Recent Progress in Defect Tolerance and Defect Passivation in Halide Perovskite Solar Cells
Yuan Yin ,Zhendong Guo ,Gaoyuan Chen ,Huifeng Zhang ,Wan-Jian Yin ,*
1 College of Physics and Optoelectronic Technology,Baoji University of Arts and Sciences,Baoji 721013,Shaanxi Province,China.
2 College of Energy,Soochow Institute for Energy and Materials InnovationS (SIEMIS),Soochow University,Suzhou 215006,Jiangsu Province,China.
Abstract:In less than a decade,metal halide perovskites (MHPs)have been demonstrated as promising solar cell materials because the photoelectric conversion efficiency (PCE)of the representative material CH3NH3PbI3 rapidly increased from 3.8% in 2009 to 25.2% in 2009.However,defects play crucial roles in the rapid development of perovskite solar cells (PSCs)because they can influence the photovoltaic parameters of PSCs,such as the open circuit voltage,short-circuit current density,fill factor,and PCE.Among a series of superior optoelectronic properties,defect tolerance,i.e.,the dominate defects are shallow and do not act as strong nonradiative recombination centers,is considered to be a unique property of MHPs,which is responsible for its surprisingly high PCE.Currently,the growth of PCE has gradually slowed,which is due to low concentrations of deep detrimental defects that can influence the performances of PSCs.To further improve the PCE and stability of PSCs,it is necessary to eliminate the impact of these minor detrimental defects in perovskites,including point defects,grain boundaries (GBs),surfaces,and interfaces,because nonradiative recombination centers seriously affect device performance,such as carrier generation and transport.Owing to its defect tolerance,most intrinsic point defects,such as VI and VMA,form shallow level traps in CH3NH3PbI3.The structural and electronic characteristics of the charged point defect VI− are similar to those of the unknown donor center in a tetrahedral semiconductor.It is a harmful defect caused by a large atomic displacement and can be passivated to strengthen chemical bonds and prevent atom migration by the addition of Br atoms.Owing to the ionic nature of MHPs and high ion migration speed,there are a large number of deep detrimental defects that can migrate to the interfaces under an electric field and influence the performance of PSCs.In addition,the ionic nature of MHPs results in surface/interface dangling bonds terminated with cations or anions;thus,deep defects can be passivated through Coulomb interactions between charged ions and passivators.Hence,the de-active deep-level traps resulting from charged defects can be passivated via coordinate bonding or ionic bonding.Usually,surface-terminated anions or cations can be passivated by corresponding cations or anions through ionic bonding,and Lewis acids or bases can be passivated through coordinated bonding.In this review,we not only briefly summarize recent research progress in defect tolerance,including the soft phonon mode and polaron effect,but also strategies for defect passivation,including ionic bonding with cations or anions and coordinated bonding with Lewis acids or bases.
Key Words:Defect tolerance; Defect passivation; Perovskite; Solar cell; Photoelectric conversion efficiency
1 Introduction
Metal halide perovskites (MHPs)are originally reported in the 1970s1.However,they did not draw widespread interest until the year around 2010 when organic-inorganic MHP CH3NH3PbI3(MAPbI3)was implemented as solar cell absorber and achieved promising efficiency.The photoelectric conversion efficiency (PCE)of MHP goes rapidly from 3.8% in 2009 to 25.2% in 20192,3.Perovskite solar cells (PSCs)have been considered as a powerful contender for the third generation photovoltaic (PV)absorber materials due to a series of superior optoelectronic properties,including excellent light absorption coefficient (~105cm−1)4,long carrier diffusion lengths in polycrystalline films (> 1 µm)5,6,high carrier mobility7,8,tunable direct bandgap9,10,high defect tolerance11and low production cost12–14.Among them,defect tolerance has been considered as a unique feature of MHPs and as the main reason why PSC has high PCE although it is fabricated in various environments15.
Although MHPs possess the above excellent photoelectric performances,its reported PCE is still far below its theoretical limit efficiency of 30.5%16–19.The energy loss is mainly attributed to nonradiative recombination of photo-excited charged carriers at internal point defects,surface and interface11,20,21.During the nonradiative recombination process,the internal electrical energy is released through phonons (heat).According to previous reports,there are two main types of defects in MHPs that have been identified as significant impact on nonradiative recombination affecting device performances.One is point defects,including intrinsic defects (vacancy,antisite substitution and interstitial)and impurities (doped ion)11,22–31,and the other is 2D defects,such as grain boundaries (GBs)and surface/interface defects31,32.The concept of ‘defect tolerance’ is discovered based on the theoretical calculations on point defects11,which result in shallow defect levels and thus have a negligible contribution on nonradiative recombination.Due to the ionic nature of MHPs and high ion migration speed,some deep detrimental defects with low concentration can migrate to interfaces under an electric field and influence the performances of PSCs25,33–36.Therefore,in order to further improve the PCE of PSCs,it is necessary to effectively passivate the defects causing deep levels.
