intrinsic instability of perovskite materials,with the focus on the moisture and thermal instability and ion migration.These two issues have not been investigated carefully in perovskite-based tandem solar cells yet,but they need to be addressed well.
5.1 Moisture and thermal instability related with MA components of perovskite
The widely employed cation,namely methylammonium(CH3NH3,MA),at A site in I-Br alloying perovskite or Sn-Pb alloying perovskite absorbers,is reported to bring about various and serious instability problems.First,its hygroscopicity makes lead perovskite to absorb water and form hydrate(CH3NH3)4PbI6·2H2O88when exposed to humid environment and may further degradation89.In addition,CH3NH3I will decompose into CH3I and NH3via so-called reverse Menshutkin reaction under thermal treatment90.Low formation energy of MAPbI3,as calculated by DFT,was also reported to be responsible for the instability when subjected to heat and light illumination91,92.According to IEC standards,perovskite materials must undergo thermal stability test at 85 °C when integrating with silicon to make tandem devices.Therefore,many works have been reported to improve stability of MA-based perovskite.Researchers found that replacing MA with formamidinium (CH(NH2)2,FA)cation or inorganic cations such as cesium (Cs)and rubidium (Rb)can effectively improve the intrinsic thermal stability of perovskite93,94,as shown in Fig.8a.Therefore,double-cation or triple-cation perovskite systems such as FAMA,FACs and FAMACs were developed to achieve better chemical stability.Lee et al.95reported FA0.9Cs0.1PbI3perovskite as an alternative to FAPbI3and MAPbI3with 19.0% efficiency.They also found that FA0.9Cs0.1PbI3was more robust against light illumination and humidity.Saliba et al.93discovered RbxCsyFA(100–x–y)PbI3perovskite without MA showed improved long-term stability.They also used polymer to passivate interface between the charge transport layer and perovskite,reaching stabilized efficiency of 20.35% and a small loss after ageing at maximum power point (MPP)for 1000 h.From the discussion above,we consider that to construct the stable perovskite-based tandem solar cells,the component of MA needs to reduce as much as possible to improve the intrinsic thermal stability.

Fig.8 (a)A schematic illustration of the structure of MA and thermally more stable Rb,Cs and FA.(b)A schematic illustration of hydrogen bond between halogen and MA/FA,and ionic bond between halogen and lead.(c)A proposed mechanism diagram of continuous elimination of Pb0 and I0 and regeneration of Eu3+-Eu2+ ion pair.
5.2 Ion migration related with defects of perovskite
It’s widely accepted that defect states in perovskite can seriously affect long-term stability of corresponding devices,even in tandem solar cells96–101.Unlike other covalently bonded stable semiconductor materials such as silicon and germanium,constituting ions of perovskite can migrate within the film or even cross the interface.This phenomenon is aggravated when defect density is high102,103,because defects always act as channels to facilitate ion migration104.The ion-migration behavior has been considered as the main course of J–V hysteresis and have profound effects on long-term stability of perovskite-based photovoltaic devices105,106.Thus,eliminating defects and suppressing ion migration play an important role in improving the working stability of perovskite-based tandem solar cells.Li et al.107proposed a novel method to passivate organic cation and lead vacancies via chemical bond modulation using Sodium fluoride (NaF).As shown in Fig.8b,the fluoride ion interacted with both organic cations via both hydrogen bonds,and lead via strong ionic bond,which effectively suppresses ion migration.The corresponding device exhibited excellent long-term stability,remaining 90% of original efficiency after 1000 h of operation test under MPP tracking.In addition,some defects will form spontaneously in perovskite layer during ageing process of device and do harm to long-term stability.For example,Pb2+and I-ions are chemically reactive to be easily converted into Pb0and I0defects respectively108.This process will severely decompose the perovskite layer and decline the performance of device during operation.Eliminating these defects is beneficial for improving long-term stability.Wang et al.108introduced the “Eu3+-Eu2+” ion pair as redox shuttle into Pb-I perovskite to simultaneously eliminate both Pb0and I0defects.As shown in Fig.8c,they found that Eu3+could oxidize Pb0to Pb2+,while the reduction product of Eu2+could reduce I0to I-and be regenerated into Eu3+itself.This method can eliminate Pb0and I0simultaneously in a cyclical transition.The full devices with Eu3+incorporated exhibited impressive performance and stability,maintaining 91% of initial PCE after 500 h MPP tracking.Other methods such as incorporating low dimensional perovskite structures to form 2D/3D hybrid structure109–111,enlarging the grain size in polycrystalline perovskite film and improving crystallinity via additives (e.g.,chlorine46–47,112–113)or solvent annealing114–118method are also effective for suppressing ion migration.
