瞬烯类似物C9H9+、C9BH9和 C8NH9中的流变键
高彩月,马媛媛,闫苗,李思殿
(山西大学 分子科学研究所,山西 太原 030006)
1 Introduction
Various molecules are known in chemistry to exhibit structural fluxionalities under certain conditions.Typical fluxional molecules include iron pentacarbonyl Fe(CO)5,phosphorus pentafluoride PF5,dimethylformamide(CH3)2NC(O)H,and protonated methane CH5+which undergo Berry pseudo-rotations through the bending,swaying,or stretching of localized two-centertwo-electron(2c-2e)σ bonds[1-6].No chemical bonds are formed or broken in the fluxional processes of these molecules.Our group recently proposed the concept of fluxional bonds(FBs)in planar B19-,tubular Ta@B20-,and cage-like B39-,as an extension of the classical localized and delocalized bonds known in chemistry[7].FBs in these species form and break constantly under certain conditions at finite temperatures.We also reported multi-center FBs in half-sandwich MB18-(M=K,Rb and Cs)[8]and NiB11-[9],planar or quasi-planarandC2vB15+(B4@B11+)[13-14],and tubular B3-[Ta@B18],B4-[Ta@B18]+,and La2[B2@B18][15-16].Typical π-FBs and σ-FBs were very recently observed in the well-known fluxional molecule bullvalene(C10H10)and its analogs C8H8,C9H10and C8BH9in rapid Cope rearrangements[17],opening the door to explore novel FBs in both stable organic molecules and important catalysis processes.
Based on extensive first-principles theory calculations and detailed bonding analyses,we report herein the existence of multi-center FBs in more bullvalene analogs including the cage-like C9H9+,C9BH9and C8NH9.Two fluxional σ-bonds were revealed in the fluxional process of C9H9+,while two fluxional πbonds and one fluxional σ-bond were identified in concerted mechanisms in both C9BH9and C8NH9.The activation energies between the ground states and transition states and the corresponding reaction rate constantskTSTare predicted to facilitate future experiments.
2 Theoretical procedure and computational method
The ground state(GS)and transition state(TS)of all molecules were optimized at the DFT-PBE0[18-20]level with the basis sets of 6-311+G(d)[21].Frequency checks were performed to ensure that all optimized structures are true GSs or TSs.Intrinsic reaction coordinate(IRC)[22-23]path analyses were also performed to confirm the TS structures.All the PBE0 calculations in this work were performed using the Gaussian 09 package[24].The activation energies in the fluxional processes were further refined at the more accurate CCSD(T)/6-311G(d)level[25-27]at PBE0 geometries using the Molpro program[28].Detailed bonding analyses were performed on the GS,TS,and GS′of the concerned species(GS′represents the second ground state in the GS→TS→GS′fluctuation process.It may be the same as the GS or it may be an intermediate state in the reaction path)using the adaptive natural density partitioning(AdNDP)program[29-30].The reaction rate constantkis calculated based on the transition-state theory(TST)[31]using the following equation:[32]

where,κ,kB,T,h,ΔG≠,andRare the transmission coefficient,Boltzmann constant,temperature,Planck constant,standard activation free energy,and molar gas constant,respectively.The transmission coefficientκcan be valued by the Wigner tunneling correction[33],κ=1+(1/24)[hIm(v≠)/kBT]2,where Im(v≠)is the imaginary frequency of the transition state.The calculated results are summarized in Table 1 to facilitate future experimental confirmations of these fluxional species.