In this review,we present recent advances on understanding defect tolerance,and summarize the achievements that have been made to improve PCE of PSCs,reduce nonradiative charge recombination,and enhance device stability by defect passivation.
2 Defects impact on performance of perovskite solar cells
Defects unavoidably exist in MHPs and are crucial factors affecting the cell performance.They could be beneficial since it will result in p- or n-type conductivity and help separate the photogenerated electrons and holes.On the other hand,they could act as the Shockley-Read-Hall (SRH)nonradiative recombination centers for trapping electrons and holes,thus reduce the carrier lifetime and VOC.Recent studies revealed that the SRH recombination is the dominant pathway of charged carrier loss in perovskite materials16,37.
In contrast to conventional solar cell absorbers,defect tolerance was considered as the unique properties of MHPs leading to its surprisingly high PCE.Taking MAPbI3as an example,we systematically studied all the possible point defects,including three interstitials (MAi,Pbi,Ii),three vacancies (VMA,VPb,VI),two cation substitutions (MAPb,PbMA)and four antisite substitutions (MAI,PbI,IMA,IPb),by the first-principles calculations11.Shallow defects with relatively low formation energy close to band edges,can accept or donate electrons are benign for absorbers.However,deep defects with relatively high formation energy near the middle of bandgap,can trap electrons or holes as SRH nonradiative recombination are harmful for absorbers.Fig.1 gives the transition levels of all possible point defects in MAPbI3.The predominant defect levels,including acceptors (VPb,MAPb)and donors (MAi,VI),are shallow.Defects with deep levels bear high formation energy thus low defect concentration that expected to have low impact on PCE.Similar conclusion are subsequently confirmed by Kim et al.22used DFT-GGA to calculate the DOS and partial charge densities of neutral defects,and Du38used DFT-PBE to calculate the point defects properties in β phase MAPbI3.In addition,Buin et al.26found that the surface with minimum energy in perovskite crystals could be completely free of defect traps.They found these point defects do not create deep gap states as well.As a result,shallow defect levels have no significant effect on carrier recombination11,39,40,which is thought to be the main reason for the unusually high solar efficiency of perovskite15.

Fig.1 The transition energy levels of (a)intrinsic acceptors and (b)intrinsic donors in MAPbI3.
Recently,Chu et al.41used nonadiabatic molecular dynamics simulations combined with time-dependent density-functional theory and proposed alternative explanation for defect tolerance in the MHPs that the characteristic low-frequency lattice phonon modes decrease the non-adiabatic coupling and weaken the overlap between the free carrier and defect states.In their calculations,some deep defect states such as Ii,MAI,MAPb,VPb,and VIexhibit low electron-hole recombination rate,therefore,they claimed the possible breakdown of SRH theory.While Kim and Walsh42disagreed with this interpretation and they thought there could be flaws in this methodology employed for electronphonon coupling calculation.
Another explanation about the high PCEs for PSC is the possible formation of large polarons,which was observed in single crystal MAPbBr3and CsPbBr3using time-resolved optical Kerr effect spectroscopy43.In conjunction with firstprinciples calculations,Miyata et al.43suggested that the formation of large polarons predominantly stems from the deformation of Pb-Br-Pb framework.However,Neukirch et al.44proposed volumetric strain in MAPbI3creates a polaron with binding energy of around 600 and 1300 meV for holes and electrons respectively using the hybrid density functional theory,such large reorganization energies indicate the existence of small polarons in organometallic perovskite materials.Hole and electron spin density distribution of optimized charged are shown in Fig.2a,b.To address the slow electron-hole recombination in MHPs,Ambrosio et al.45simulated the evolution of extra electrons and holes through advanced ab initio molecular dynamics,emphasizes that the separate polaronic localization of electrons and holes is the key feature for achieving excellent photovoltaic properties,the charge localization for a hole and an electron are shown in Fig.2c,d.

Fig.2 Hole (a)and electron (b)spin density distribution of charged MAPbI3 clusters.Structural rearrangements leading to charge localization (c)for an extra hole and (d)for an extra electron.