6 Instability related to interconnecting layer
In a typical 2T configuration,two sub-cells are electrically and mechanically connected by the interconnecting layer,which can be classified into two types:recombination layer and tunnel junction.The recombination layer,usually transparent electrode such as ITO,possesses excellent electrical conductivity and transmittance.Tunnel junction is composed of heavily n-doped and p-doped layer,facilitating electrons and holes recombination.In addition to the instability of perovskite subcells,the instability of interconnecting layers are also crucial to perovskite-based tandem solar cell.In this section,we briefly summarized the instability caused by interconnecting layer,including the instability caused by fabrication process and interfacial reaction between interconnecting layer and perovskite.
6.1 Instability caused by fabrication process of interconnecting layer
The recombination layer is widely used in perovskite/perovskite tandem configuration,which usually contains an inorganic transparent electrode such as sputtered ITO or an organic polymer layer such as PEDOT:PSS.In the process of sputtering ITO,high temperature and the sputter particles with high energy could cause a severe damage to the perovskite sub-cell underneath.To solve this problem,McGehee et al.119introduced aluminum doped ZnO (AZO)nanoparticles as “buffer layer” to protect the underlying electron transport layer and perovskite active layer from damage during the subsequent sputter process of ITO.The buffer layer had proper bandgap and work function,enabling itself as an electronselective contact simultaneously.The initial efficiency of semitransparent perovskite device without AZO buffer layer (6% efficiency)was worse than device with buffer layer (12.3% efficiency).This is most likely due to the damage during sputter process of ITO.The semitransparent device had a T80lifetime of 124 h when operated at 100 °C under LED illumination.Introducing MoOXas a buffer layer is also reported previously120.The evaporated MoOXlayer,used to be the passivated holeselective contact in silicon heterojunction solar cell,has been proven to be able to protect perovskite active layer underneath from sputter damage.
In addition to the protection from sputter process,a careful protection from solvent damage to perovskite during spincoating process is also vital.It is difficult to use orthogonal solvents method to fabricate two perovskite sub-cells in a solution-processed perovskite/perovskite tandem.This is because the perovskite precursor is able to dissolve the previously spin-coated perovskite active layer underneath.Thus,the interconnecting layer must be designed to have robust resistance against subsequent solution-based deposition process.Chang et al.121designed an interconnecting layer composed of cross-linked PTAA and hexamethonium bromide (HMB)doped PC61BM in perovskite/perovskite tandem.They used 1,2-bis[4-(azidomethyl)phenyl]-1,2-diphenylethene (TPE-MN3)to crosslink PTAA and increase the thickness of PTAA,in order to improve the solvent resistance.Besides,the tris-[1-(trifluoroethanoyl)-2-(trifluoro-methyl)ethane1,2-dithiolene](Mo(tfdCOCF3)3)was used to dope PTAA to improve electrical conductivity of a thicker PTAA film.After encapsulation with alumina (Al2O3),a 2T tandem device remained more than 70% of its initial efficiency after 2100 h under MPP tracking in ambient air (30 °C,60% relative humidity).PEDOT:PSS is widely used as hole transport layer and can also be served as transparent electrode in recombination layer due to its high conductivity and transparency.However,it is usually dispersed in water,which would cause damage to the perovskite layer if it is directly spin-coated on perovskite sub-cell.Zhou et al.122demonstrated a dry-transferred method to deposit PEDOT:PSS in interconnecting layer as transparent electrode.This is an effective method to avoid directly spin-coating aqueous solution.In summary,the interconnecting layer needs to be modified to protect perovskite active layers underneath from damage of fabrication process of functional layers sequentially deposited.