3 Results and discussion
3.1 Structures and stabilities
The closed-shellC3vGS( 11),C2vTS( 22)andC3vGS'( 33)of C9H9+in Fig.1 are constructed by removing one CH group from the experimentally knownC3vGS,C2vTS,andC3vGS′of C10H10[17],respectively.Similarly,C3vGS( 44),CsTS( 55)andCsGS'( 66)of C9BH9can be obtained by substituting one CH group in C10H10with a trivalent B atom.CsGS( 77),CsTS( 88),andCsGS'( 99)of C8NH9are achieved by substituting one C atom in C9H9+with a trivalent N(Fig.1).There exists a three-center bonding interaction between C3,C4 and C5 in theC3vGS( 11)which is the true GS of the monocation with no imaginary vibra-tional frequency.The corresponding degenerateC3vGS'( 33)can be obtained by turning theC3vGS( 11)structure up-down.TheC2vC9H9+( 22)as the transition state betweenC3vGS( 11)andC3vGS′( 33)has one imaginary frequency at-544.33 cm-1.As can be seen from the bond lengths indicated in Fig.1,the distance between C3-C4 at the bottom withrC3-C4=1.81 Å in the GS has been shortened torC3-C4=1.57 Å in the TS,while the C1-C2 single bond on the top withrC1-C2=1.51 Å in the GS has been elongated torC1-C2=1.57 Å in the TS.That is,a C3-C4 single σ bond is formed in the TS while the C1-C2 σ bond in the GS is to be broken.From the TSC2vC9H9+( 22)to the GS'C3vC9H9+( 33),the opposite process occurs.In the fluxional process of GS→TS→GS',the upward and downward sways of C5 induce the structural fluctuation of the monocation.The calculated activation energy of ΔEa=22.87 kcal·mol-1at CCSD(T)from the GS to the TS of C9H9+approximately doubles the corresponding value of ΔEa=12.9 kcal·mol-1calculated for bullvalene C10H10at the same level of theory[17].

Fig.1 Optimized structures of the ground state(GS/GS')and transition state(TS)of(a)C9H9+,(b)C9BH9and(c)C8NH9,with the lowest vibrational frequenciesvminat PBE0 level and relative energies ΔEaat the single-point CCSD(T)//PBE0/6-311+G(d)indicated.The bond lengths are labelled in Å
TheC3vGS( 44)of C9BH9contains three equivalent C-C σ bonds on the top and three equivalent C=C double bonds on the waist.Its transition stateCsC9BH9( 55)has one imaginary frequency at-254.58 cm-1.The C1-C2 distancerC1-C2=1.53 Å inC3vC9BH9( 44)has been elongated torC1-C2=2.15 Å inCsC9BH9( 55),while C5-C6 distancerc5-c6=2.65 Å in the GS has been simultaneously shortened torc5-c6=2.21 Å in the TS.The calculated C1-C3/C2-C4(1.39 Å)and C3-C5/C4-C6(1.40 Å)distances in theCsTS have practically the same bond lengths.This structural fluctuation indicates the transfer of one 2c-2e σ bond on C1-C2 in the GS into one 4c-2e σ bond on C1-C2 and C5-C6 in the TS and two π bonds from two 2c-2e π bonds over C3-C5/C4-C6 in the GS to two 3c-2e π bonds over C1C3C5/C2C4C6 in the TS.The intermediateCsGS′( 66)lies 6.87 kcal·mol-1higher than theC3vGS( 44)in energy.It contains a newly formed C5-C6 σ bond at the bottom,with the C1-C2 interaction on the top broken.
As shown in Fig.1,C8NH9possesses similar cage-like GS,TS,and GS′structures with C9BH9,withCsC8NH9( 77)being 13.10 and 5.51 kcal·mol-1more stable thanCsC8NH9( 88)andCsC8NH9( 99),respectively.The calculated activation energies of C9BH9and C8NH9are 14.42 and 13.10 kcal·mol-1from their GSs to TSs at CCSD(T)level,respectively,close to the energy barrier of 12.90 kcal·mol-1calculated at the same level for C10H10[17].The predicted rate constantskTSTof C9H9+,C9BH9and C8NH9from their GSs to TSs and from TSs to the GSs'at 298 K in Table 1 clearly indicates that C9BH9and C8NH9fluctuate much more quickly than C9H9+due to the huge differences in their activation free energies ΔG≠.It is also noticed that both the intermediate statesCsC9BH9( 66)andCsC8NH9( 99)with higher rate constantskTSTare short-lived species compared to their ground statesC3vC9BH9( 44)andCsC8NH9( 77),respectively.
3.2 Fluxional bonds in C9H9+
AdNDP bonding analyses recover both localized and delocalized bonds of the concerned species.As shown in Fig.2(a),theC3vGS possesses 21 2c-2e σ bonds including 12 C-C σ single bonds and 9 C-H σ single bonds with the occupation numbers of ON=1.94-1.99|e|and 1 3c-2e σ bond with ON=1.96|e|,among which the 3c-2e σ bond on C3,C4,and C5 at the bottom has been converted into a 2c-2e C3-C4 σ bond inC2vTS.An opposite process occurs from TS → GS′where the 2c-2e C1-C2 σ bond is converted into a 3c-2e σ bond on C1,C2,and C5 on the top inC3vGS′.Thus,C9H9+undergoes a GS(1 2c-2e σ +1 3c-2e σ)→ TS(2 2c-2e σ)→ GS′(1 2c-2e σ +1 3c-2e σ)bonding fluctuation which mainly involves two fluxional σ-bonds in a concerted mechanism,as shown in Fig.2(b).Such a process repeats itself at 950 K in molecular dynamics simulations(Video S1 in the Supporting Information).