3 Defects passivation in perovskites
Defect tolerance explains the high efficiency of PSCs in various synthesis environments15,46.Currently,the growth of PCE gradually slows down.In order to further improve the PCE and stability of PSCs,it is necessary to eliminate relatively low concentration of detrimental defects in perovskite including point defects,GBs,surfaces and interfaces,as nonradiative recombination centers seriously affects the carrier generation and transport47.Fig.3 illustrates the primary defects on the surface or GBs of standard perovskite crystals and various approaches to passivate these defects48.In these defects,undercoordinated ion species (I−and Pb2+),Pb-I antisite (PbI3−)and metal Pb cluster may create deep level traps,while intrinsic point defects (VIand VMA)are reported to form shallow level traps.Meanwhile,defect passivation introduce hydrophobic functional groups,therefore,may enhance the stability of PSCs49.For example,Guo et al.50presented a simple and effective passivation strategy at surface and GBs in MA-base perovskite based on PEA molecules,as illustrated in Fig.4g.The PCE and stability of PSCs has been effectively improved,that only 5% efficiency loss after 800 h storage at a humidity of 50% + 5% at room temperature.
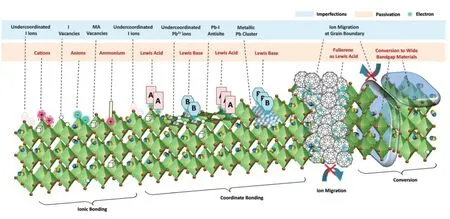
Fig.3 Defects in PSCs films and their passivation by ionic bonding,coordinated bonding,et al.
3.1 Point defects passivation
As early as 2014,based on first-principles calculation,Michael et al.51found that stable Pb dimer and I trimer can be formed under specific charge states of some intrinsic defects,as shown in Fig.4b,c,which significantly reduced the lattice energy and led to the formation of deep trap state in the band gap.In addition,Du38performed defect calculation and showed that the intrinsic point defect Iiis a low energy deep trap,which could be a nonradiative recombination center.Recent theoretical studies by Yang et al.51have found that DY center is easy to form in MAPbBr3of Bi-doped perovskite,thus limiting n-type doping.Ming et al.52stated that some noble and transition metal impurities such as Au,Ag,Cu could form and diffuse in MAPbI3and can be used for the optimal metal electrodes.Klug et al.53deemed that the lead-cobalt compositions can efficiently improve the power conversion efficiency when less than 6% of Pb2+ions are replaced by Co2+.
Recently,Wang et al.54found that the structural and electronic characteristics of VI−in MAPbI3are similar to the DX in tetrahedral semiconductor,which is a harmful defect caused by a large atomic displacement,as shown in Fig.4d–f.The addition of Br can passivate this defect,strengthen chemical bonds,and prevent the migration of atoms.Time-dependent density functional theory calculations within nonadiabatic molecular dynamics has shown that the carrier lifetime of MAPbI3is increased from 3.2 to 19 ns (MAPb(I0.96Br0.04)3).Wang et al.55systematically studied the conditions of MHP in chemical environment of various molecular functional groups,and found that the stable PCE can reached 22.6% after the treatment of theophylline,as illustrated in Fig.4a.Therefore,defect passivation is efficient for the reduction of nonradiative recombination.

Fig.4 (a)Stabilized PCE and current density as a function of time; Pb dimer of VI− (b)and I trimer of IMA0 (c); schematic illustration of nondimer (d)and dimer (e)structure for VI; (f)the formation mechanism of defect levels for DX center in MAPbI3;(g)schematic illustration of GBs passivation for enhanced moisture tolerance.
3.2 Surface/interface defect passivation
Defects are observed abundant at interface/surface and GBs,with concentration 2–4 orders higher than that in bulk56.Surface/interface defects and GBs are considered as the main origins for nonradiative recombination.Recently,Ni et al.56demonstrated deep defect density of 1.0 × 1017cm−3at the interfaces,which were two or three orders of magnitude greater than point defects in film (5.0 × 1014cm−3).
For surfaces/interfaces or GBs in perovskites,the crystal structure is suddenly interrupted and there are a large amount of localized surface states bringing in deep level traps.Usually,these deep states are described by dangling bonds,which can be a major nonradiative recombination pathway in the MHPs.Therefore,the effective passivation of the extended surface defects is one of the cores for avoiding nonradiative recombination to improve the PCE of PSCs57–59.Recently,a lot of efforts on defect passivation have been made to effectively control and eliminate nonradiative recombination centers60.