6.2 Interfacial reaction between interconnecting layer and perovskite
Most recombination layer used in perovskite/perovskite tandems contains an n-type and a p-type layer,that is,carrier transport layers in perovskite sub-cells.They are used to extract electrons and holes from different sub-cells,by directly contacting with perovskite active layer and electrodes.The interfacial reaction between carrier transport layers in recombination layer and perovskite can also result in instability of perovskite-based tandems.As discussed above,Huang et al.31demonstrated a simplified interconnecting layer composed of C60and SnO1.76,improving the photostability of perovskite/perovskite tandem device.The SnO1.76film was fabricated via low-temperature atomic layer deposition (ALD)process,serving as hole transport layer in narrow bandgap Sn-Pb perovskite subcell.It is found that the photocurrent output of single junction Sn-Pb perovskite solar cell with SnO1.76as hole transport layer was more stable than device utilized commonly used PEDOT:PSS under MPP condition.The improved stability could be attributed to the replacement of PEDOT:PSS,as PEDOT:PSS could cause damage to stability of single junction perovskite solar cell through the corrosion of ITO and the interfacial reaction with Sn-Pb perovskite85,123.In addition,the strategies reported in the single junction perovskite solar cell to suppress the interfacial chemical reaction between perovskite and metal electrode (such as I‒and Ag),may also be helpful for improving the stability of perovskite-based tandem solar cell.
7 Summary and outlook
Perovskite-based tandem solar cells have demonstrated impressive power conversion efficiency that can satisfy the requirement for commercial applications.However,long-term stability of perovskite-based tandem devices is still the most serious problem for its economic feasibility.In this review,we have first summarized the recent advances in the stability of perovskite-based tandem solar cells,including perovskite/silicon and perovskite/perovskite tandem devices.Then,we specially discussed the phase instability of wide bandgap perovskite,atmospheric instability of narrow bandgap perovskite and the potential intrinsic instability issue in tandem solar cells,as well as the corresponding strategies to tackle these instability issues.At last,we briefly discussed instability issues related with the interconnecting layer.We highlight that intrinsic instability of perovskite sub-cells is the major cause for the instability of perovskite-based tandem solar cells.
At present,perovskite/silicon tandems are considered as the most attractive perovskite-based configuration.The semitransparent perovskite sub-cell with wide bandgap perovskites as absorbers can be utilized as a supplement for single junction solar cell,reducing energy loss and further improving efficiency.Considering that the perovskite material is very sensitive to the external environment,encapsulation may be an evitable method to improve device stability,which needs to be further explored.Perovskite-based tandem devices have demonstrated excellent PCEs,leaving the potential long-term instability as the major obstacle.At present,there is few publications devoted to understanding improving the long-term stability of perovskite-based tandem devices.Researchers are currently focusing on improving the instability of individual parts of perovskite-based tandem devices,i.e.,the phase instability of top sub-cell based on mixed halide perovskite or atmospheric instability of Sn-Pb sub-cell in all-perovskite tandem devices.It needs to be further explored that whether two sub-cells would affect each other especially in 2T configuration during working conditions,resulting in additional instability problem.Future work should pay more attention on the overall instability issues of perovskite-based tandem devices during working conditions.
In addition,standard testing procedures and protocols for perovskite-based tandem stability needs to be established.Recently,a consensus on procedures for testing perovskite solar cell stability has been published124.This guideline is beneficial for setting a standard to compare stability data from different works.A consensus that pays more attention to the perovskitebased tandem devices is also needed in the future.We expect that with the more and deeper understanding of the origin of perovskite-based tandem devices’ instability,lifetime up to 20 years can be achieved via modification of perovskite materials and optimization of tandem device structure.
- 物理化学学报的其它文章
- SCN-doped CsPbI3 for Improving Stability and Photodetection Performance of Colloidal Quantum Dots
- 电泳法制备的致密氧化锡薄膜及其在高稳定性钙钛矿太阳能电池中的应用
- Highly Moisture Resistant 5-Aminovaleric Acid Crosslinked CH3NH3PbBr3 Perovskite Film with ALD-Al2O3 Protection
- Structural,Thermodynamical and Electronic Properties of All-Inorganic Lead Halide Perovskites
- 基于易升华添加剂辅助合成纯相富铯CH(NH2)2)xCs1−xPbI3钙钛矿
- 锡基钙钛矿太阳能电池研究进展