3.3 Fluxional bonds in C9BH9and C8NH9
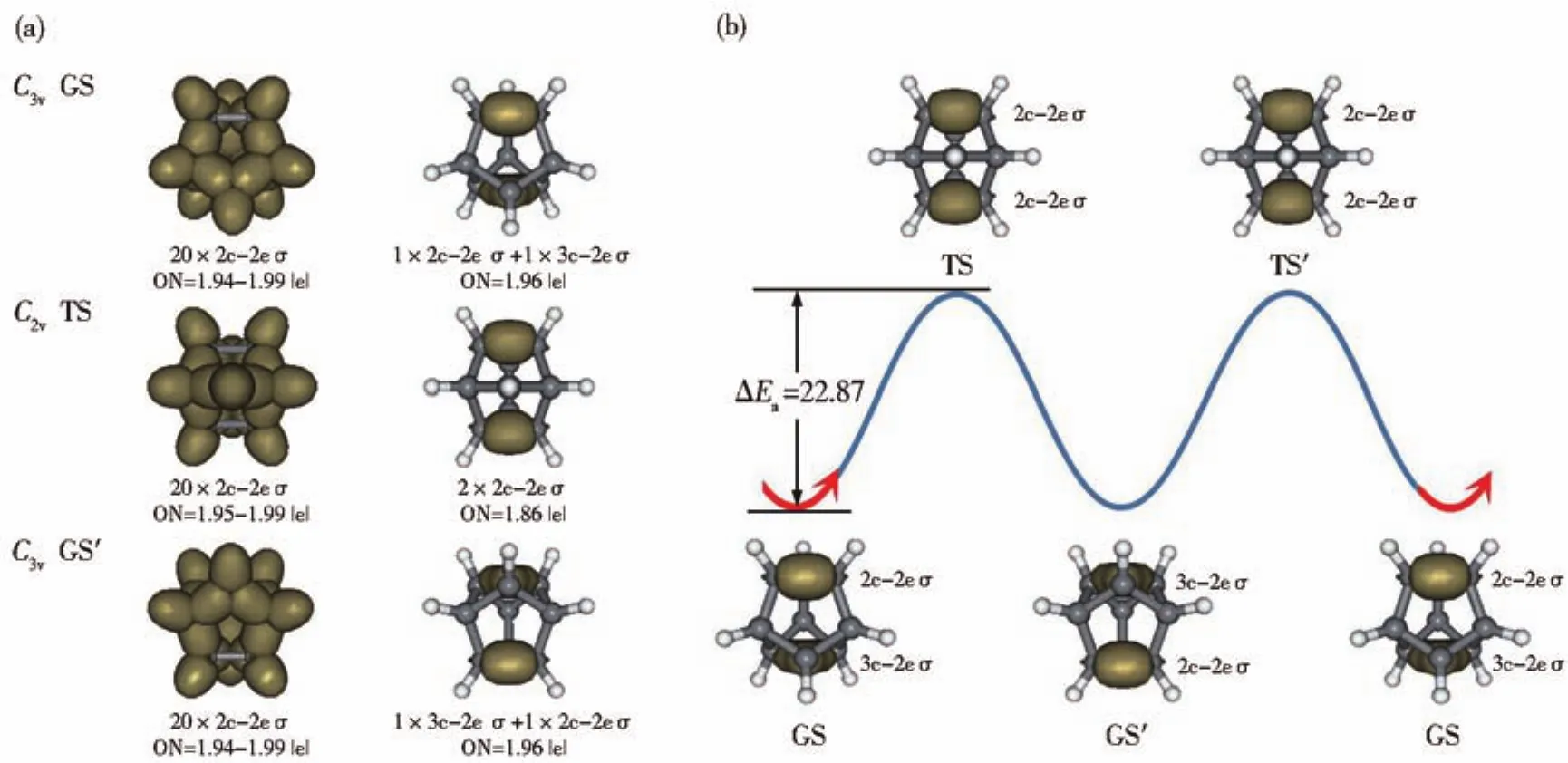
Fig.2 (a)AdNDP bonding patterns of the GS/GS' and TS of C9H9+.(b)σ-bonding fluctuation of C9H9+in the fluxional process of GS→TS→GS'→TS'→GS,with the energy barrier ΔEaindicated in kcal·mol-1
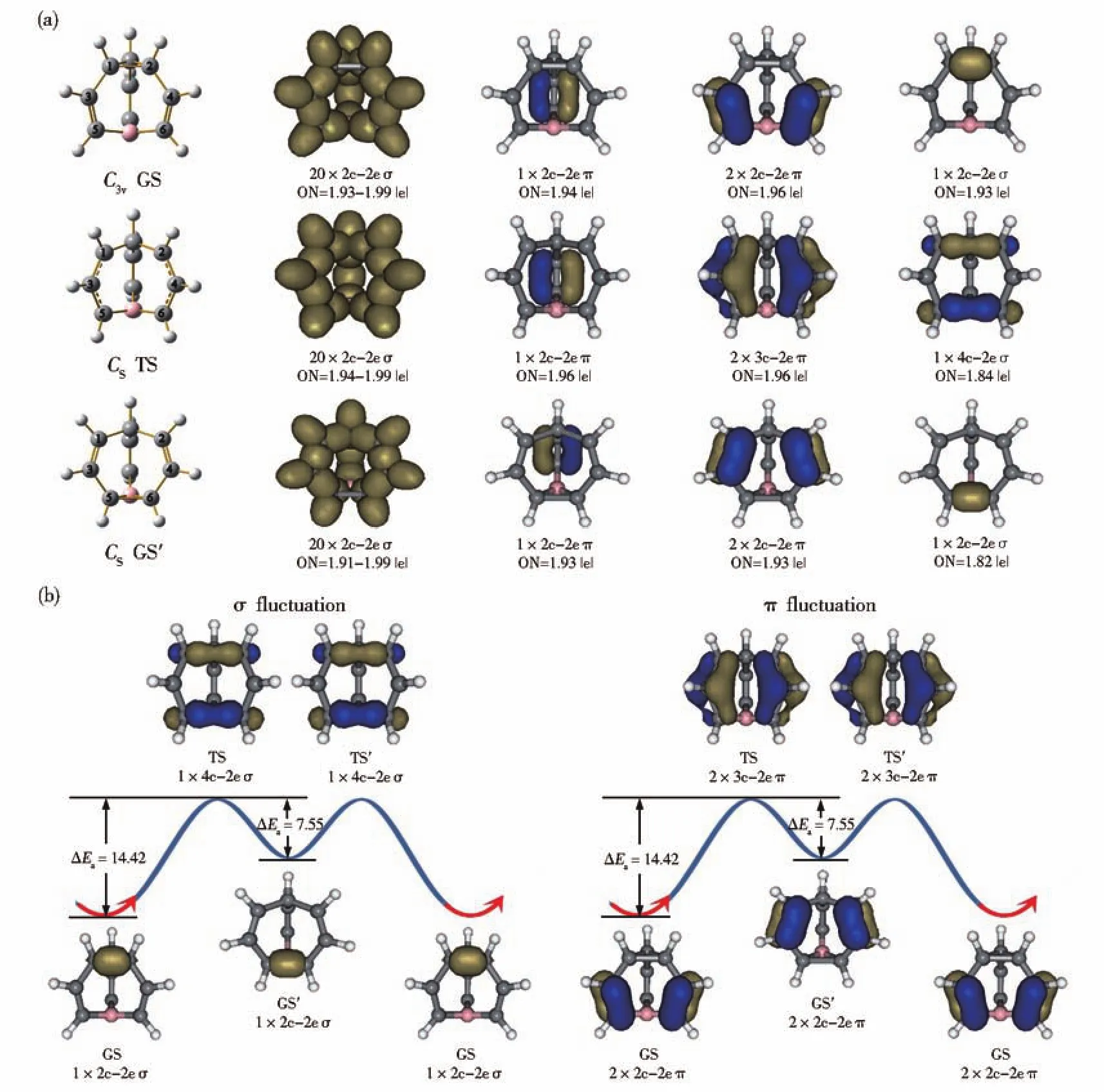
Fig.3 (a)AdNDP bonding patterns of the GS/GS' and TS of C9BH9.(b)σ and π bonding fluctuations of C9BH9in the fluxional process ofGS→TS→GS'→TS'→GS,with the energy barrier ΔEaindicated in kcal·mol-1
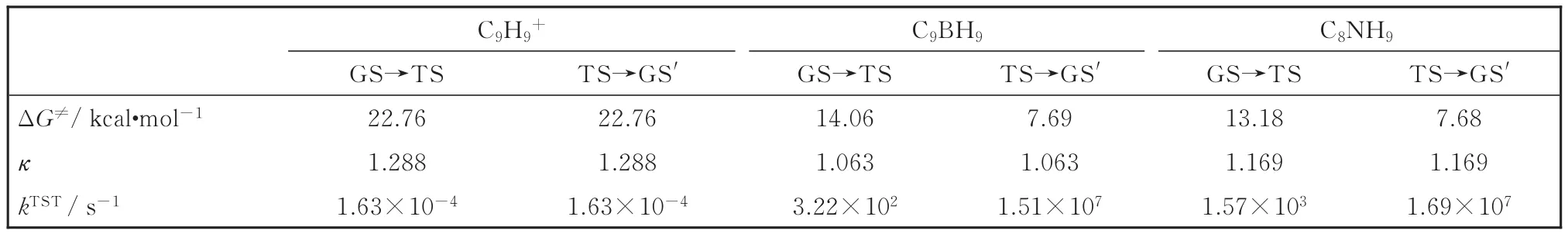
Table 1 Calculated standard activation free energies ΔG≠,transmission coefficientκ,and rate constantskTSTof C9H9+,C9BH9and C8NH9in the fluxional processes of GS→TS and TS→GS'at the single-point CCSD(T)//PBE0/6-311+G(d)level.
As indicated in Fig.3(a),the bonding fluctuation of C9BH9appears to be similar with that of bullvalene[17].It mainly involves two fluxional π bonds and one fluxional σ bond in the fluxional process.FromC3vGS toCsTS,two 2c-2e π bonds over C3-C5 and C4-C6 are converted into two 3c-2e π