The ionic nature of MHP’s lattice results in surface/interface dangling bond terminated with cations or anions,making it possible to realize defect passivation through the Coulomb interaction between charged ions and charged functional groups.The de-active deep level traps resulting from charged defects can be passivated via coordinate bonding or ionic bonding,which is different from that of covalent bond-based semiconductors like silicon.For example,the excess electrons of undercoordinated I−and Pb-I antisite (PbI3−)defects can be passivated by cations through ionic bonding61–65or by Lewis acid through coordinate bonding66,67.Additionally,the undercoordinated Pb2+at the perovskite surfaces/interfaces and GBs has an ability to accept electrons serving as Lewis base.
3.2.1 Ionic bonding passivation with cations and anions
A common approach to passivate ionic compounds is introducing charged ions in perovskites that have opposite charge to the dangling ions.
The introduction of metal ions and organic molecules usually have effective passivating effects on anionic defects,such as undercoordinated I−,PbI3−in halide perovskites.Bi et al.61reported that metal sodium ions (Na+)were capable of diffusing from substrates into GBs,which contributes to enhanced PL lifetimes and PCE improvement by passivation effect on the GBs in perovskites.Son et al.57later systematically studies the passivation effect of all alkali metal ions,as shown in Fig.5a.All the metal iodide,including LiI,NaI,KI,RbI and CsI,were doped with same dose in perovskite and the hysteresis nearly disappears only when KI was doped.Additionally,Abdi-Jalebi et al.62reported that the K+could accumulate the passivation of extended defects and produce internal Photoluminescence Quantum Yields (PLQYs)than 95% when the KI was added to a Cs0.06FA0.79MA0.15Pb(I0.85Br0.15)3perovskite precursor,as illustrated in Fig.5b and Table 1.Meanwhile,the PCE of perovskite thin films is high to 21.5% and the initial PCE over 80% after 300 h.Ionic interactions between K+and undercoordinated Br−and I−at the surface and GBs contribute to the improvement of PLQYs,PCE and stability,as illustrated in Fig.5c.Beyond metal cation,a number of small organic molecules with alkylammonium functionalities,such as butylammonium (BA+),phenylethylammonium (PEA+),octylammonium (OA+)and diammonium derivatives,have been used as passivators for their ability to enhance device performance and stability63,68–72.
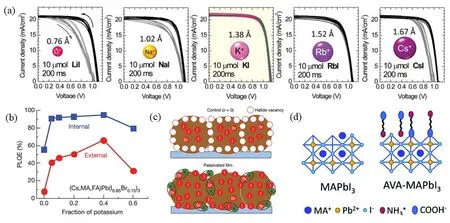
Fig.5 J–V curves (a)of PSCs based on doped with LiI,NaI,KI,RbI and CsI; PLQEs (b)of KI passivated PSCs with the increasing fraction of K+; (c)schematic of VI control in case of excess I; (d)schematic of AVA passivation at termination of MAPbI3.

Table 1 Summarization of defect passivation by ionic bonding and coordinate bonding for PSCs:passivators,structures,perovskite materials,passivation functional groups,target defects,and device parameters without (C)and with passivation (P).
Contrary to cations,anions are added to passivate the dominate cation defects,such as undercoordinated Pb2+and VI.One reported anion defect passivator of PSCs is halide ion Cl−,which is introduced through PbCl2,NH4Cl,MaCl,FACl and CsCl73–78.The additional Cl−could improve the device performance of PSCs by improving electronic properties,which can be indicated by the defect activation energy reduced from 74.4 to 21.6 meV79.Theoretical simulation by Nan et al.80suggested the introducing of a tiny amount of Cl−can remit the deep trap states created by VPband VI.Another reported anion halide ion I−could compensate the VIat the GBs to inhibit the nonradiative recombination pathways61,65.Additionally,as the halide ion F−possesses the strongest electronegativity and can form strong ionic bonds with Pb2+and hydrogen bonds with MA+,which have been verified by Li et al.81using DFT calculations.Beyond halogen anions,halogen-like organic anions can passivate the nonradiative recombination as well,among which -COOH−passivates the positively charged defects in perovskite films though ionic bonding,while another halogen-like organic anion -COOH−passivates the positively charged defects via hydrogen bonding,which is illustrated in Fig.5d82,83.Other anions that produce similar effects are also found to be SCN−and O2−84,85.
3.2.2 Coordinate bonding passivation with Lewis acid and base
Lewis acids and bases refer to a class of molecules or ions that have been used for the passivation of undercoordinated defects.A Lewis acid accepts a foreign electron pair,while a Lewis base gives an electron pair,which could combine with undercoordinated defects by coordinate covalent bond to form Lewis complexes without electron transfer.The selective interactions between Lewis acid or base functional groups and the perovskite crystal sites are showed in Fig.6a,where Lewis acid passivates I−or Pb-I antisite (PbI3−),while Lewis base can be strongly bonded with Pb2+.The passivation of harmful defects by Lewis acid or base have been proven to be an efficiency methodology for improving the performance and stability of PSCs.Xu et al.86firstly reported Fullerene (C60)derivatives PCBM reacts with I−at the GBs in perovskites materials and which promotes electrons extraction.This interaction is in accord with the mechanism for passivation by a Lewis acid.Their DFT calculations proposed this interaction between PCBM and surface or GBs can be shown by hybridization,as illustrated in Fig.6b86and Table I.As shown in Fig.6c,Nobel et al.87firstly proposed that thiophene or pyridine would work as a Lewis base to passivate undercoordinated Pb2+in perovskites.DFT calculations show strong evidence that these Lewis bases have strong coordination with undercoordinated Pb2+,as shown in Fig.6d,which can reduce the recombination and improve lifetimes to enhance perovskite performance and stability.
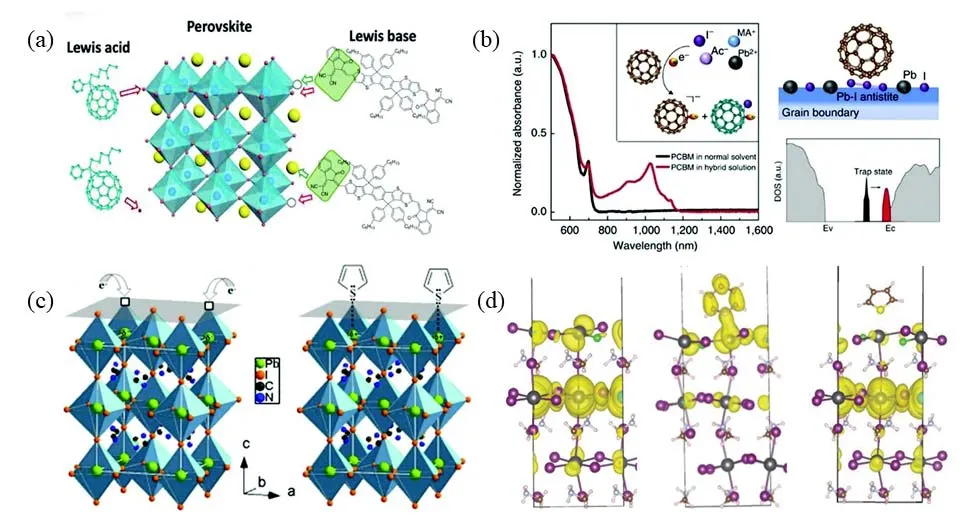
Fig.6 (a)Selective interactions between Lewis functional groups and perovskite; (b)interaction between PCBM and perovskite ions;(c)selective of pyridine and thiophene passivating undercoordinated Pb2+; (d)charge density of bulk,Cl− doped and surface passivation in MAPbI3.
4 Summary and outlook
PSCs have been considered as a powerful contender for a series of superior optoelectronic properties,and defect tolerance has been considered as a unique feature of MHPs and as the main reason why PSC has high PCE although it is fabricated in various environments.In summary,we discussed the defect tolerance impact on performance of PSCs that major point defects are shallow levels and are benign for nonradiative recombination88.However,although the PCE of PSCs exceeding 25%,but this value is still lower than its theorical limit of 30.5%.In order to further improve the PCE and stability of PSCs,defect passivation has been proven as an efficient way to eliminate relatively low concentration of detrimental defects including point defects,GBs,surfaces,and interfaces.Here we summarized the research progresses of defect passivation for high efficiency PSCs in recent years,and expounded the passivation mechanism and passivators based on ionic bonding and coordinate bonding.Effective defect passivation can improve the performance of PSCs by promoting the PCE,reducing the nonradiative recombination,and enhancing the device stability.Of course,more detail works about the coupling of bulk defects and GBs,surfaces,or interface defects is still needed in the future.
- 物理化学学报的其它文章
- SCN-doped CsPbI3 for Improving Stability and Photodetection Performance of Colloidal Quantum Dots
- 电泳法制备的致密氧化锡薄膜及其在高稳定性钙钛矿太阳能电池中的应用
- Highly Moisture Resistant 5-Aminovaleric Acid Crosslinked CH3NH3PbBr3 Perovskite Film with ALD-Al2O3 Protection
- Structural,Thermodynamical and Electronic Properties of All-Inorganic Lead Halide Perovskites
- 基于易升华添加剂辅助合成纯相富铯CH(NH2)2)xCs1−xPbI3钙钛矿
- 锡基钙钛矿太阳能电池研究进展