bonds over C1C3C5 and C2C4C6 along two edges in the front,while the 2c-2e C1-C2 σ bond on the top is transferred into a 4c-2e σ bond on C1-C2 and C5-C6.The remaining 20 2c-2e σ bonds on the surface and 1 2c-2e π bond at the back are kept basically unchanged in the fluxional process.An opposite process takes place from theCsTS toCsGS'.The fluctuation of C9BH9thus involves basically two fluxional π-bonds(GS(2 2c-2e π)→ TS(2 3c-2e π)→ GS′(2 2c-2e π))and one fluxional σ-bond(GS(1 2c-2e σ)→ TS(1 4c-2e σ)→ GS′(1 2c-2e σ))in concerted mechanisms(Fig.3(b)).We observed this process in molecular dynamics simulations at 800 K(Video S2).
Fig.S1(a)shows the AdNDP bonding pattern of C8NH9and Fig.S1(b)demonstrates the σ and π bonding fluctuations in it.Similar to C10H10and C9BH9,the bonding fluctuation of C8NH9mainly involves two fluxional π -bonds(GS(2 2c-2e π) → TS(2 3c-2e π) → GS′(2 2c-2e π))and one fluxional σ-bond(GS(1 2c-2e σ) → TS(1 4c-2e σ) → GS′(1 2c-2e σ)),while the remaining 19 2c-2e σ bonds remain basically unchanged.The breakage and formation of two πbonds and one σ-bond of C8NH9occurred at 800 K in molecular dynamics simulations(Video S3).To better visualize the bonding changes in these processes,we simulate the bonding fluctuations of C9H9+,C9BH9,and C8NH9in Video S4-S6 in the Supporting Information.
4 Summary
We have optimized the GS,TS,and GS′structures of bullvalene analogs C9H9+,C9BH9,and C8NH9,and analyzed the fluxional bonds in them which facilitate the fluxionality of these cage-like species,unveiling the σ bonding fluxional nature of C9H9+(GS(1 2c-2e σ +1 3c-2e σ)→ TS(2 2c-2e σ)→ GS′(1 2c-2e σ+1 3c-2e σ))and σ + π dual bonding fluctuation in both C9BH9and C8NH9(GS(2 2c-2e π) → TS(2 3c-2e π)→ GS′(2 2c-2e π)plus GS(1 2c-2e σ)→ TS(1 4c-2e σ) → GS′(1 2c-2e σ))in concerted mechanisms.More theoretical and experimental investigations on related fluxional molecules are currently in progresses.Fluxional bonds are expected to exist in various intramolecular rearrangements in organic organic chemistry and may have important applications in both chemical catalysis and nanomaterials designs.

